Metabonomic Response to Milk Proteins after a Single Bout of Heavy Resistance Exercise Elucidated by 1H Nuclear Magnetic Resonance Spectroscopy
Abstract
:1. Introduction
2. Results
2.1. Post-Exercise Effect

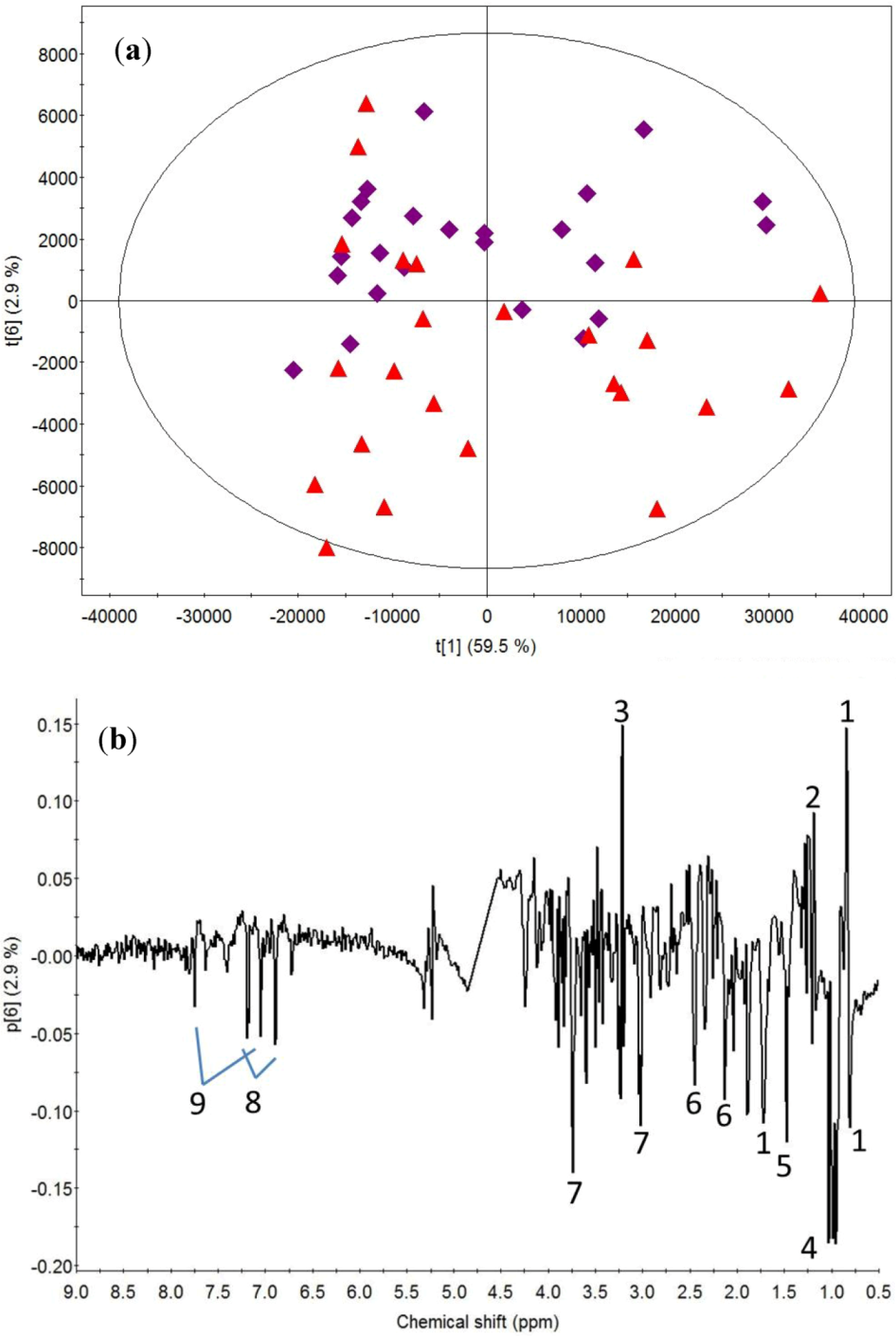
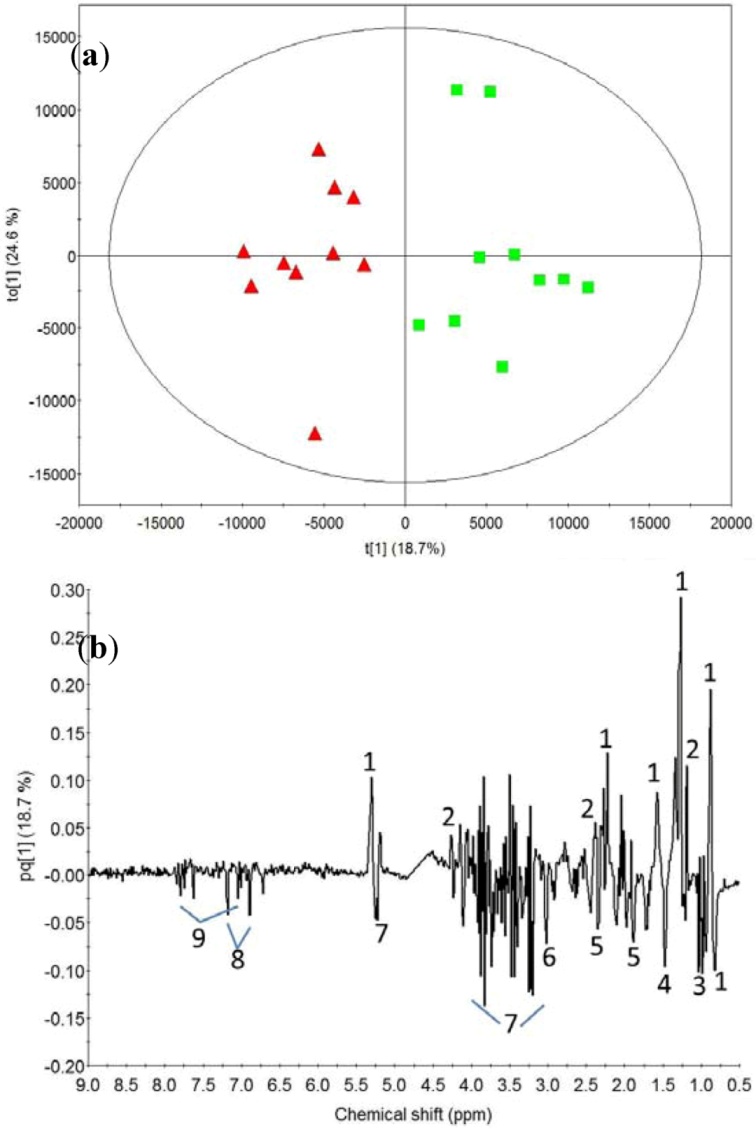
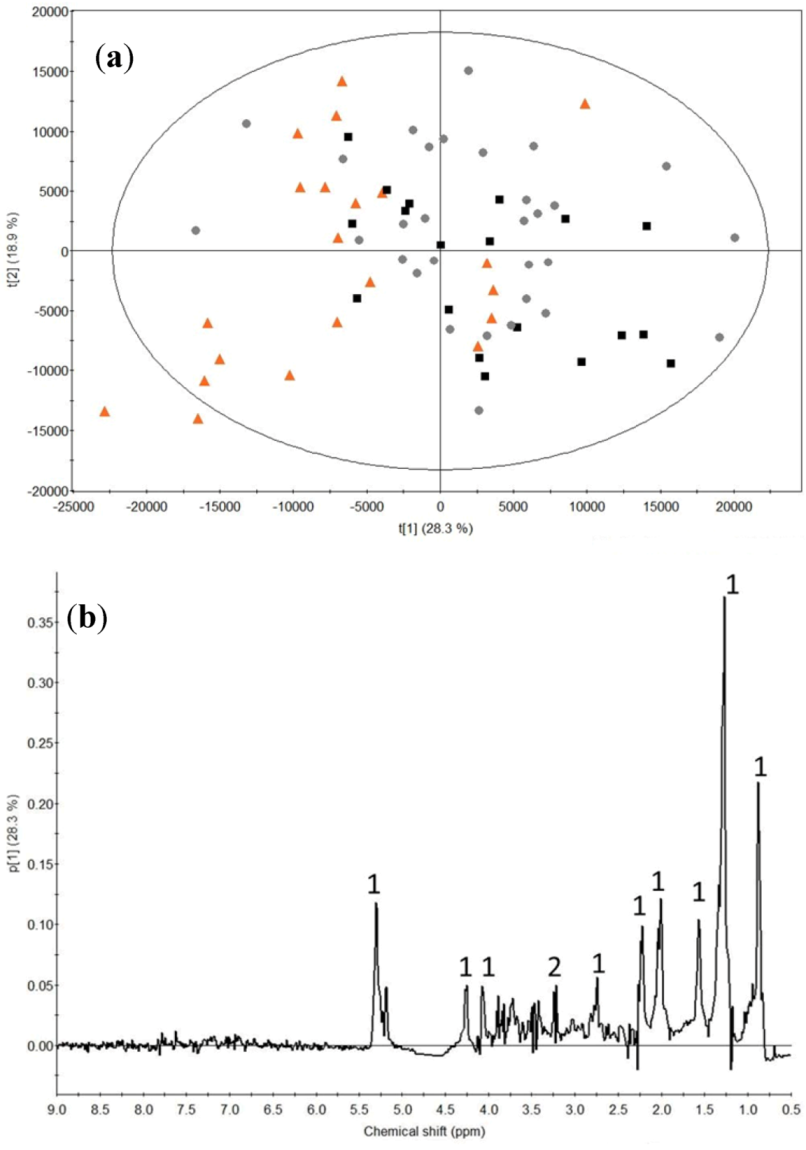
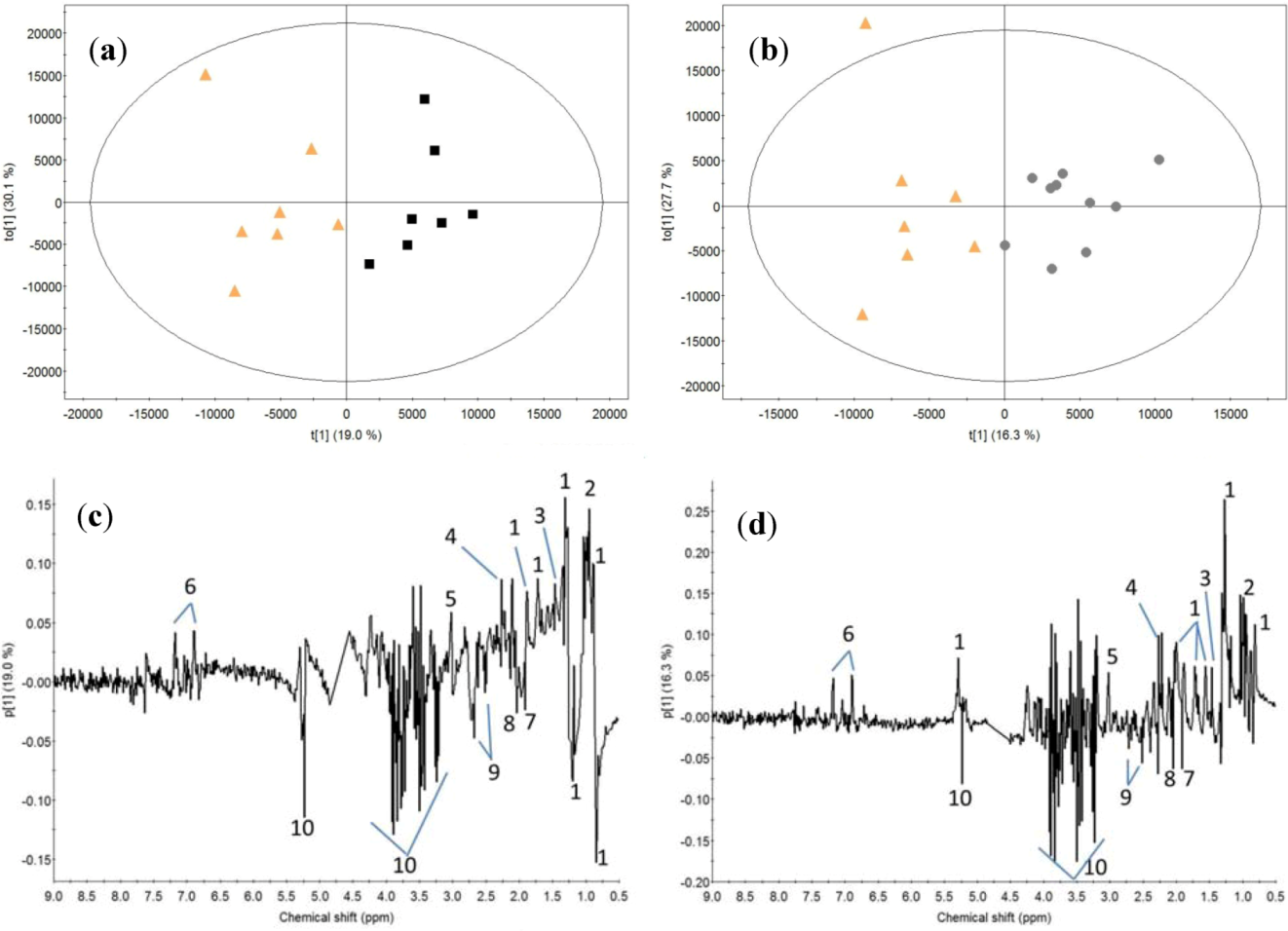
2.2. Milk Protein Effect
3. Discussion
3.1. Post-Exercise Effect
3.2. Milk Protein Effect
4. Experimental Section
4.1. Subjects
4.2. Pre-Tests and Food Registration
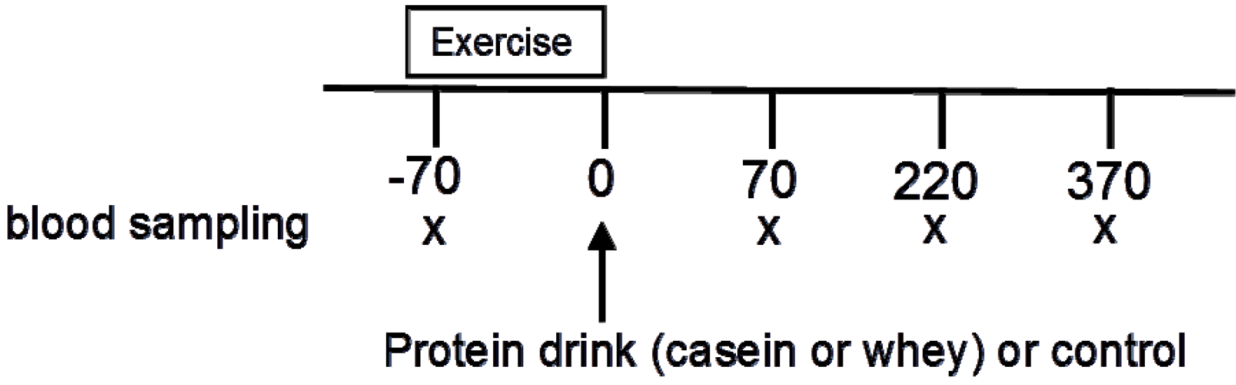
4.3. Experimental Protocol
4.4. NMR Measurements
4.5. Data Handling
5. Conclusions
Acknowledgments
Conflict of Interest
References
- Biolo, G.; Maggi, S.P.; Williams, B.D.; Tipton, K.D.; Wolfe, R.R. Increased Rates of Muscle Protein-Turnover and Amino-Acid-Transport After Resistance Exercise in Humans. Am. J. Physiol. Endocrinol. Metabol. 1995, 268, E514–E520. [Google Scholar]
- Phillips, S.M.; Tipton, K.D.; Aarsland, A.; Wolf, S.E.; Wolfe, R.R. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am. J. Physiol. Endocrinol. Metabol. 1997, 273, E99–E107. [Google Scholar]
- Phillips, S.M.; Tipton, K.D.; Ferrando, A.A.; Wolfe, R.R. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am. J. Physiol. Endocrinol. Metabol. 1999, 276, E118–E124. [Google Scholar]
- Farnfield, M.M.; Trenerry, C.; Carey, K.A.; Cameron-Smith, D. Plasma amino acid response after ingestion of different whey protein fractions. Int. J. Food Sci. Nutr. 2009, 60, 476–486. [Google Scholar] [CrossRef]
- Tipton, K.D.; Elliott, T.A.; Cree, M.G.; Wolf, S.E.; Sanford, A.P.; Wolfe, R.R. Ingestion of casein and whey proteins result in muscle anabolism after resistance exercise. Med. Sci Sports Exerc. 2004, 36, 2073–2081. [Google Scholar]
- Wilkinson, S.B.; Tarnopolsky, M.A.; MacDonald, M.J.; MacDonald, J.R.; Armstrong, D.; Philips, S.M. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am. J. Clin. Nutr. 2007, 85, 1031–1040. [Google Scholar]
- Calbet, J.A.L.; Holst, J.J. Gastric emptying, gastric secretion and enterogastrone response after administration of milk proteins or their peptide hydrolysates in humans. Eur. J. Nutr. 2004, 43, 127–139. [Google Scholar] [CrossRef]
- Savalle, B.; Miranda, G.; Pelissier, J.P. Invitro Simulation of Gastric Digestion of Milk-Proteins. J. Agric. Food Chem. 1989, 37, 1336–1340. [Google Scholar] [CrossRef]
- Boirie, Y.; Dangin, M.; Gachon, P.; Vasson, M.P.; Maubois, J.L.; Beaufrere, B. Slow and fast dietary proteins differently modulate postprandial protein accretion. Prog. Natl. Acad. Sci. USA 1997, 94, 14930–14935. [Google Scholar] [CrossRef]
- Reitelseder, S.; Agergaard, J.; Doessing, S.; Helmark, I.C.; Lund, P.; Kristensen, N.B.; Frystyk, J.; Flyvbjerg, A.; Schjerling, P.; van Hall, G.; Kjaer, M.; Holm, L. Whey and casein labeled with L-[1-C-13]leucine and muscle protein synthesis: effect of resistance exercise and protein ingestion. Am. J. Physiol. Endocrinol. Metabol. 2011, 300, E231–E242. [Google Scholar]
- Tang, J.E.; Moore, D.R.; Kujbida, G.W.; Tarnopolsky, M.A.; Phillips, S.M. Ingestion of whey hydrolysate, casein, or soy protein isolate: effects on mixed muscle protein synthesis at rest and following resistance exercise in young men. J. Appl. Physiol. 2009, 107, 987–992. [Google Scholar] [CrossRef]
- Tessari, P.; Kiwanuka, E.; Cristini, M.; Zaramella, M.; Enslen, M.; Zurlo, C.; Garcia-Rodenas, C. Slow versus fast proteins in the stimulation of beta-cell response and the activation of the entero-insular axis in type 2 diabetes. Diabetes-Metab. Res. Rev. 2007, 23, 378–385. [Google Scholar]
- Nicholson, J.K.; Connelly, J.; Lindon, J.C.; Holmes, E. Metabonomics: a platform for studying drug toxicity and gene function. Nat. Rev. Drug Discov. 2002, 1, 153–161. [Google Scholar] [CrossRef]
- Bertram, H.C.; Hoppe, C.; Petersen, B.O.; Duus, J.O.; Molgaard, C.; Michaelsen, K.F. An NMR-based metabonomic investigation on effects of milk and meat protein diets given to 8-year-old boys. Br. J. Nutr. 2007, 97, 758–763. [Google Scholar] [CrossRef]
- Bertram, H.C.; Duus, J.O.; Petersen, B.O.; Hoppe, C.; Larnkjaer, A.; Schack-Nielsen, L.; Molgaard, C.; Michaelsen, K.F. Nuclear magnetic resonance-based metabonomics reveals strong sex effect on plasma metabolism in 17-year-old Scandinavians and correlation to retrospective infant plasma parameters. Metab.-Clin. Exp. 2009, 58, 1039–1045. [Google Scholar]
- Martin, J.C.; Canlet, C.; Delplanque, B.; Agnani, G.; Lairon, D.; Gottardi, G.; Bencharif, K.; Gripois, D.; Thaminy, A.; Paris, A. (1)H NMR metabonomics can differentiate the early atherogenic effect of dairy products in hyperlipidemic hamsters. Atherosclerosis 2009, 206, 127–133. [Google Scholar] [CrossRef] [Green Version]
- Yde, C. C.; Westerhuis, J. A.; Bertram, H. C.; Bach Knudsen, K.E. Application of NMR-based metabonomics suggests a relationship between betaine absorption and elevated creatine plasma concentrations in catheterised sows. Br. J. Nutr. 2012, 107, 1603–1615. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, G.J.; Jiye, A.; Ma, B.; Dua, Y.; Zhu, L.L.; Wu, D. Metabonomic profiling of diet-induced hyperlipidaemia in a rat model. Biomarkers 2010, 15, 205–216. [Google Scholar] [CrossRef]
- Kirwan, G.M.; Coffey, V.G.; Niere, J.O.; Hawley, J.A.; Adams, M.J. Spectroscopic correlation analysis of NMR-based metabonomics in exercise science. Anal. Chim. Acta 2009, 652, 173–179. [Google Scholar] [CrossRef]
- Enea, C.; Seguin, F.; Petitpas-Mulliez, J.; Boildieu, N.; Boisseau, N.; Delpech, N.; Diaz, V.; Eugene, M.; Dugue, B. H-1 NMR-based metabolomics approach for exploring urinary metabolome modifications after acute and chronic physical exercise. Anal. Bioanal. Chem. 2010, 396, 1167–1176. [Google Scholar]
- Le Moyec, L.; Mille-Hamard, L.; Triba, M.N.; Breuneval, C.; Petot, H.; Billat, V.L. NMR metabolomics for assessment of exercise effects with mouse biofluids. Anal. Bioanal. Chem. 2012, 404, 593–602. [Google Scholar]
- Yan, B.; A, J.Y.; Wang, G.J.; Lu, H.L.; Huang, X.P.; Liu, Y.; Zha, W.B.; Hao, H.P.; Zhang, Y.; Liu, L.S.; Gu, S.H.; Huang, Q.; Zheng, Y.T.; Sun, J.G. Metabolomic investigation into variation of endogenous metabolites in professional athletes subject to strength-endurance training. J. Appl. Physiol. 2009, 106, 531–538. [Google Scholar]
- Huang, C.C.; Lin, W.T.; Hsu, F.L.; Tsai, P.W.; Hou, C.C. Metabolomics investigation of exercise-modulated changes in metabolism in rat liver after exhaustive and endurance exercises. Eur. J. Appl. Physiol. 2010, 108, 557–566. [Google Scholar] [CrossRef]
- Miccheli, A.; Marini, F.; Capuani, G.; Miccheli, A.T.; Delfini, M.; Di Cocco, M.E.; Puccetti, C.; Paci, M.; Rizzo, M.; Spataro, A. The Influence of a Sports Drink on the Postexercise Metabolism of Elite Athletes as Investigated by NMR-Based Metabolomics. J. Am. Coll. Nutr. 2009, 28, 553–564. [Google Scholar]
- Otvos, J.D.; Jeyarajah, E.J.; Bennett, D.W. Quantification of Plasma-Lipoproteins by Proton Nuclear-Magnetic-Resonance Spectroscopy. Clin. Chem. 1991, 37, 377–386. [Google Scholar]
- AlaKorpela, M.; Korhonen, A.; Keisala, J.; Horkko, S.; Korpi, P.; Ingman, L.P.; Jokisaari, J.; Savolainen, M.J.; Kesaniemi, Y.A. H-1 Nmr-Based Absolute Quantitation of Human Lipoproteins and Their Lipid Contents Directly from Plasma. J. Lipid Res. 1994, 35, 2292–2304. [Google Scholar]
- Meiboom, S.; Gill, D. Modified Spin-Echo Method for Measuring Nuclear Relaxation Times. Rev. Sci Instrum 1958, 29, 688–691. [Google Scholar] [CrossRef]
- Ala-Korpela, M.; Lankinen, N.; Salminen, A.; Suna, T.; Soininen, P.; Laatikainen, R.; Ingman, P.; Jauhiainen, M.; Taskinen, M.R.; Heberger, K.; Kaski, K. The inherent accuracy of H-1 NMR spectroscopy to quantify plasma lipoproteins is subclass dependent. Atherosclerosis 2007, 190, 352–358. [Google Scholar] [CrossRef]
- AlaKorpela, M. H-1 NMR spectroscopy of human blood plasma. Prog. Nucl. Mag. Res. Spectrosc. 1995, 27, 475–554. [Google Scholar] [CrossRef]
- Lindon, J.C.; Nicholson, J.K.; Everett, J.R. NMR spectroscopy of biofluids. Ann. Rep. NMR Spectrosc. 1999, 33, 1–88. [Google Scholar]
- Wishart, D.S.; Knox, C.; Guo, A.C.; Eisner, R.; Young, N.; Gautam, B.; Hau, D.D.; Psychogios, N.; Dong, E.; Bouatra, S.; et al. Nucleic Acids Res. 2009, 37, D603–D610. [CrossRef]
- Bro, R.; Smilde, A.K. Centering and scaling in component analysis. J. Chemometr. 2003, 17, 16–33. [Google Scholar]
- Yde, C.C.; Jansen, J.J.; Theil, P.K.; Bertram, H.C.; Bach Knudsen, K.E. Different metabolic and absorption patterns of betaine in response to dietary intake of whole-wheat grain, wheat aleurone or rye aleurone in catheterized pigs. Eur. Food Res. Tech. 2012, 235, 939–949. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yde, C.C.; Ditlev, D.B.; Reitelseder, S.; Bertram, H.C. Metabonomic Response to Milk Proteins after a Single Bout of Heavy Resistance Exercise Elucidated by 1H Nuclear Magnetic Resonance Spectroscopy . Metabolites 2013, 3, 33-46. https://doi.org/10.3390/metabo3010033
Yde CC, Ditlev DB, Reitelseder S, Bertram HC. Metabonomic Response to Milk Proteins after a Single Bout of Heavy Resistance Exercise Elucidated by 1H Nuclear Magnetic Resonance Spectroscopy . Metabolites. 2013; 3(1):33-46. https://doi.org/10.3390/metabo3010033
Chicago/Turabian StyleYde, Christian Clement, Ditte Bruun Ditlev, Søren Reitelseder, and Hanne Christine Bertram. 2013. "Metabonomic Response to Milk Proteins after a Single Bout of Heavy Resistance Exercise Elucidated by 1H Nuclear Magnetic Resonance Spectroscopy " Metabolites 3, no. 1: 33-46. https://doi.org/10.3390/metabo3010033
APA StyleYde, C. C., Ditlev, D. B., Reitelseder, S., & Bertram, H. C. (2013). Metabonomic Response to Milk Proteins after a Single Bout of Heavy Resistance Exercise Elucidated by 1H Nuclear Magnetic Resonance Spectroscopy . Metabolites, 3(1), 33-46. https://doi.org/10.3390/metabo3010033




