Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought
Abstract
:1. Introduction
2. Results
2.1. Plant Performance and Soil Nutrients under Different Biochar Levels
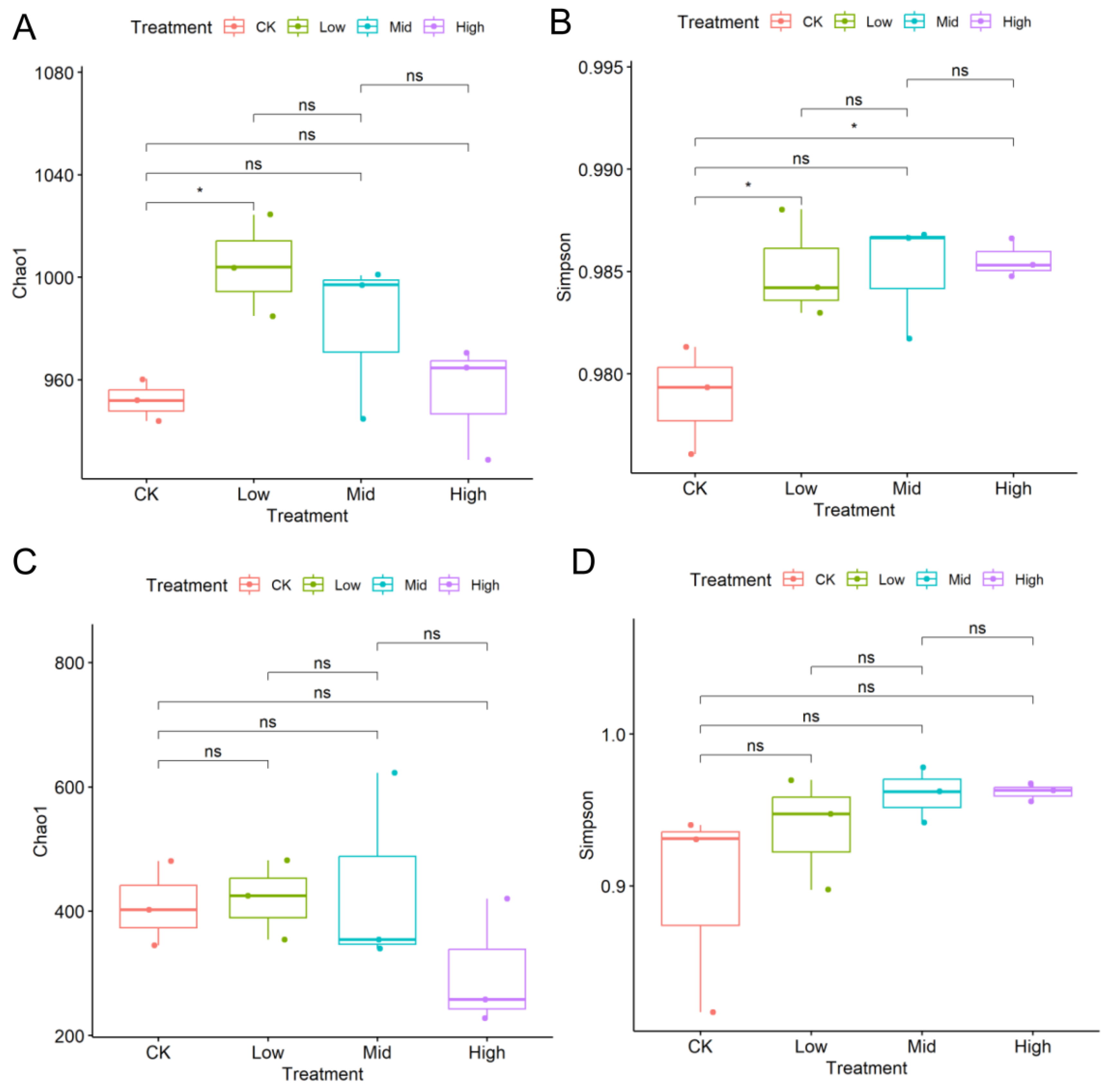
2.2. Soil Microbial Community Composition and Diversity under Different Biochar Levels
2.3. Analyses of Assembly Processes and Networks Based on the Soil Microbial Community across Treatments
2.4. Response of Soil Microbial Community, Dominants and Key Sub Community to Environmental Factors
2.5. Soil Metabolome under Different Biochar Levels
3. Discussion
3.1. Biochar Ameliorates the Negative Effects of Drought on Plant Performance and C, P and N Nutrient Cycling
3.2. Biochar Ameliorates the Negative Effects of Drought on the Soil Microbial Community
3.3. Biochar Content Influences the Soil Metabolome
4. Materials and Methods
4.1. Experimental Design
4.2. Measurements of Soil Chemical Properties
4.3. Soil Total DNA Extraction and High-Throughput Sequencing
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- IPCC. Climate Change 2021: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2021. [Google Scholar]
- Huntington, T.G. Evidence for intensification of the global water cycle: Review and synthesis. J. Hydrol. 2006, 319, 83–95. [Google Scholar] [CrossRef]
- Furtak, K.; Wolinska, A. The impact of extreme weather events as a consequence of climate change on the soil moisture and on the quality of the soil environment and agriculture—A review. Catena 2023, 231, 15. [Google Scholar] [CrossRef]
- Touma, D.; Ashfaq, M.; Nayak, M.A.; Kao, S.C.; Diffenbaugh, N.S. A multi-model and multi-index evaluation of drought characteristics in the 21st century. J. Hydrol. 2015, 526, 196–207. [Google Scholar] [CrossRef]
- Piao, S.L.; Ciais, P.; Huang, Y.; Shen, Z.H.; Peng, S.S.; Li, J.S.; Zhou, L.P.; Liu, H.Y.; Ma, Y.C.; Ding, Y.H.; et al. The impacts of climate change on water resources and agriculture in China. Nature 2010, 467, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Zhou, L.Y.; Nie, Y.Y.; Fu, Y.L.; Du, Z.G.; Shao, J.J.; Zheng, Z.M.; Wang, X.H. Similar responses of soil carbon storage to drought and irrigation in terrestrial ecosystems but with contrasting mechanisms: A meta-analysis. Agric. Ecosyst. Environ. 2016, 228, 70–81. [Google Scholar] [CrossRef]
- Bornø, M.L.; Müller-Stöver, D.S.; Liu, F.L. Biochar modifies the content of primary metabolites in the rhizosphere of well-watered and drought-stressed Zea mays L. (maize). Biol. Fertil. Soils 2022, 58, 633–647. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant drought stress: Effects, mechanisms and management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef]
- Kim, Y.; Chung, Y.S.; Lee, E.; Tripathi, P.; Heo, S.; Kim, K.H. Root Response to Drought Stress in Rice (Oryza sativa L.). Int. J. Mol. Sci. 2020, 21, 22. [Google Scholar] [CrossRef]
- Tardieu, F.; Simonneau, T.; Muller, B. The Physiological Basis of Drought Tolerance in Crop Plants: A Scenario-Dependent Probabilistic Approach. Annu. Rev. Plant Biol. 2018, 69, 733–759. [Google Scholar] [CrossRef]
- Preece, C.; Penuelas, J. Rhizodeposition under drought and consequences for soil communities and ecosystem resilience. Plant Soil 2016, 409, 1–17. [Google Scholar] [CrossRef]
- Korup, K.; Laerke, P.E.; Baadsgaard, H.; Andersen, M.N.; Kristensen, K.; Munnich, C.; Didion, T.; Jensen, E.S.; Martensson, L.M.; Jorgensen, U. Biomass production and water use efficiency in perennial grasses during and after drought stress. GCB Bioenergy 2018, 10, 12–27. [Google Scholar] [CrossRef]
- Hahn, C.; Luscher, A.; Ernst-Hasler, S.; Suter, M.; Kahmen, A. Timing of drought in the growing season and strong legacy effects determine the annual productivity of temperate grasses in a changing climate. Biogeosciences 2021, 18, 585–604. [Google Scholar] [CrossRef]
- King, C.A.; Purcell, L.C. Soybean nodule size and relationship to nitrogen fixation response to water deficit. Crop Sci. 2001, 41, 1099–1107. [Google Scholar] [CrossRef]
- Esfahani, M.N.; Sulieman, S.; Schulze, J.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Tran, L.S.P. Mechanisms of physiological adjustment of N2 fixation in Cicer arietinum L. (chickpea) during early stages of water deficit: Single or multi-factor controls. Plant J. 2014, 79, 964–980. [Google Scholar] [CrossRef] [PubMed]
- Pimratch, S.; Jogloy, S.; Vorasoot, N.; Toomsan, B. Effect of drought stress on traits related to N-2 fixation in eleven peanut (Arachis hypogaea L.) genotypes differing in degrees of resistance to drought. Asian J. Plant Sci. 2008, 7, 334–342. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Jin, Y.G.; Ma, R.Y.; Kong, D.L.; Zhu-Barker, X.; Horwath, W.R.; Niu, S.L.; Wang, H.; Xiao, X.; Liu, S.W.; et al. Drought shrinks terrestrial upland resilience to climate change. Glob. Ecol. Biogeogr. 2020, 29, 1840–1851. [Google Scholar] [CrossRef]
- Tao, F.; Huang, Y.Y.; Hungate, B.A.; Manzoni, S.; Frey, S.D.; Schmidt, M.W.I.; Reichstein, M.; Carvalhais, N.; Ciais, P.; Jiang, L.F.; et al. Microbial carbon use efficiency promotes global soil carbon storage. Nature 2023, 618, 981–985. [Google Scholar] [CrossRef]
- Qu, Q.; Xu, H.W.; Ai, Z.M.; Wang, M.G.; Wang, G.L.; Liu, G.B.; Geissen, V.; Ritsema, C.J.; Xue, S. Impacts of extreme weather events on terrestrial carbon and nitrogen cycling: A global meta-analysis. Environ. Pollut. 2023, 319, 120996. [Google Scholar] [CrossRef]
- Manzoni, S.; Schimel, J.P.; Porporato, A. Responses of soil microbial communities to water stress: Results from a meta-analysis. Ecology 2012, 93, 930–938. [Google Scholar] [CrossRef]
- Hu, W.G.; Ran, J.Z.; Dong, L.W.; Du, Q.J.; Ji, M.F.; Yao, S.R.; Sun, Y.; Gong, C.M.; Hou, Q.Q.; Gong, H.Y.; et al. Aridity-driven shift in biodiversity-soil multifunctionality relationships. Nat. Commun. 2021, 12, 5350. [Google Scholar] [CrossRef]
- Citerne, N.; Wallace, H.M.; Lewis, T.; Reverchon, F.; Omidvar, N.; Hu, H.W.; Shi, X.Z.; Zhou, X.H.; Zhou, G.Y.; Farrar, M.; et al. Effects of Biochar on Pulse C and N Cycling After a Short-term Drought: A Laboratory Study. J. Soil Sci. Plant Nutr. 2021, 21, 2815–2825. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Knight, C.G.; Nicolitch, O.; Williams, A. Harnessing rhizosphere microbiomes for drought-resilient crop production. Science 2020, 368, 270–274. [Google Scholar] [CrossRef] [PubMed]
- De Vries, F.T.; Williams, A.; Stringer, F.; Willcocks, R.; McEwing, R.; Langridge, H.; Straathof, A.L. Changes in root-exudate-induced respiration reveal a novel mechanism through which drought affects ecosystem carbon cycling. New Phytol. 2019, 224, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Hart, A.T.; Merlin, M.; Wiley, E.; Landhausser, S.M. Splitting the Difference: Heterogeneous Soil Moisture Availability Affects Aboveground and Belowground Reserve and Mass Allocation in Trembling Aspen. Front. Plant Sci. 2021, 12, 654159. [Google Scholar] [CrossRef] [PubMed]
- Rüger, L.; Ganther, M.; Freudenthal, J.; Jansa, J.; Heintz-Buschart, A.; Tarkka, M.T.; Bonkowski, M. Root cap is an important determinant of rhizosphere microbiome assembly. New Phytol. 2023, 239, 1434–1448. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J. A handful of carbon. Nature 2007, 447, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.W.; Zhang, Y.J.; Zong, Y.J.; Hu, Z.Q.; Wu, S.; Zhou, J.; Jin, Y.G.; Zou, J.W. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. GCB Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Wang, J.Y.; Xiong, Z.Q.; Kuzyakov, Y. Biochar stability in soil: Meta-analysis of decomposition and priming effects. GCB Bioenergy 2016, 8, 512–523. [Google Scholar] [CrossRef]
- Zhu, B.J.; Wan, B.B.; Liu, T.; Zhang, C.Z.; Cheng, L.Z.; Cheng, Y.H.; Tian, S.Y.; Chen, X.Y.; Hu, F.; Whalen, J.K.; et al. Biochar enhances multifunctionality by increasing the uniformity of energy flow through a soil nematode food web. Soil Biol. Biochem. 2023, 183, 109056. [Google Scholar] [CrossRef]
- Yu, J.; Deem, L.M.; Crow, S.E.; Deenik, J.L.; Penton, C.R. Biochar application influences microbial assemblage complexity and composition due to soil and bioenergy crop type interactions. Soil Biol. Biochem. 2018, 117, 97–107. [Google Scholar] [CrossRef]
- Yao, Q.; Liu, J.J.; Yu, Z.H.; Li, Y.S.; Jin, J.; Liu, X.B.; Wang, G.H. Three years of biochar amendment alters soil physiochemical properties and fungal community composition in a black soil of northeast China. Soil Biol. Biochem. 2017, 110, 56–67. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, X.; Liang, A.J.; Li, Y.Y.; Li, X.Y.; Li, D.P.; Hou, N. Insight into the soil aggregate-mediated restoration mechanism of degraded black soil via biochar addition: Emphasizing the driving role of core microbial communities and nutrient cycling. Environ. Res. 2023, 228, 115895. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cao, L.X.; Zhang, R.D. Bacterial and fungal taxon changes in soil microbial community composition induced by short-term biochar amendment in red oxidized loam soil. World J. Microbiol. Biotechnol. 2014, 30, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Liu, X.Y.; Zheng, J.W.; Zhang, B.; Lu, H.F.; Chi, Z.Z.; Pan, G.X.; Li, L.Q.; Zheng, J.F.; Zhang, X.H.; et al. Biochar soil amendment increased bacterial but decreased fungal gene abundance with shifts in community structure in a slightly acid rice paddy from Southwest China. Appl. Soil Ecol. 2013, 71, 33–44. [Google Scholar] [CrossRef]
- Herrmann, L.; Lesueur, D.; Robin, A.; Robain, H.; Wiriyakitnateekul, W.; Brau, L. Impact of biochar application dose on soil microbial communities associated with rubber trees in North East Thailand. Sci. Total Environ. 2019, 689, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Han, Z.Q.; Zheng, F.W.; Wu, S.; Wang, J.Y.; Wang, J.D.; Zhang, H.; Zhang, Y.C.; Liu, S.W.; Li, S.Q.; et al. Biochar reduced soil nitrous oxide emissions through suppressing fungal denitrification and affecting fungal community assembly in a subtropical tea plantation. Agric. Ecosyst. Environ. 2022, 326, 107784. [Google Scholar] [CrossRef]
- Bahram, M.; Hildebrand, F.; Forslund, S.K.; Anderson, J.L.; Soudzilovskaia, N.A.; Bodegom, P.M.; Bengtsson-Palme, J.; Anslan, S.; Coelho, L.P.; Harend, H.; et al. Structure and function of the global topsoil microbiome. Nature 2018, 560, 233–237. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Li, G.L.C.; Chen, J.J.; Johnson, D. Direction of plant-soil feedback determines plant responses to drought. Glob. Chang. Biol. 2022, 28, 3995–3997. [Google Scholar] [CrossRef]
- Allsup, C.M.; George, I.; Lankau, R.A. Shifting microbial communities can enhance tree tolerance to changing climates. Science 2023, 380, 835–840. [Google Scholar] [CrossRef]
- Feng, H.C.; Fu, R.X.; Luo, J.Y.; Hou, X.Q.; Gao, K.; Su, L.; Xu, Y.; Miao, Y.Z.; Liu, Y.P.; Xu, Z.H.; et al. Listening to plant’s Esperanto via root exudates: Reprogramming the functional expression of plant growth-promoting rhizobacteria. New Phytol. 2023, 239, 2307–2319. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Murray, J.D.; Poole, P.S.; Downie, J.A. The Rules of Engagement in the Legume-Rhizobial Symbiosis. Annu. Rev. Genet. 2011, 45, 119–144. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.T.; Zhu, Y.Y.; Chang, H.Z.; Wang, C.H.; Yang, J.; Shi, J.C.; Gao, J.P.; Yang, W.B.; Lan, L.Y.; Wang, Y.R.; et al. An SHR-SCR module specifies legume cortical cell fate to enable nodulation. Nature 2021, 589, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.M.; Sun, X.; Cao, B.C.; Chiariello, N.R.; Docherty, K.M.; Field, C.B.; Gao, Q.; Gutknecht, J.L.M.; Guo, X.; He, G.H.; et al. Long-term elevated precipitation induces grassland soil carbon loss via microbe-plant-soil interplay. Glob. Chang. Biol. 2023, 29, 5429–5444. [Google Scholar] [CrossRef] [PubMed]
- Lian, T.; Cheng, L.; Liu, Q.; Yu, T.; Cai, Z.; Nian, H.; Hartmann, M. Potential relevance between soybean nitrogen uptake and rhizosphere prokaryotic communities under waterlogging stress. ISME Commun. 2023, 3, 71. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D. Microbial drought resistance may destabilize soil carbon. Trends Microbiol. 2023, 31, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Afaf, A.; Alosaimi, J.S.R.; Alharby, H.F.; Alayafi, A.A.M. The Importance of Initial Application of Biochar on Soil Fertility to Improve Growth and Productivity of Tomato Plants (Solanum lycopersicum L.) Under Drought Stress. Gesunde Pflanz. 2023, 10, 2515–2524. [Google Scholar] [CrossRef]
- Ali, S.; Rizwan, M.; Qayyum, M.F.; Ok, Y.S.; Ibrahim, M.; Riaz, M.; Arif, M.S.; Hafeez, F.; Al-Wabel, M.I.; Shahzad, A.N. Biochar soil amendment on alleviation of drought and salt stress in plants: A critical review. Environ. Sci. Pollut. Res. 2017, 24, 12700–12712. [Google Scholar] [CrossRef]
- Baselga, A. Partitioning abundance-basedmultiple-site dissimilarity into components: Balanced variation in abundance and abundance gradients. Methods Ecol. Evol. 2017, 8, 799–808. [Google Scholar] [CrossRef]
- Baselga, A.; Orme, C.D.L. betapart: An R package for the study of beta diversity. Methods Ecol. Evol. 2012, 3, 808–812. [Google Scholar] [CrossRef]
- Zhang, C.L.; Chen, J.H.; Huang, W.X.; Song, X.Q.; Niu, J. Transcriptomics and Metabolomics Reveal Purine and Phenylpropanoid Metabolism Response to Drought Stress in Dendrobium sinense, an Endemic Orchid Species in Hainan Island. Front. Genet. 2021, 12, 692702. [Google Scholar] [CrossRef]
- Diaz, P.; Betti, M.; Sanchez, D.H.; Udvardi, M.K.; Monza, J.; Marquez, A.J. Deficiency in plastidic glutamine synthetase alters proline metabolism and transcriptomic response in Lotus japonicus under drought stress. New Phytol. 2010, 188, 1001–1013. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Canqui, H. Biochar and Soil Physical Properties. Soil Sci. Soc. Am. J. 2017, 81, 687–711. [Google Scholar] [CrossRef]
- Razzaghi, F.; Obour, P.B.; Arthur, E. Does biochar improve soil water retention? A systematic review and meta-analysis. Geoderma 2020, 361, 114055. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.T.; Andersen, M.N.; Liu, F.L. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Guo, L.L.; Bornø, M.L.; Niu, W.Q.; Liu, F.L. Biochar amendment improves shoot biomass of tomato seedlings and sustains water relations and leaf gas exchange rates under different irrigation and nitrogen regimes. Agric. Water Manag. 2021, 245, 106580. [Google Scholar] [CrossRef]
- Homyak, P.M.; Allison, S.D.; Huxman, T.E.; Goulden, M.L.; Treseder, K.K. Effects of Drought Manipulation on Soil Nitrogen Cycling: A Meta-Analysis. J. Geophys. Res. Biogeosci. 2017, 122, 3260–3272. [Google Scholar] [CrossRef]
- Del Grosso, S.J.; Parton, W.J.; Mosier, A.R.; Ojima, D.S.; Kulmala, A.E.; Phongpan, S. General model for N2O and N2 gas emissions from soils due to dentrification. Glob. Biogeochem. Cycles 2000, 14, 1045–1060. [Google Scholar] [CrossRef]
- Kuypers, M.M.M.; Marchant, H.K.; Kartal, B. The microbial nitrogen-cycling network. Nat. Rev. Microbiol. 2018, 16, 263–276. [Google Scholar] [CrossRef]
- Smith, K.A.; Ball, T.; Conen, F.; Dobbie, K.E.; Massheder, J.; Rey, A. Exchange of greenhouse gases between soil and atmosphere: Interactions of soil physical factors and biological processes. Eur. J. Soil Sci. 2003, 54, 779–791. [Google Scholar] [CrossRef]
- Gessler, A.; Jung, K.; Gasche, R.; Papen, H.; Heidenfelder, A.; Borner, E.; Metzler, B.; Augustin, S.; Hildebrand, E.; Rennenberg, H. Climate and forest management influence nitrogen balance of European beech forests: Microbial N transformations and inorganic N net uptake capacity of mycorrhizal roots. Eur. J. For. Res. 2005, 124, 95–111. [Google Scholar] [CrossRef]
- Pradhan, D.; Bertin, D.; Sinclair, T.R.; Nogueira, M.A.; Livingston, D.; Carter, T. Microsphere stem blockage as a screen for nitrogen-fixation drought tolerance in soybean. Physiol. Plant. 2021, 172, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Lumactud, R.A.; Dollete, D.; Liyanage, D.K.; Szczyglowski, K.; Hill, B.; Thilakarathna, M.S. The effect of drought stress on nodulation, plant growth, and nitrogen fixation in soybean during early plant growth. J. Agron. Crop Sci. 2022, 10, 345–354. [Google Scholar] [CrossRef]
- Oldroyd, G.E.D.; Leyser, O. A plant’s diet, surviving in a variable nutrient environment. Science 2020, 368, eaba0196. [Google Scholar] [CrossRef] [PubMed]
- Kiers, E.T.; Rousseau, R.A.; West, S.A.; Denison, R.F. Host sanctions and the legume-rhizobium mutualism. Nature 2003, 425, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Reid, J.B.; Foo, E. The Art of Self-Control—Autoregulation of Plant-Microbe Symbioses. Front. Plant Sci. 2018, 9, 988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.Q.; Shen, R.F. Aluminum-Nitrogen Interactions in the Soil-Plant System. Front. Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Emmett, B.A.; Beier, C.; Estiarte, M.; Tietema, A.; Kristensen, H.L.; Williams, D.; Penuelas, J.; Schmidt, I.; Sowerby, A. The response of soil processes to climate change: Results from manipulation studies of shrublands across an environmental gradient. Ecosystems 2004, 7, 625–637. [Google Scholar] [CrossRef]
- Schimel, J.; Balser, T.C.; Wallenstein, M. Microbial stress-response physiology and its implications for ecosystem function. Ecology 2007, 88, 1386–1394. [Google Scholar] [CrossRef]
- Zhou, Z.H.; Wang, C.K.; Luo, Y.Q. Response of soil microbial communities to altered precipitation: A global synthesis. Glob. Ecol. Biogeogr. 2018, 27, 1121–1136. [Google Scholar] [CrossRef]
- Sun, Y.; Chen, H.Y.H.; Jin, L.; Wang, C.T.; Zhang, R.T.; Ruan, H.H.; Yang, J.Y. Drought stress induced increase of fungi: Bacteria ratio in a poplar plantation. Catena 2020, 193, 9. [Google Scholar] [CrossRef]
- Fierer, N.; Schimel, J.P.; Holden, P.A. Influence of drying-rewetting frequency on soil bacterial community structure. Microb. Ecol. 2003, 45, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Toth, Z.; Tancsics, A.; Kriszt, B.; Kroel-Dulay, G.; Onodi, G.; Hornung, E. Extreme effects of drought on composition of the soil bacterial community and decomposition of plant tissue. Eur. J. Soil Sci. 2017, 68, 504–513. [Google Scholar] [CrossRef]
- Liao, X.H.; Zhao, J.; Xu, L.; Tang, L.; Li, J.N.; Zhang, W.; Xiao, J.; Xiao, D.; Nie, Y.P.; Zou, D.S.; et al. Arbuscular mycorrhizal fungi increase the interspecific competition between two forage plant species and stabilize the soil microbial network during a drought event: Evidence from the field. Appl. Soil Ecol. 2023, 185, 104805. [Google Scholar] [CrossRef]
- Gao, C.; Xu, L.; Montoya, L.; Madera, M.; Hollingsworth, J.; Chen, L.; Purdom, E.; Singan, V.; Vogel, J.; Hutmacher, R.B.; et al. Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nat. Commun. 2022, 13, 3867. [Google Scholar] [CrossRef] [PubMed]
- Walter, J.; Kreyling, J.; Singh, B.K.; Jentsch, A. Effects of extreme weather events and legume presence on mycorrhization of Plantago lanceolata and Holcus lanatus in the field. Plant Biol. 2016, 18, 262–270. [Google Scholar] [CrossRef]
- Liang, C.F.; Zhu, X.L.; Fu, S.L.; Mendez, A.; Gasco, G.; Paz-Ferreiro, J. Biochar alters the resistance and resilience to drought in a tropical soil. Environ. Res. Lett. 2014, 9, 064013. [Google Scholar] [CrossRef]
- Hernandez, D.J.; David, A.S.; Menges, E.S.; Searcy, C.A.; Afkhami, M.E. Environmental stress destabilizes microbial networks. ISME J. 2021, 15, 1722–1734. [Google Scholar] [CrossRef]
- De Vries, F.T.; Griffiths, R.I.; Bailey, M.; Craig, H.; Girlanda, M.; Gweon, H.S.; Hallin, S.; Kaisermann, A.; Keith, A.M.; Kretzschmar, M.; et al. Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 2018, 9, 3033. [Google Scholar] [CrossRef]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, E.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Balestrini, R.; Chitarra, W.; Fotopoulos, V.; Ruocco, M. Potential Role of Beneficial Soil Microorganisms in Plant Tolerance to Abiotic Stress Factors. In Proceedings of the 3rd Annual Meeting of the COST-Action-FP1305-Biolink on Linking Belowground Biodiversity and Ecosystem Function in European Forests, Rome, Italy, 17–19 November 2015; pp. 191–207. [Google Scholar]
- Van der Heijden, M.G.A.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef]
- Lenoir, I.; Fontaine, J.; Sahraoui, A.L.H. Arbuscular mycorrhizal fungal responses to abiotic stresses: A review. Phytochemistry 2016, 123, 4–15. [Google Scholar] [CrossRef] [PubMed]
- Staudinger, C.; Mehmeti-Tershani, V.; Gil-Quintana, E.; Gonzalez, E.M.; Hofhansl, F.; Bachmann, G.; Wienkoop, S. Evidence for a rhizobia-induced drought stress response strategy in Medicago truncatula. J. Proteom. 2016, 136, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-dependent response of Trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Mastouri, F.; Bjorkman, T.; Harman, G.E. Trichoderma harzianum Enhances Antioxidant Defense of Tomato Seedlings and Resistance to Water Deficit. Mol. Plant-Microbe Interact. 2012, 25, 1264–1271. [Google Scholar] [CrossRef] [PubMed]
- Veach, A.M.; Chen, H.H.; Yang, Z.K.; Labbe, A.D.; Engle, N.L.; Tschaplinski, T.J.; Schadt, C.W.; Cregger, M.A. Plant Hosts Modify Belowground Microbial Community Response to Extreme Drought. mSystems 2020, 5, e00092-20. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Pan, K.W.; Olatunji, O.A.; Li, Z.L.; Chen, W.K.; Zhang, A.P.; Song, D.G.; Sun, X.M.; Huang, D.; Tan, X. Specific legumes allay drought effects on soil microbial food web activities of the focal species in agroecosystem. Plant Soil 2019, 437, 455–471. [Google Scholar] [CrossRef]
- Marino, D.; Frendo, P.; Ladrera, R.; Zabalza, A.; Puppo, A.; Arrese-Igor, C.; Gonzalez, E.M. Nitrogen fixation control under drought stress. Localized or systemic? Plant Physiol. 2007, 143, 1968–1974. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; Sinclair, T.R.; Purcell, L.C. Symbiotic N-2 fixation response to drought. J. Exp. Bot. 1999, 50, 143–155. [Google Scholar] [CrossRef]
- Cerezini, P.; Kuwano, B.H.; Grunvald, A.K.; Hungria, M.; Nogueira, M.A. Soybean tolerance to drought depends on the associated Bradyrhizobium strain. Braz. J. Microbiol. 2020, 51, 1977–1986. [Google Scholar] [CrossRef]
- Santos, M.A.; Vargas, M.A.T.; Hungria, M. Characterization of soybean Bradyrhizobium strains adapted to the Brazilian savannas. Fems Microbiol. Ecol. 1999, 30, 261–272. [Google Scholar] [CrossRef]
- Raza, S.; Jornsgard, B.; Abou-Taleb, H.; Christiansen, J.L. Tolerance of Bradyrhizobium sp. (Lupini) strains to salinity, pH, CaCO3 and antibiotics. Lett. Appl. Microbiol. 2001, 32, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Boscari, A.; Mandon, K.; Poggi, M.C.; Le Rudulier, D. Functional expression of Sinorhizobium meliloti BetS, a high-affinity betaine transporter, in Bradyrhizobium japonicum USDA110. Appl. Environ. Microbiol. 2004, 70, 5916–5922. [Google Scholar] [CrossRef] [PubMed]
- Clements, C.F.; Worsfold, N.T.; Warren, P.H.; Collen, B.; Clark, N.; Blackburn, T.M.; Petchey, O.L. Experimentally testing the accuracy of an extinction estimator: Solow’s optimal linear estimation model. J. Anim. Ecol. 2013, 82, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Wipf, H.M.L.; Bui, T.N.; Coleman-Derr, D. Distinguishing Between the Impacts of Heat and Drought Stress on the Root Microbiome of Sorghum bicolor. Phytobiomes J. 2021, 5, 166–176. [Google Scholar] [CrossRef]
- Baker, M.E.; King, R.S. A new method for detecting and interpreting biodiversity and ecological community thresholds. Methods Ecol. Evol. 2010, 1, 25–37. [Google Scholar] [CrossRef]
- Pereira, L.B.; Gambarini, V.M.D.; de Menezes, A.B.; Ottoboni, L.M.M.; Vicentini, R. Responses of the sugarcane rhizosphere microbiota to different levels of water stress. Appl. Soil Ecol. 2021, 159, 103817. [Google Scholar] [CrossRef]
- Goyal, R.K.; Habtewold, J.Z. Evaluation of Legume-Rhizobial Symbiotic Interactions Beyond Nitrogen Fixation That Help the Host Survival and Diversification in Hostile Environments. Microorganisms 2023, 11, 1454. [Google Scholar] [CrossRef] [PubMed]
- Brown, R.W.; Chadwick, D.R.; Zang, H.D.; Jones, D.L. Use of metabolomics to quantify changes in soil microbial function in response to fertiliser nitrogen supply and extreme drought. Soil Biol. Biochem. 2021, 160, 108351. [Google Scholar] [CrossRef]
- Guhr, A.; Horn, M.A.; Weig, A.R. Vitamin B-2 (riboflavin) increases drought tolerance of Agaricus bisporus. Mycologia 2017, 109, 860–873. [Google Scholar] [CrossRef]
- He, L.L.; Zhong, Z.K.; Yang, H.M. Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef]
- Wang, H.F.; Zheng, H.; Jiang, Z.X.; Dai, Y.H.; Liu, G.C.; Chen, L.; Luo, X.X.; Liu, M.H.; Wang, Z.Y. Efficacies of biochar and biochar-based amendment on vegetable yield and nitrogen utilization in four consecutive planting seasons. Sci. Total Environ. 2017, 593, 124–133. [Google Scholar] [CrossRef]
- Sloan, W.T.; Lunn, M.; Woodcock, S.; Head, I.M.; Nee, S.; Curtis, T.P. Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environ. Microbiol. 2006, 8, 732–740. [Google Scholar] [CrossRef]
- Mo, Y.Y.; Peng, F.; Gao, X.F.; Xiao, P.; Logares, R.; Jeppesen, E.; Ren, K.X.; Xue, Y.Y.; Yang, J. Low shifts in salinity determined assembly processes and network stability of microeukaryotic plankton communities in a subtropical urban reservoir. Microbiome 2021, 9, 128. [Google Scholar] [CrossRef]


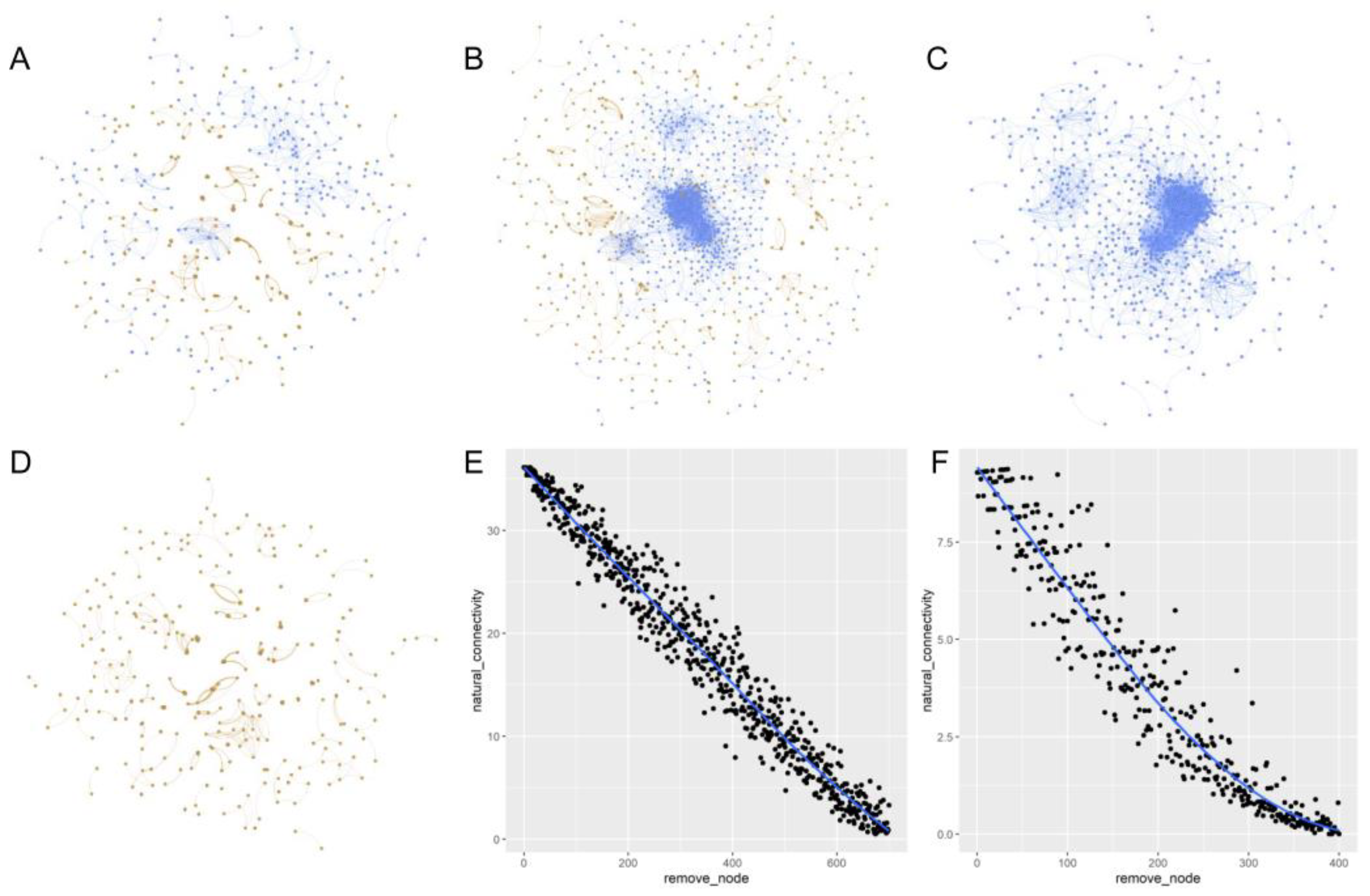


| Topological Properties | Networks | |||
|---|---|---|---|---|
| Overall (p < 0.001) | Overall (p < 0.05) | Bacterial (p < 0.05) | Fungal (p < 0.05) | |
| Number of nodes | 548 | 1252 | 777 | 414 |
| Number of edges | 1160 | 5993 | 4763 | 918 |
| Average degree | 4.234 | 9.573 | 12.260 | 4.435 |
| Network diameter | 10.614 | 18.023 | 16.126 | 4.661 |
| Network density | 0.00774 | 0.00765 | 0.01580 | 0.01074 |
| Connectivity | 9.028 | 35.778 | 36.101 | 9.295 |
| Modularity | 0.9547 | 0.5941 | 0.4560 | 0.9536 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, K.; Liu, Q.; Zhang, J.; Zhang, G.; Li, G. Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought. Plants 2023, 12, 4155. https://doi.org/10.3390/plants12244155
He K, Liu Q, Zhang J, Zhang G, Li G. Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought. Plants. 2023; 12(24):4155. https://doi.org/10.3390/plants12244155
Chicago/Turabian StyleHe, Kang, Qiangbo Liu, Jialei Zhang, Guanchu Zhang, and Guolin Li. 2023. "Biochar Enhances the Resistance of Legumes and Soil Microbes to Extreme Short-Term Drought" Plants 12, no. 24: 4155. https://doi.org/10.3390/plants12244155








