Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Study Area
2.2. Field Surveys
2.3. Remote Sensing by Sentinel-2 Satellite Images
2.4. Data Processing in GIS
2.5. Spectral Vegetation Indices
2.5.1. NDVI (Normalized Difference Vegetation Index)
2.5.2. NDMI (Normalized Difference Moisture Index)
2.5.3. NDRE (Normalized Difference Red Edge)
2.5.4. NBR (Normalized Burn Ratio)
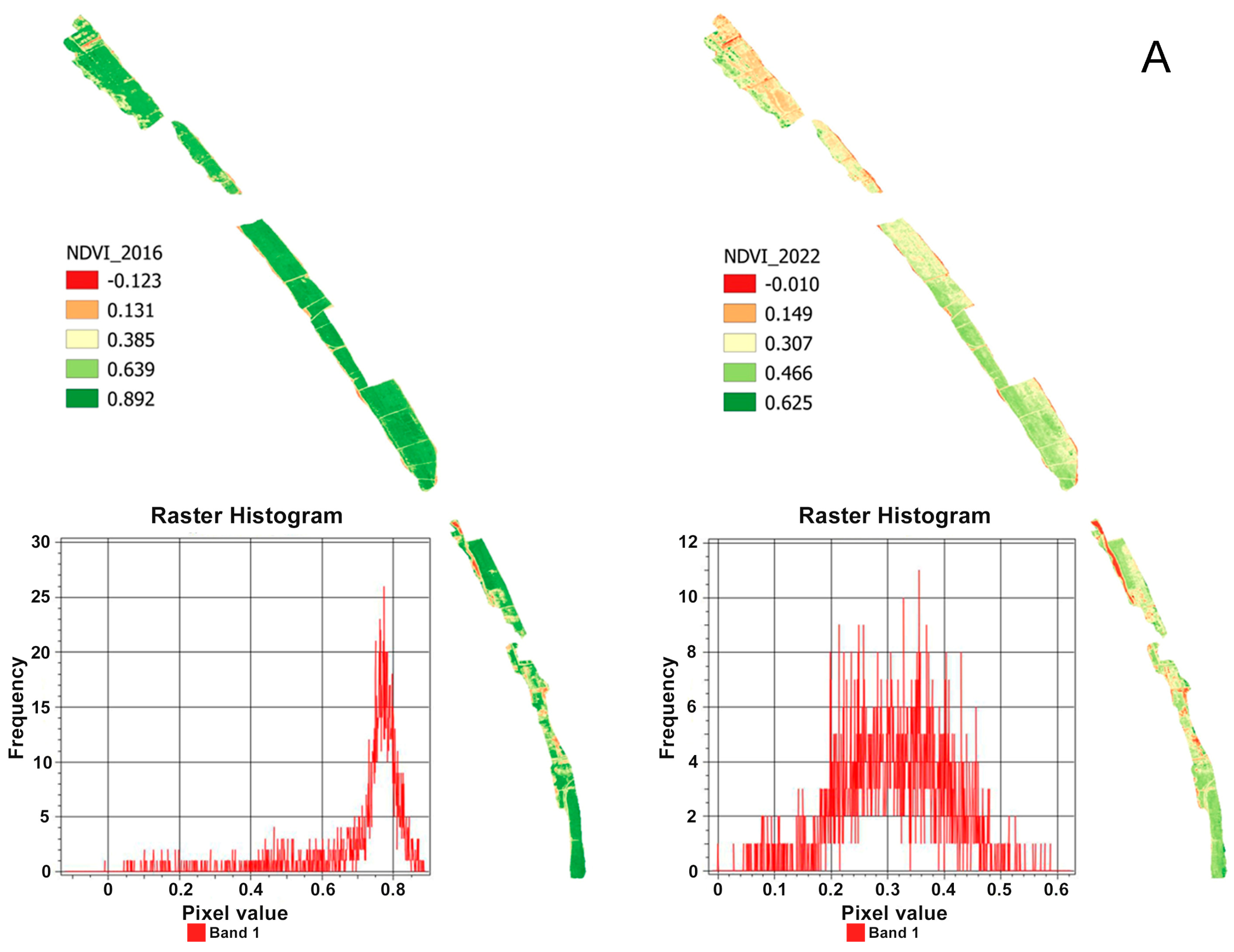
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Calandrelli, M.M.; Calandrelli, R. The district tourism lake of Castel Volturno: An example of territorial requalification of abandoned quarries. In Engineering Geology for Society and Territory: Urban Geology, Sustainable Planning and Landscape Exploitation; Lollino, G., Manconi, A., Guzzetti, F., Culshaw, M., Bobrowsky, P.T., Luino, F., Eds.; Springer: Cham, Switzerland, 2015; Volume 5, pp. 1315–1319. [Google Scholar]
- Rispo, M.; Calandrelli, R.; Calandrelli, M.M. L’uso delle tecnologie digitali per il monitoraggio forestale: La pineta del litorale domitio [The use of digital technologies for forest monitoring: The pine forest of the Domitian coast]. In Proceedings of the 10th National Congress of the Italian Society of Sylviculture and Forest Ecology, Florence, Italy, 15–18 September 2015; p. 101. (In Italian). [Google Scholar]
- Calandrelli, M.M.; Migliozzi, A. Storia di degrado e di rinascita di una pineta costiera [History of degradation and rebirth of a coastal pine forest]. Forest 2023, 20, 73–80. [Google Scholar] [CrossRef]
- D’Amico, G.; Francini, S.; Parisi, F.; Vangi, E.; De Santis, E.; Travaglini, D.; Chirici, G. Multitemporal optical remote sensing to support forest health condition assessment of Mediterranean pine forests in Italy. In Proceedings of the Global Challenges for a Sustainable Society: EURECA-PRO 2022; Springer Proceedings in Earth and Environmental Sciences Series; Benítez-Andrades, J.A., García-Llamas, P., Taboada, Á., Estévez-Mauriz, L., Baelo, R., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Del Perugia, B.; Travaglini, D.; Bottalico, F.; Nocentini, S.; Rossi, P.; Salbitano, F.; Sanesi, G. Le pinete litoranee di pino domestico (Pinus pinea L.) sono un paesaggio costiero in via di estinzione? Un caso di studio in Regione Toscana. Ital. For. E Mont. 2017, 72, 83–101. (In Italian) [Google Scholar]
- Nierhaus-Wunderwald, D.; Forster, B. Coleotteri Corticicoli sui Pini; Notizie per la pratica; Istituto Federale di Ricerca WSL: Birmensdorf, Switzerland, 2000; Volume 31, pp. 1–12. (In Italian) [Google Scholar]
- Garonna, A.P.; Scarpato, S.; Vicinanza, F.; Espinosa, B. First report of Toumeyella parvicornis (Cockerell) in Europe (Hemiptera, Coccidae). Zootaxa 2015, 3949, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Garonna, A.P.; Foscari, A.; Russo, E.; Jesu, G.; Somma, S.; Cascone, P.; Guerrieri, E. The spread of the non-native pine tortoise scale Toumeyella parvicornis (Hemiptera: Coccidae) in Europe: A major threat to Pinus pinea in Southern Italy. iForest 2018, 11, 628–634. [Google Scholar] [CrossRef]
- Campania Region. Available online: http://www.agricoltura.regione.campania.it/difesa/toumeyella.html (accessed on 20 June 2023).
- Cooper, D.D.; Cranshaw, W. Seasonal biology and associated natural enemies of two Toumeyella spp. Colorado. Southwest. Entomol. 2004, 29, 39–45. [Google Scholar]
- Stone, C.; Mohammed, C. Application of remote sensing technologies for assessing planted forests damaged by insect pests and fungal pathogens: A review. Curr. For. Rep. 2017, 3, 75–92. [Google Scholar] [CrossRef]
- Somma, S.; Notaro, L.; Russo, E.; Jesu, G.; De Leva, G.; Griffo, R.; Garonna, A.P. Distribution of Toumeyella parvicornis (Cockerell) nine year after its introduction in Campania Region, Italy. In Proceedings of the XXVII Italian Congress of Entomology, Palermo, Italy, 12–16 June 2023. (In Italian). [Google Scholar]
- Muñoz-Adalia, E.J.; Sanz-Ros, A.V.; Flores-Pacheco, J.A.; Hantula, J.; Diez, J.J.; Vainio, E.J.; Fernández, M. Sydowia polyspora dominates fungal communities carried by two Tomicus species in pine plantations threatened by Fusarium circinatum. Forests 2017, 8, 127. [Google Scholar] [CrossRef]
- Sabbatini Peverieri, G.; Capretti, P.; Tiberi, R. Associations between Tomicus destruens and Leptographium spp. in Pinus pinea and P. pinaster stands in Tuscany, central Italy. For. Pathol. 2006, 36, 14–20. [Google Scholar] [CrossRef]
- Dori-Bachash, M.; Avrahami-Moyal, L.; Protasov, A.; Mendel, Z.; Freeman, S. The occurrence and pathogenicity of Geosmithia spp. and common blue-stain fungi associated with pine bark beetles in planted forests in Israel. Eur. J. Plant Pathol. 2015, 143, 627–639. [Google Scholar] [CrossRef]
- Tiberi, R.; Branco, M.; Bracalini, M.; Croci, F.; Panzavolta, T. Cork oak pests: A review of insect damage and management. Ann. For. Sci. 2016, 73, 219–232. [Google Scholar] [CrossRef]
- Abd El-Ghany, N.M.; Abd El-Aziz, S.E.; Marei, S.S. A review: Application of remote sensing as a promising strategy for insect pests and diseases management. In Environmental Science and Pollution Research; Springer: Berlin/Heidelberg, Germany, 2020; pp. 1–13. [Google Scholar]
- Faccoli, M.; Piscedda, A.; Salvato, P.; Simonato, M.; Masutti, L.; Battisti, A. Genetic structure and phylogeography of pine shoot beetle populations (Tomicus destruens and T. piniperda, Coleoptera Scolytidae) in Italy. Ann. For. Sci. 2005, 62, 361–368. [Google Scholar] [CrossRef]
- Keenan, R.J. Climate change impacts and adaptation in forest management: A review. Ann. For. Sci. 2015, 72, 145–167. [Google Scholar] [CrossRef]
- Bernier, P.-Y.; Schoene, D. Adapting forests and their management to climate change: An overview. Unasylva 2009, 60, 5–11. [Google Scholar]
- Sabbatini Peverieri, G.; Faggi, M.; Marziali, L.; Panzavolta, T.; Bonuomo, L.; Tiberi, R. Use of attractant and repellent substances to control Tomicus destruens (Coleoptera: Scolytidae) in Pinus pinea and P. pinaster pine forests of Tuscany. Entomologica 2004, 38, 91–102. [Google Scholar]
- Choi, W.I.; Park, Y.S. Monitoring, assessment and management of forest insect pests and diseases. Forests 2019, 10, 865. [Google Scholar] [CrossRef]
- Barros, P.P.; Schutze, I.X.; Iost Filho, F.H.; Yamamoto, P.T.; Fiorio, P.R.; Demattê, J.A. Monitoring Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae) infestation in soybean by proximal sensing. Insects 2021, 12, 47. [Google Scholar] [CrossRef]
- Riley, J.R. Remote sensing in entomology. Ann. Rev. Entomol. 1989, 34, 247–271. [Google Scholar] [CrossRef]
- Hall, R.J.; Castilla, G.; White, J.C.; Cooke, B.J.; Skakun, R.S. Remote sensing of forest pest damage: A review and lessons learned from a Canadian perspective. Can. Entomol. 2016, 148, S296–S356. [Google Scholar] [CrossRef]
- Torres, P.; Rodes-Blanco, M.; Viana-Soto, A.; Nieto, H.; García, M. The role of remote sensing for the assessment and monitoring of forest health: A systematic evidence synthesis. Forests 2021, 12, 1134. [Google Scholar] [CrossRef]
- Baldo, M.; Malacarne, D.F.; Morao, G.; Caldart, S.; Codato, D.; Gianoli, F.; Pappalardo, S.E.; Menegon, S.; Dallago, G.; De Marchi, M. Sentinel-2 come supporto per il monitoraggio e la gestione di agroecosistemi vinicoli [Sentinel-2 a support for the monitoring and management of wine agroecosystems]. In Proceedings of the Conference Associazioni Scientifiche per le Informazioni Territoriali ed Ambientali, Salerno, Italy, 21–23 November 2017; pp. 75–82. (In Italian). [Google Scholar]
- Ives, W.G.H.; Wong, H.R. Tree and shrub insects of the prairie provinces. In Northern Forestry Centre Information Report NOR-X-292; Canadian Forestry Service: Edmonton, AB, USA, 1988. [Google Scholar]
- Armstrong, J.A.; Ives, W.G.H. Insectes Forestiers Ravageurs au Canada; Ressources Naturelles Canada—Service Canadien des Forêts; Direction des Sciences et du Développement Durable: Ottawa, ON, Canada, 1995; p. 732. [Google Scholar]
- Gitelson, A.A.; Kaufman, Y.J.; Stark, R.; Rundquist, D. Novel algorithms for remote estimation of vegetation fraction. Remote Sens. Environ. 2002, 80, 76–87. [Google Scholar] [CrossRef]
- Spadoni, G.L.; Cavalli, A.; Congedo, L.; Munafò, M. Analysis of Normalized Difference Vegetation Index (NDVI) multi-temporal series for the production of forest cartography. Remote Sens. Appl. Soc. Environ. 2020, 20, 100419. [Google Scholar] [CrossRef]
- Barbarella, M.; De Giglio, M.; Greggio, N. Dati ASTER per la valutazione dello stato di salute di aree costiere vegetate interessate da salinizzazione. In Proceedings of the 17th National Conference Associazioni Scientifiche per le Informazioni Territoriali ed Ambientali, Riva del Garda, Italy, 5–7 November 2013; pp. 87–94. (In Italian). [Google Scholar]
- Meera Gandhi, G.; Parthiban, S.; Nagaraj Thummalu, D.; Christy, A. Ndvi: Vegetation change detection using remote sensing and gis–A case study of Vellore District. Proc. Comp. Sci. 2015, 57, 1199–1210. [Google Scholar] [CrossRef]
- Spinsi, A.; Boratelli, L.; Marletto, V. Indici vegetazionali da satellite per il monitoraggio in continuo del territorio. Ital. J. Agrometeor. 2012, 3, 49–55. [Google Scholar]
- Cayrol, P.; Chehbouni, A.; Kergoat, L.; Dedieu, G.; Mordelet, P.; Nouvellon, Y. Grassland modeling and monitoring with Spot-4 Vegetation instrument during the 1997–1999 SALSA experiment. Agric. For. Meteor. 2000, 105, 91–115. [Google Scholar] [CrossRef]
- Rani, M.; Kumar, P.; Pandey, P.C.; Srivastava, P.K.; Chaudhary, B.S.; Tomar, V.; Mandal, V.P. Multi-temporal NDVI and surface temperature analysis for Urban Heat Island inbuilt surrounding of sub-humid region: A case study of two geographical regions. Remote Sens. Applic. Soc. Environ. 2018, 10, 163–172. [Google Scholar] [CrossRef]
- Lan, Y.; Zhang, H.; Lacey, R.; Hoffmann, W.C.; Wu, W. Development of an integrated sensor and instrumentation system for measuring crop conditions. Agric. Engin. J. 2009, 11, 11–15. [Google Scholar]
- El-Shikha, D.M.; Waller, P.; Hunsaker, D.; Clarke, T.; Barnes, E. Ground-based remote sensing for assessing water and nitrogen status of broccoli. Agric. Water Manag. 2007, 92, 183–193. [Google Scholar] [CrossRef]
- Hmimina, G.; Dufrêne, E.; Pontailler, J.-Y.; Delpierre, N.; Aubinet, M.; Caquet, B.; de Grandcourt, A.; Burban, B.; Flechard, C.; Granier, A.; et al. Evaluation of the potential of MODIS satellite data to predict vegetation phenology in different biomes: An investigation using ground-based NDVI measurements. Remote Sens. Environ. 2013, 132, 145–158. [Google Scholar] [CrossRef]
- Bechtel, A.; Puttmann, W.; Carlson, T.N.; Ripley, D.A. On the relation between NDVI fractional vegetation cover, and leaf area index. Remote Sens. Environ. 1997, 62, 241–252. [Google Scholar]
- AL-Gaadi, K.A.; Hassaballa, K.A.; Tola, E.; Kayad, A.G.; Madugundu, R.; Alblewi, B.; Assyrians, F. Prediction of potato crop yield using precision agriculture techniques. PLoS ONE 2016, 11, e0162219. [Google Scholar] [CrossRef]
- Di Natale, M.; Di Ronza, S.; Eramo, C. Verifica delle opere di difesa e di riqualificazione ambientale del litorale Domitio-Indagine Sperimentale. Studi Costieri 2012, 20, 47–57. (In Italian) [Google Scholar]
- Rispo, V.; Digilio, A.; Calandrelli, M.M. Tutelare il capitale naturale con il remote sensing. In Atti del Convegno “Urbanistica Informazioni”; INU: Napoli, Italy, 2022; pp. 400–402. (In Italian) [Google Scholar]
- Migliozzi, A.; Stinca, A. La Piana Campana: Trasformazioni del paesaggio. In Dal Degrado alla Bellezza; Simeone, M.M., Ed.; Edizioni Scientifiche Italiane: Napoli, Italy, 2012; pp. 89–100. (In Italian) [Google Scholar]
- Zhang, B.; Ye, H.; Lu, W.; Huang, W.; Wu, B.; Hao, Z.; Sun, H. A spatiotemporal change detection method for monitoring pine wilt disease in a complex landscape using high-resolution remote sensing imagery. Remote Sens. 2021, 13, 2083. [Google Scholar] [CrossRef]
- European Space Agency. S2 MSI Product Specification. Available online: https://sentinel.esa.int/documents/247904/349490/S2_MSI_Product_Specification.pdf (accessed on 15 April 2021).
- European Space Agency. Sentinel-2 User Handbook; ESA Standard Document; ESA: Paris, France, 2015; Available online: https://sentinel.esa.int/documents/247904/685211/Sentinel-2_User_Handbook (accessed on 15 April 2021).
- Gascon, F.; Bouzinac, C.; Thépaut, O.; Jung, M.; Francesconi, B.; Louis, J.; Lonjou, V.; Lafrance, B.; Massera, S.; Gaudel-Vacaresse, A.; et al. Copernicus Sentinel-2A calibration and products validation status. Remote Sens. 2017, 9, 584. [Google Scholar] [CrossRef]
- Li, J.; Roy, D.P. A global analysis of Sentinel-2a, Sentinel-2b and Landsat-8 data revisit intervals and implications for terrestrial monitoring. Remote Sens. 2017, 9, 902. [Google Scholar] [CrossRef]
- Kurbatova, A.I.; Bouchhima, R.A.; Grigorets, E.A.; Tsymbarovich, P.R.; Ksibi, M. Methodology for mapping soil salinity and halophyte cover using remote sensing data in Kerkennah, Tunisia. Euro-Mediterr. J. Environ. Integr. 2021, 6, 51. [Google Scholar] [CrossRef]
- Congedo, L. Semi-Automatic Classification Plugin Documentation. 2016. Available online: https://doi.org/10.13140/RG.2.2.29474.02242/1 (accessed on 20 October 2020).
- CLC2016. Available online: https://land.copernicus.eu/pan-european/corine-land-cover (accessed on 22 September 2020).
- Zeng, Y.; Hao, D.; Huete, A.; Dechant, B.; Berry, J.; Chen, J.M.; Joiner, J.; Frankenberg, C.; Bond-Lamberty, B.; Ryu, Y.; et al. Optical vegetation indices for monitoring terrestrial ecosystems globally. Nat. Rev. Earth Environ. 2022, 3, 477–493. [Google Scholar] [CrossRef]
- Ferchichi, A.; Abbes, A.B.; Barra, V.; Farah, I.R. Forecasting vegetation indices from spatio-temporal remotely sensed data using deep learning-based approaches: A systematic literature review. Ecol. Inform. 2022, 68, 101552. [Google Scholar] [CrossRef]
- Gu, L.; Shuai, Y.; Shao, C.; Xie, D.; Zhang, Q.; Li, Y.; Yang, J. Angle effect on typical optical remote sensing indices in vegetation monitoring. Remote Sens. 2021, 13, 1699. [Google Scholar] [CrossRef]
- Rouse, J.W.; Haas, R.W.; Schell, J.A.; Deering, D.W.; Harlan, J.C. Monitoring the Vernal Advancement and Retrogradation (Green Wave Effect) of Natural Vegetation. NASA/GSFC Type III Final Rep; Goddard Space Flight Center: Greenbelt, MD, USA, 1974; Volume 371, pp. 1–112. [Google Scholar]
- JARS. Remote Sensing Note. Japan Association on Remote Sensing. 1993. Available online: http://www.jars1974.net/pdf/rsnote_e.html (accessed on 21 April 2021).
- Walsh, S.J.; Crawford, T.W.; Welsh, W.F.; Crews-Meyer, K.A. A multiscale analysis of LULC and NDVI variation in Nang Rong district, northeast Thailand. Agric. Ecosyst. Environ. 2001, 85, 47–64. [Google Scholar] [CrossRef]
- Moraca, M.; Pepe, A. Studio comparativo tra lo stato dei luoghi prima e dopo l’incendio del Vesuvio tramite analisi satellitare. GEOMedia 2018, 5, 1–21. (In Italian) [Google Scholar]
- Gao, B.C. NDWI—A normalized difference water index for remote sensing of vegetation liquid water from space. Remote Sens. Environ. 1995, 58, 257–266. [Google Scholar] [CrossRef]
- EOS Data Analytics. NDMI. 2023. Available online: https://eos.com/make-an-analysis/ndmi/ (accessed on 19 January 2023).
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, IN, USA, 16–19 July 2000; pp. 6–20. [Google Scholar]
- EOS Data Analytics. NDRE. 2023. Available online: https://eos.com/industries/agriculture/ndre/ (accessed on 21 November 2023).
- Key, C.H.; Benson, N.C. Landscape assessment: Sampling and analysis methods. In FIREMON: Fire Effects Monitoring and Inventory System; Lutes, D.C., Keane, R.E., Caratti, J.F., Key, C.H., Benson, N.C., Sutherland, S., Gangi, L.J., Eds.; General Technical Reports: RMRS-GTR-164; LA1-LA55 ISBN; USDA Forest Service: Washington, DC, USA, 2006. [Google Scholar]
- USGS. Available online: https://un-spider.org/advisory-support/recommended-practices/recommended-practice-burn-severity/Step-by-Step/QGIS-sentinel2 (accessed on 17 March 2023).
- Bennett, W.H. Insect Pests of Southern Pines; US Department of Agriculture: Washington, DC, USA, 1972. [Google Scholar]
- Wermelinger, B.; Rigling, A.; Schneider Mathis, D.; Dobbertin, M. Assessing the role of bark-and wood-boring insects in the decline of Scots pine (Pinus sylvestris) in the Swiss Rhone valley. Ecol. Entomol. 2008, 33, 239–249. [Google Scholar] [CrossRef]
- Choi, W.I.; Nam, Y.; Lee, C.Y.; Choi, B.K.; Shin, Y.J.; Lim, J.-H.; Koh, S.-H.; Park, Y.-S. Changes in major insect pests of pine forests in Korea over the last 50 years. Forests 2019, 10, 692. [Google Scholar] [CrossRef]
- Hennessy, A.; Clarke, K.; Lewis, M. Hyperspectral classification of plants: A review of waveband selection generalisability. Remote Sens. 2020, 12, 113. [Google Scholar] [CrossRef]
- Akyol, A.; Orucu, O.K. Investigation and evaluation of stone pine (Pinus pinea L.) current and future potential distribution under climate change in Turkey. Cerne 2019, 25, 415–423. [Google Scholar] [CrossRef]
- Kim, S.-R.; Lee, W.-K.; Lim, C.-H.; Kim, M.; Kafatos, M.C.; Lee, S.-H.; Lee, S.-S. Hyperspectral analysis of pine wilt disease to determine an optimal detection index. Forests 2018, 9, 115. [Google Scholar] [CrossRef]
- Bertin, S.; Ilardi, F.; Scapini, C.; Simoni, S.; Roversi, P.F. Alien pest Toumeyella parvicornis (Cockerell) (Hemiptera: Coccidae) on Pinus pinea L.: Short time evaluation of endotherapic treatment. Redia 2022, 105, 11–16. [Google Scholar] [CrossRef]
- Balla, A.; Silini, A.; Cherif-Silini, H.; Chenari Bouket, A.; Moser, W.K.; Nowakowska, J.A.; Oszako, T.; Benia, F.; Belbahri, L. The Threat of Pests and Pathogens and the Potential for Biological Control in Forest Ecosystems. Forests 2021, 12, 1579. [Google Scholar] [CrossRef]
- Joshi, C.; De Leeuw, J.; van Duren, I.C. Remote sensing and GIS applications for mapping and spatial modelling of invasive species. In Proceedings of the International Society for Photogrammetry and Remote Sensing, Istanbul, Turkey, 12–23 July 2004; pp. 669–677. [Google Scholar]
- Taddeo, S. Leveraging time series of satellite and aerial images to promote the long-term monitoring of restored plant communities. Appl. Veg. Sci. 2022, 25, e12664. [Google Scholar] [CrossRef]
- Ezaidi, S.; Aydda, A.; Kabbachi, B.; Althuwaynee, O.F.; Ezaidi, A.; Haddou, M.A.; Idoumskine, I.; Thorpe, J.; Park, H.J.; Kim, S.W. Multi-temporal Landsat-derived NDVI for vegetation cover degradation for the period 1984–2018 in part of the Arganeraie Biosphere Reserve (Morocco). Remote Sens. Appl. Soc. Environ. 2022, 27, 100800. [Google Scholar] [CrossRef]
- IPCC-Intergovernmental Panel on Climate Change. 2023. Available online: https://www.ipcc.ch/report/sixth-assessment-report-cycle/ (accessed on 17 August 2023).







| Spectral Band | Central Wavelength (nm) | Spatial Resolution-GSD (m) |
|---|---|---|
| B1: Ultra Blue (coastal, aerosol) | 443 | 60 |
| B2: Blue | 490 | 10 |
| B3: Green | 560 | 10 |
| B4: Red | 665 | 10 |
| B5: Red-edge 1 | 705 | 20 |
| B6: Red-edge 2 | 740 | 20 |
| B7: Red-edge 3 | 783 | 20 |
| B8: Near-infrared (NIR) | 842 | 10 |
| B8A: Near-infrared narrow | 865 | 20 |
| B9: Water vapor | 945 | 60 |
| B10: Short-wave infrared (SWIR) | 1375 | 60 |
| B11: Short-wave infrared (SWIR) | 1610 | 20 |
| B12: Short-wave infrared (SWIR) | 2190 | 20 |
| N | Identifier Data |
|---|---|
| 1 | S2A_MSIL1C_20160523T095032_N0202_R079_T33TVF_20160523T095404 |
| 2 | S2A_MSIL1C_20170518T095031_N0205_R079_T33TVF_20170518T095716 |
| 3 | S2A_MSIL2A_20180526T100031_N0208_R122_T33TVF_20180526T161700 |
| 4 | S2B_MSIL2A_20190523T095039_N0212_R079_T33TVF_20190523T124054 |
| 5 | S2A_MSIL2A_20200522T095041_N0214_R079_T33TVF_20200522T122957 |
| 6 | S2A_MSIL2A_20210507T095031_N0300_R079_T33TVF_20210507T151839 |
| 7 | S2A_MSIL2A_20220515T100031_N0400_R122_T33TVF_20220515T141508 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicoletti, R.; De Masi, L.; Migliozzi, A.; Calandrelli, M.M. Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing. Plants 2024, 13, 182. https://doi.org/10.3390/plants13020182
Nicoletti R, De Masi L, Migliozzi A, Calandrelli MM. Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing. Plants. 2024; 13(2):182. https://doi.org/10.3390/plants13020182
Chicago/Turabian StyleNicoletti, Rosario, Luigi De Masi, Antonello Migliozzi, and Marina Maura Calandrelli. 2024. "Analysis of Dieback in a Coastal Pinewood in Campania, Southern Italy, through High-Resolution Remote Sensing" Plants 13, no. 2: 182. https://doi.org/10.3390/plants13020182








