Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status
Abstract
:1. Introduction
2. Results and Discussion
2.1. Plant Nutrient Uptake Experiment
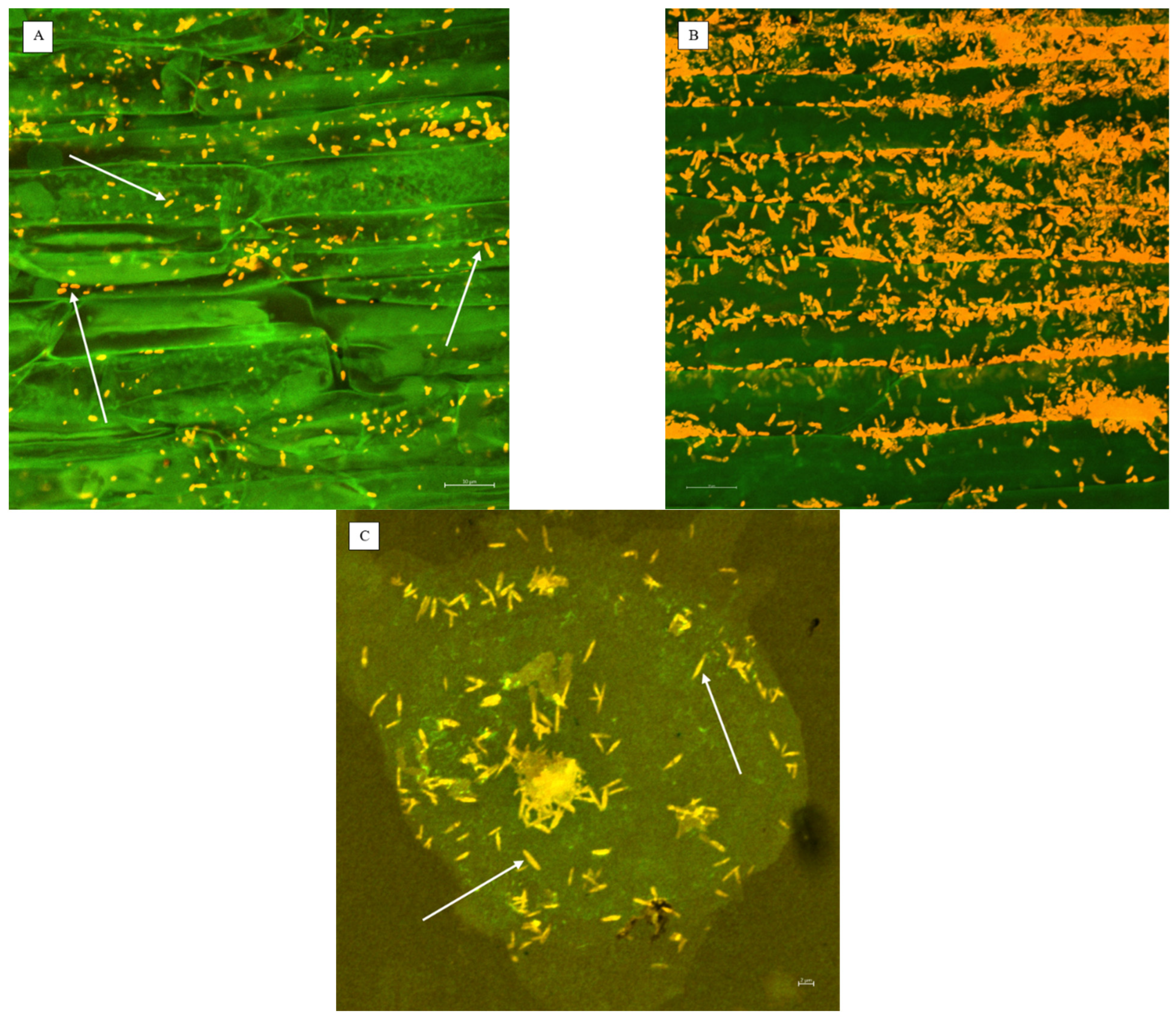
2.1.1. Microscopic Detection
2.1.2. Biometric Parameters, Dry Weight
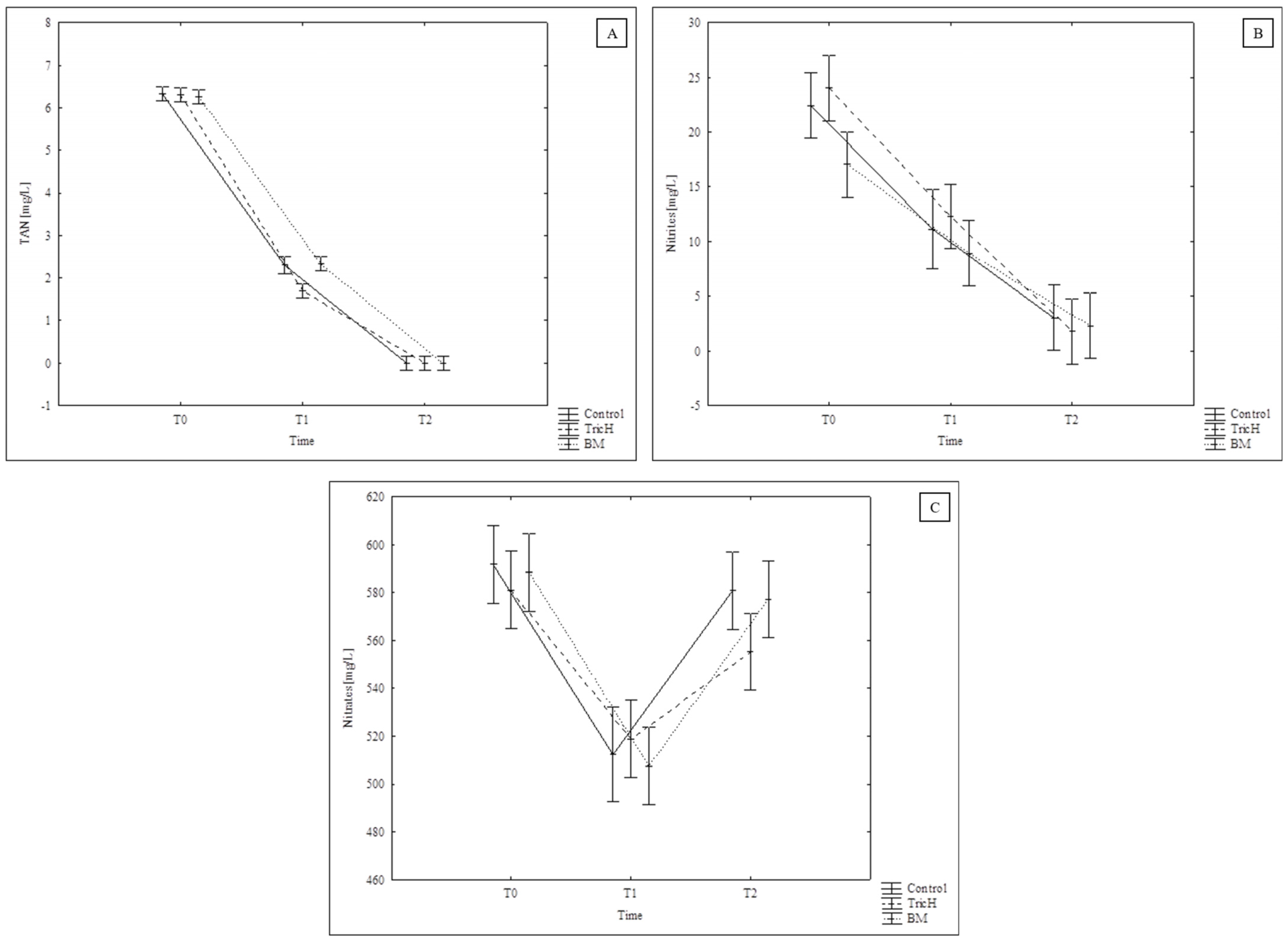
2.1.3. Nutrient Solution Parameters
2.1.4. Nutrient Uptake and Levels in Plants
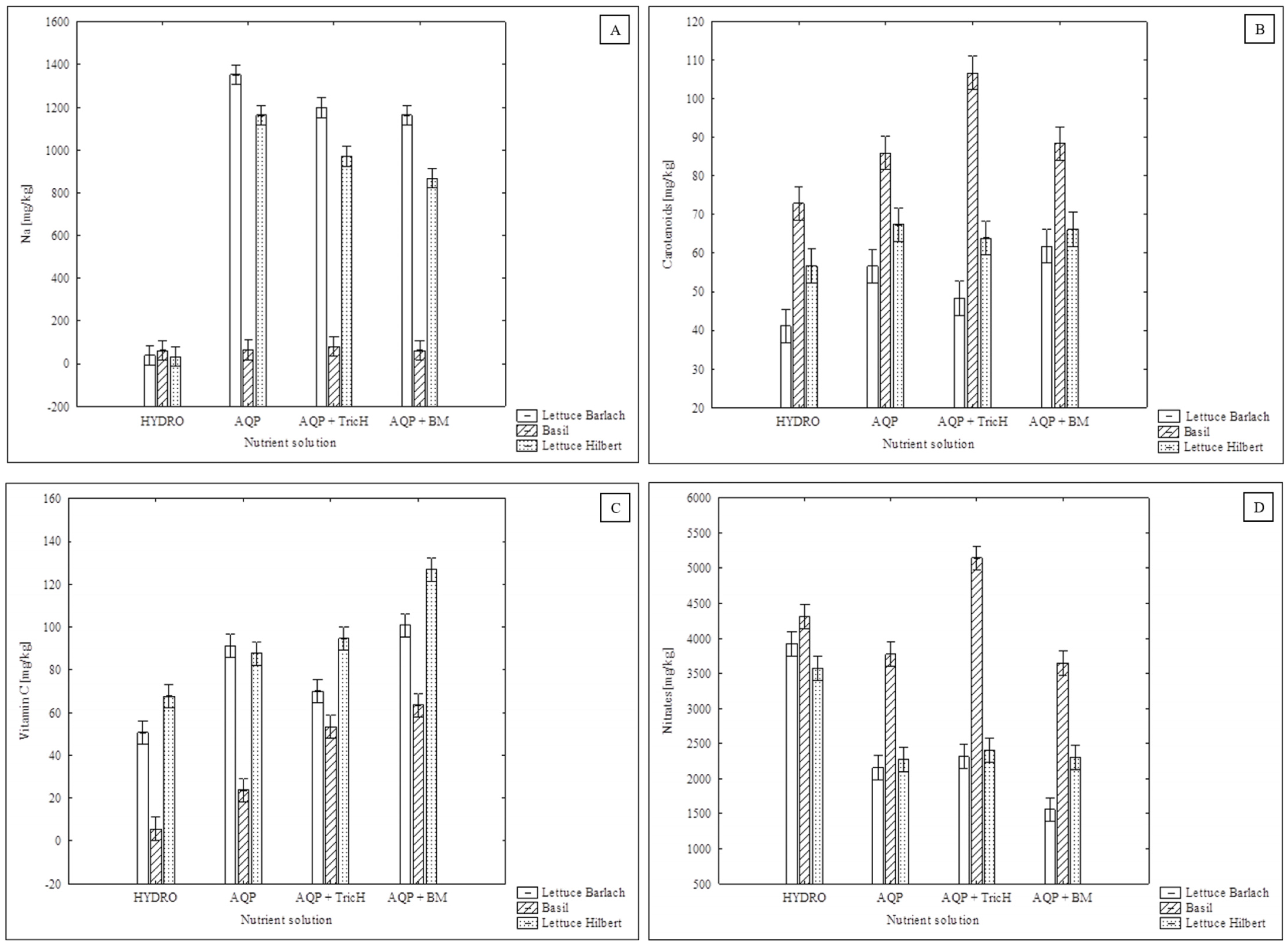
2.1.5. Chlorophyll, Carotenoids, and Vitamin C
2.1.6. Nitrates
2.2. Biofilter Experiment
3. Materials and Methods
3.1. Plant Nutrient Uptake Experiment
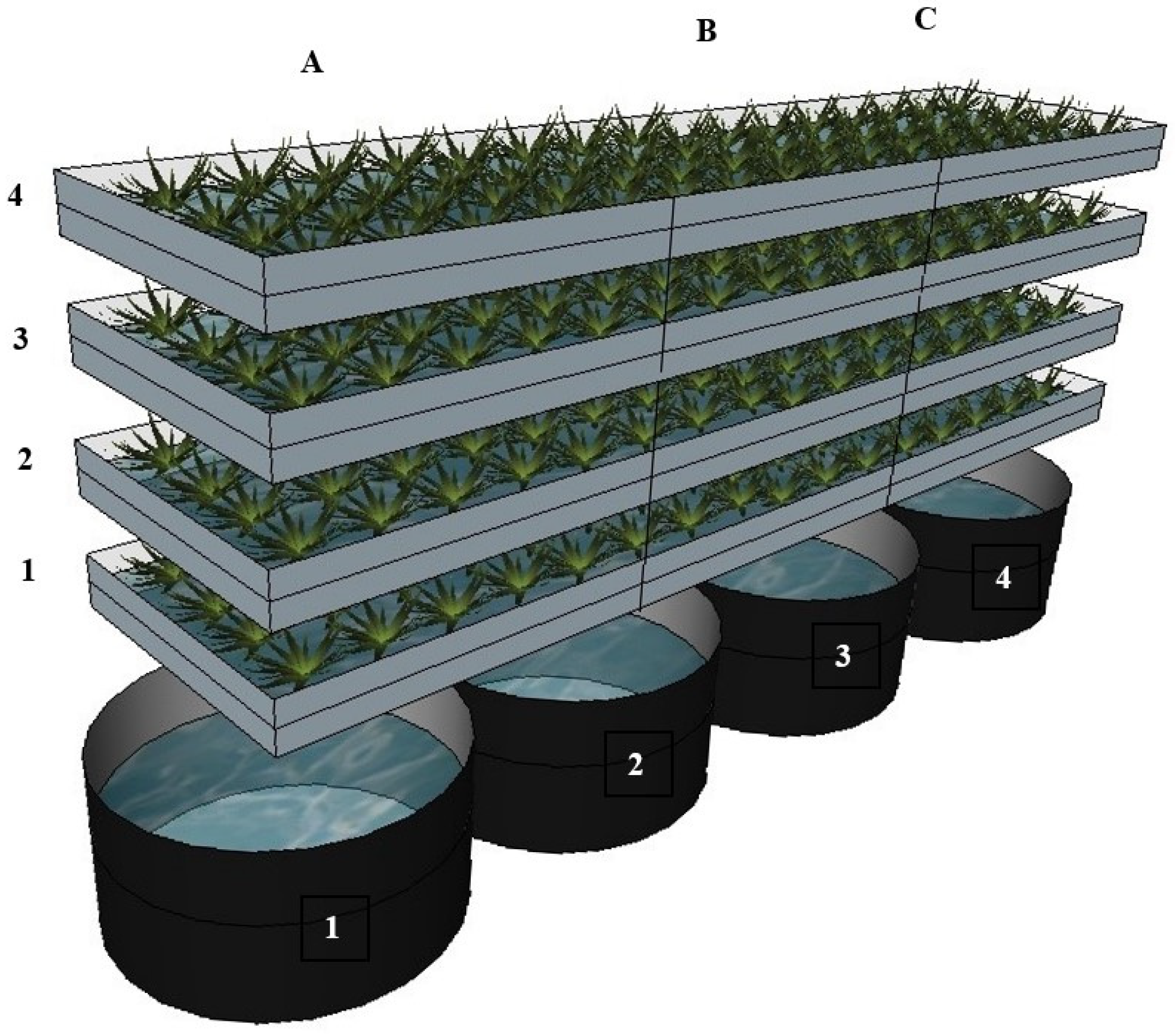
3.1.1. System Setup and Cultivation Conditions
3.1.2. Experimental Design
- (1)
- HYDRO (control)—hydroponic nutrient solution (commercial fertilizer for leafy vegetables, made up of two components: JUNGLE garden G1 0.4% N, 2% P2O5 and 4.5% K2O and JUNGLE garden BASE 7% N, 11.2% CaO and 0.22% Fe, Numazon Ltd., Brno).
- (2)
- AQP—aquaponic nutrient solution derived from a recirculation aquaculture system (RAS) with rainbow trout (Oncorhynchus mykiss, Walbaum).
- (3)
- AQP + TricH—the aquaponic nutrient solution inoculated with Trichoderma harzianum.
- (4)
- AQP + BM—the aquaponic nutrient solution inoculated with Bacillus mojavensis.
3.1.3. Inoculation
3.1.4. Course of the Experiment
3.1.5. Microscopic Detection
3.1.6. Analytical Methodology
3.2. Biofilter Experiment
3.3. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Goddek, S.; Delaide, B.; Mankasingh, U.; Ragnarsdottir, K.V.; Jijakli, H.; Thorarinsdottir, R. Challenges of Sustainable and Commercial Aquaponics. Sustainability 2015, 7, 4199–4224. [Google Scholar] [CrossRef]
- Yep, B.; Zheng, Y. Aquaponic trends and challenges—A review. J. Clean. Prod. 2019, 228, 1586–1599. [Google Scholar] [CrossRef]
- Kasozi, N.; Tandlich, R.; Fick, M.; Kaiser, H.; Wilhelmi, B. Iron supplementation and management in aquaponic systems: A review. Aquac. Rep. 2019, 15, 100221. [Google Scholar] [CrossRef]
- Barteleme, R.P.; Oyserman, B.O.; Blom, J.E.; Sepulveda-Villet, O.J.; Newton, R.J. Stripping Away the Soil: Plant Growth Promoting Microbiology Opportunities in Aquaponics. Front. Microbiol. 2018, 9, 8. [Google Scholar] [CrossRef] [PubMed]
- Kloas, W.; Gross, R.; Baganz, D.; Graupner, J.; Monsees, H.; Schmidt, U.; Staaks, G.B.O.; Suhl, J.; Tschirner, M.; Wittstock, B.; et al. A new concept for aquaponic systems to improve sustainability, increase productivity, and reduce environmental impacts. Aquac. Environ. Interact. 2015, 7, 179–192. [Google Scholar] [CrossRef]
- Roosta, H.R.; Hamidpour, M. Effects of foliar application of some macro- and micro-nutrients on tomato plants in aquaponic and hydroponic systems. Sci. Hortic. 2011, 129, 396–402. [Google Scholar] [CrossRef]
- Robaina, L.; Pirhonen, J.; Mente, E.; Sánchez, J.; Goosen, N. Fish Diets in Aquaponics. In Aquaponics Food Production Systems—Combined Aquaculture and Hydroponic Production Technologies for the Future; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: New York, NY, USA, 2019; ISBN 978-3-030-15943-6. [Google Scholar] [CrossRef]
- Mitra, D.; Andjelkovic, S.; Manisha; Panneerselvam, P.; Chauhan, M.; Senapati, A.; Vasic, T.; Ganeshamurthy, A.N.; Verma, D.; Arya, P.; et al. Plant growth promoting microorganisms (PGPMs) helping in sustainable agriculture: Current perspective. Int. J. Agric. Sci. Vet. Med. 2019, 7, 50–74. [Google Scholar]
- Bugbee, B. Nutrient Management in Recirculating Hydroponic Culture. Acta Hortic. 2004, 648, 99–112. [Google Scholar] [CrossRef]
- Waghunde, R.; Shelake, R.M.; Sabalpara, A.N. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Mukherjee, M.; Mukherjee, P.K.; Horwitz, B.A.; Zachow, C.; Berg, G.; Zeilinger, S. Trichoderma–Plant–Pathogen Interactions: Advances in Genetics of Biological Control. Indian J. Microbiol. 2012, 52, 522–529. [Google Scholar] [CrossRef]
- Sharma, B.L.; Singh, S.P.; Sharma, M.L. Bio-degradation of Crop Residues by Trichoderma Species vis-à vis Nutrient Quality of the Prepared Compost. Sugar Tech. 2012, 14, 174–180. [Google Scholar] [CrossRef]
- Amira, D. Bioconversion of empty fruit bunches (EFB) and palm oil mill effluent (POME) into compost using Trichoderma virens. Afr. J. Biotechnol. 2011, 10, 81. [Google Scholar] [CrossRef]
- Vázquez, M.B.; Barrera, V.; Bianchinotti, V. Molecular identification of three isolates of Trichoderma harzianum isolated from agricultural soils in Argentina, and their abilities to detoxify in vitro metsulfuron methyl. Botany 2015, 93, 793–800. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a novel plant growth regulator and systemic resistance elicitor from Trichoderma harzianum. Plant Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Halim, A.M.-A.; Shivanand, P.; Krishnamoorthy, S.; Taha, H. A review on the biological properties of Trichoderma spp. as a prospective biocontrol agent and biofertilizer. J. Appl. Biol. Biotechnol. 2023, 11, 5. [Google Scholar] [CrossRef]
- Jaroszuk-Ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, Gibberellin) and ACC Deaminase In Vitro Synthesized by the Mycoparasitic Trichoderma DEMTkZ3A0 Strain and Changes in the Level of Auxin and Plant Resistance Markers in Wheat Seedlings Inoculated with this Strain Conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef]
- Yedida, I.; Srivastava, A.; Kapulnik, Y.; Chet, I. Effect of Trichoderma harzianum on microelement concentrations and increased growth of cucumber plants. Plant Soil 2001, 235, 235–242. [Google Scholar] [CrossRef]
- Kahil, A.A.; Hassan, F.A.S.; Ali, E.F. Influence of bio-fertilizers on growth, yield and anthocyanin content of Hibiscus sabdariffa L. plant under Taif region conditions. Ann. Res. Rev. Biol. 2017, 17, 1–15. [Google Scholar] [CrossRef]
- Hassan, F.; Ali, E.F.; Mahfouz, S.A. Comparison between different fertilization sources, irrigation frequency and their combinations on the growth and yield of the coriander plant. Aust. J. Appl. Basic Sci. 2012, 6, 600–615. [Google Scholar]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for Plant Growth Promotion and Stress Resilience: What Have We Learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.; Shahid, M.; Zeyad, M.T.; Bukhari, N.A.; Alkhataf, F.; Hatamleh, A.A.; Ali, S. Bacillus mojavensis, a Metal-Tolerant Plant Growth-Promoting Bacterium, Improves Growth, Photosynthetic Attributes, Gas Exchange Parameters, and Alkalo-Polyphenol Contents in Silver Nanoparticle (Ag-NP)-Treated Withania somnifera L. (Ashwagandha). ACS Omega 2022, 7, 13878–13893. [Google Scholar] [CrossRef] [PubMed]
- Emmanuel, O.C.; Babalola, O.O. Productivity and quality of horticultural crops through co-inoculation of arbuscular mycorrhizal fungi and plant growth promoting bacteria. Microbiol. Res. 2020, 239, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Keller-Pearson, M.; Liu, Y.; Peterson, A.; Pederson, K.; Willems, L.; Ané, J.-M.; Silva, E.M. Inoculation with arbuscular mycorrhizal fungi has a more significant positive impact on the growth of open-pollinated heirloom varieties of carrots than on hybrid cultivars under organic management conditions. Agric. Ecosyst. Environ. 2020, 289, 106712. [Google Scholar] [CrossRef]
- Rasouli, F.; Amini, T.; Asadi, M.; Hassanpouraghdam, M.B.; Aazami, M.A.; Ercisli, S.; Skrovankova, S.; Mlcek, J. Growth and Antioxidant Responses of Lettuce (Lactuca sativa L.) to Arbuscular Mycorrhiza Inoculation and Seaweed Extract Foliar Application. Agronomy 2022, 12, 401. [Google Scholar] [CrossRef]
- Boomsma, C.R.; Vyn, T.J. Maize drought tolerance: Potential improvements through arbuscular mycorrhizal symbiosis? Field Crops Res. 2008, 108, 14–31. [Google Scholar] [CrossRef]
- Cheng, S.; Zou, Y.-N.; Kuča, K.; Hashem, A.; Fathi, E.; Allah, A.; Wu, Q.-S. Elucidating the Mechanisms Underlying Enhanced Drought Tolerance in Plants Mediated by Arbuscular Mycorrhizal Fungi. Front. Microbiol. 2021, 12, 809473. [Google Scholar] [CrossRef]
- Santander, C.; Sanhueza, M.; Olave, J.; Borie, F.; Valentine, A.; Cornejo, P. Arbuscular Mycorrhizal Colonization Promotes the Tolerance to Salt Stress in Lettuce Plants through an Efficient Modification of Ionic Balance. J. Soil Sci. Plant Nutr. 2019, 19, 321–331. [Google Scholar] [CrossRef]
- Ma, J.; Janoušková, M.; Li, Y.; Yu, X.; Yan, Y.; Zou, Z.; He, C. Impact of arbuscular mycorrhizal fungi (AMF) on cucumber growth and phosphorus uptake under cold stress. Funct. Plant Biol. 2015, 42, 1158–1167. [Google Scholar] [CrossRef]
- Chen, M.; Arato, M.; Borghi, L.; Nouri, E.; Reinhardt, D. Beneficial Services of Arbuscular Mycorrhizal Fungi—From Ecology to Application. Front. Plant Sci. 2018, 9, 1270. [Google Scholar] [CrossRef] [PubMed]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Control of greenhouse tomato root rot [Pythium ultimum] in hydroponic systems, using plant-growth-promoting microorganisms. Can. J. Plant Pathol. 2006, 28, 475–483. [Google Scholar] [CrossRef]
- Cerozi, B.S.; Fitzsimmons, K. Use of Bacillus spp. to enhance phosphorus availability and serve as a plant growth promoter in aquaponics systems. Sci. Hortic. 2016, 211, 277–282. [Google Scholar] [CrossRef]
- Sheridan, C.; Depuydt, P.; Ro, M.D.; Petit, C.; Gysegem, E.V.; Delaere; Petal, R.; Pascale, S.D.; Ventorino, V.; Meyer, T.D.; et al. Microbial Community Dynamics and Response to Plant Growth-Promoting Microorganisms in the Rhizosphere of Four Common Food Crops Cultivated in Hydroponics. Microb. Ecol. 2017, 73, 378–393. [Google Scholar] [CrossRef] [PubMed]
- Dhawi, F. The Role of Plant Growth-Promoting Microorganisms (PGPMs) and Their Feasibility in Hydroponics and Vertical Farming. Metabolites 2023, 13, 247. [Google Scholar] [CrossRef] [PubMed]
- Mourouzidou, S.; Ntinas, G.K.; Tsaballa, A.; Monokrousos, N. Introducing the Power of Plant Growth Promoting Microorganisms in Soilless Systems: A Promising Alternative for Sustainable Agriculture. Sustainability 2023, 15, 5959. [Google Scholar] [CrossRef]
- Goddek, S.; Vermeulen, T. Comparison of Lactuca sativa growth performance in conventional and RAS-based hydroponic systems. Aquac. Int. 2018, 26, 1377–1386. [Google Scholar] [CrossRef]
- Gillespie, D.P.; Kubota, C.; Miller, S.A. Effects of Low pH of Hydroponic Nutrient Solution on Plant Growth, Nutrient Uptake, and Root Rot Disease Incidence of Basil (Ocimum basilicum L.). HortScience 2020, 55, 1251–1258. [Google Scholar] [CrossRef]
- Goddek, S.; Keesman, K.J. The necessity of desalination technology for designing and sizing multi-loop. Desalination 2018, 428, 76–85. [Google Scholar] [CrossRef]
- Andrzejak, R.; Janowska, B. Trichoderma spp. Improves Flowering, Quality, and Nutritional Status of Ornamental Plants. Int. J. Mol. Sci. 2022, 23, 15662. [Google Scholar] [CrossRef]
- Lennard, W.; Goddek, S. Aqaponics: The Basics. In Aquaponics Food Production Systems—Combined Aquaculture and Hydroponic Production Technologies for the Future; Chapter 5; Goddek, S., Joyce, A., Kotzen, B., Burnell, G.M., Eds.; Springer International Publishing: New York, NY, USA, 2019; ISBN 978-3-030-15943-6. [Google Scholar] [CrossRef]
- Yang, T.; Kim, H.-J. Characterizing Nutrient Composition and Concentration in Tomato-, Basil- and Lettuce-Based Aquaponic and Hydroponic Systems. Water 2020, 12, 1259. [Google Scholar] [CrossRef]
- Sanchez, F.A.; Vivian-Rogers, V.R.; Urakawa, H. Tilapia recirculating aquaculture systems as a source of plant growth promoting bacteria. Aquac. Res. 2019, 50, 2054–2065. [Google Scholar] [CrossRef]
- Pokluda, R.; Kobza, F. Skleníky, Fóliovníky, Využití a Pěstební Technologie, 1st ed.; Profi Press s.r.o.: Praha, Czech Republic, 2011; ISBN 978-80-86726-46-5. [Google Scholar]
- Rakocy, J.E.; Masser, M.P.; Losordo, T.M. Recirculating Aquaculture Tank Production Systems: Aquaponics—Integrating Fish and Plant Culture. SRAC Publ. 2006, 454. [Google Scholar]
- Cerozi, B.S.; Fitzimmons, K. Phosphorus dynamics modeling and mass balance in an aquaponics system. Agric. Syst. 2017, 153, 94–100. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Lopez-Baltazar, J.; Parent, L.E.; Tremblay, N.; Gosselin, A. Sulfate accumulation and calcium balance in hydroponic tomato culture. J. Plant Nutr. 2007, 25, 1585–1597. [Google Scholar] [CrossRef]
- Loyless, J.C.; Malone, R.F. A Sodium Bicarbonate Dosing Methodology for pH Management in Freshwater-Recirculating Aquaculture Systems. Progress. Fish-Cult. 1997, 59, 198–205. [Google Scholar] [CrossRef]
- Uddin, A.F.; Ahmad, H.; Hassan, R.; Mahbuba, S.; Roni, M.Z. Effects of Trichoderma spp. on growth and yield characters of Bari tomato-14. Int. J. Bus. Soc. Sci. Res. 2016, 4, 117–122. [Google Scholar]
- Vukelić, I.D.; Prokić, L.T.; Racić, G.M.; Pešić, M.B.; Bojović, M.M.; Sierka, E.M.; Kalaji, H.M.; Panković, D. Effects of Trichoderma harzianum on Photosynthetic Characteristics and Fruit Quality of Tomato Plants. Int. J. Mol. Sci. 2021, 22, 6961. [Google Scholar] [CrossRef]
- Jamil, A. Antifungal and plant growth promoting activity of Trichoderma spp. against Fusarium oxysporum f. sp. lycopersici colonizing tomato. J. Plant Prot. Res. 2021, 61, 243–253. [Google Scholar] [CrossRef]
- Chen, D.; Hou, Q.; Jia, L.; Sun, K. Combined Use of Two Trichoderma Strains to Promote Growth of Pakchoi (Brassica chinensis L.). Agronomy 2021, 11, 726. [Google Scholar] [CrossRef]
- Prajakta, B.M.; Suvarna, P.P.; Raghvendra, S.P.; Alok, R.R. Potential biocontrol and superlative plant growth promoting activity of indigenous Bacillus mojavensis PB-35 (R11) of soybean (Glycine max) rhizosphere. SN Appl. Sci. 2019, 1, 1143. [Google Scholar] [CrossRef]
- Kasozi, N.; Kaiser, H.; Wilhelmi, B. Effect of Bacillus spp. on Lettuce Growth and Root Associated Bacterial Community in a Small-Scale Aquaponics System. Agronomy 2021, 11, 947. [Google Scholar] [CrossRef]
- Pandey, C.; Bajpai, V.K.; Negi, Y.K.; Rather, I.A.; Maheshwari, D.K. Effect of plant growth promoting Bacillus spp. on nutritional properties of Amaranthus hypochondriacus grains. Saudi J. Biol. Sci. 2018, 25, 1066–1071. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.N.; Dwivedi, P.; Sarma, B.K.; Singh, G.S.; Singh, H.B. A novel function of N-signaling in plants with special reference to Trichoderma interaction influencing plant growth, nitrogen use efficiency, and cross talk with plant hormones. 3 Biotech 2019, 9, 109. [Google Scholar] [CrossRef]
- Day, J.A.; Diener, C.; Otwell, A.E.; Tams, K.E.; Bebout, B.; Detweiler, A.M.; Lee, M.D.; Scott, M.T.; Ta, W.; Ha, M.; et al. Lettuce (Lactuca sativa) productivity influenced by microbial inocula under nitrogen-limited conditions in aquaponics. PLoS ONE 2021, 16, e0247534. [Google Scholar] [CrossRef] [PubMed]
- Brentrup, F.; Palliere, C. Nitrogen Use Efficiency as an Agro-Environmental Indicator. OECD Booklet Interior Template. Available online: https://www.oecd.org/greengrowth/sustainable-agriculture/44810433.pdf (accessed on 28 August 2023).
- Marsic, N.K.; Osvald, J. Effects of different nitrogen levels on lettuce growth and nitrate accumulation in iceberg lettuce (Lactuca sativa var. capitata L.) grown hydroponically under greenhouse conditions. Gartenbauwissenschaft 2002, 67, 128–134. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Posada, L.F.; Alvarez, J.C.; Hu, C.-H.; de-Bashan, L.E.; Bashan, Y. Construction of probe of the plant growth-promoting bacteria Bacillus subtilis useful for fluorescence in situ hybridization. J. Microbiol. Methods 2016, 128, 125–129. [Google Scholar] [CrossRef]
- Zbíral, J. Analýza Rostlinného Materiálu, Jednotné Pracovní Postupy, 2nd ed.; Ústřední Kontrolní a Zkušební Ústav Zemědělský: Brno, Czechia, 2002; ISBN 8086548155. [Google Scholar]
- Holm, G. Chlorophyll Mutations in Barley. Acta Agric. Scand. 1954, 4, 457–471. [Google Scholar] [CrossRef]


| Crop | Biometric Parameter | Variant of Nutrient Solution | |||
|---|---|---|---|---|---|
| HYDRO | AQP | AQP + TricH | AQP + BM | ||
| ‘Barlach’ lettuce | Total weight of plants [g] | 162.18 (±17.97) A | 116.13 (±15.17) B | 125.19 (±13.11) AB | 120.14 (±10.93) B |
| Above-ground biomass [g] | 152.78 (±17.00) A | 103.32 (±14.50) B | 116.00 (±10.68) AB | 110.89 (±9.48) B | |
| Root biomass [g] | 9.40 (±1.33) a | 12.81 (±1.42) a | 9.19 (±2.66) a | 9.25 (±1.97) a | |
| Root:shoot ratio | 0.06 (±0.01) a | 0.13 (±0.02) a | 0.08 (±0.02) a | 0.08 (±0.01) a | |
| Number of leaves [pcs] | 63 (±8) a | 46 (±8) b | 46 (±5) b | 73 (±8) a | |
| Dry weight [%] | 4.23 (±0.16) a | 5.07 (±0.60) a | 4.49 (±0.45) a | 4.93 (±0.21) a | |
| Basil | Total weight of plants [g] | 69.15 (±18.47) A | 48.21 (±8.70) B | 43.25 (±5.21) B | 52.88 (±10.91) AB |
| Above-ground biomass [g] | 53.36 (±12.94) A | 34.21 (±6.48) B | 30.95 (±3.27) B | 38.63 (±7.61) AB | |
| Root biomass [g] | 15.78 (±6.00) A | 14.00 (±3.09) A | 12.29 (±2.36) A | 14.26 (±3.83) A | |
| Root:shoot ratio | 0.29 (±0.05) b | 0.42 (±0.09) a | 0.40 (±0.07) a | 0.37 (±0.06) a | |
| Number of leaves [pcs] | 50 (±6) b | 49 (±9) b | 71 (±7) a | 71 (±13) a | |
| Dry weight [%] | 7.30 (±0.89) a | 8.44 (±0.71) a | 8.63 (±0.96) a | 8.50 (±0.69) a | |
| ‘Hilbert’ lettuce | Total weight of plants [g] | 108.17 (±25.64) a | 95.60 (±21.98) a | 90.86 (±30.89) a | 94.58 (±25.27) a |
| Above-ground biomass [g] | 100.65 (±23.57) a | 85.38 (±18.65) a | 83.22 (±28.19) a | 86.36 (±22.57) a | |
| Root biomass [g] | 7.51 (±3.31) a | 10.21 (±3.59) a | 7.65 (±3.03) a | 8.23 (±3.58) a | |
| Root:shoot ratio | 0.08 (±0.03) a | 0.12 (±0.03) a | 0.09 (±0.02) a | 0.09 (±0.03) a | |
| Number of leaves [pcs] | 42 (±4) AB | 35 (±4) B | 39 (±3) B | 64 (±11) A | |
| Dry weight [%] | 4.40 (±0.61) a | 5.18 (±0.41) a | 4.71 (±0.37) a | 4.92 (±0.49) a | |
| Nutrient Solution | HYDRO | AQP | AQP + TricH | AQP + BM | ||||
|---|---|---|---|---|---|---|---|---|
| Time | T0 | T1 | T0 | T1 | T0 | T1 | T0 | T1 |
| pH | 6.10 (±0.00) | 6.07 (±0.10) | 7.00 (±0.10) | 7.17 (±0.06) | 7.00 (±0.10) | 7.63 (±0.06) | 7.00 (±0.10) | 7.73 (±0.06) |
| EC [mS/cm] | 1.6 (±0.0) | 1.0 (±0.0) | 1.4 (±0.0) | 1.1 (±0.0) | 1.4 (±0.0) | 1.2 (±0.0) | 1.4 (±0.0) | 1.1 (±0.0) |
| NO3− [mg/L] | 528 (±14) | 317 (±6) | 627 (±12) | 450 (±10) | 627 (±12) | 483 (±12) | 627 (±12) | 420 (±0) |
| Total ammonia N [mg/L] | 6.77 (±0.35) | 0.16 (±0.04) | << | << | << | 0.16 (±0.02) | << | 0.29 (±0.11) |
| PO42− [mg/L] | 98.3 (±3.0) | 97.5 (±2.0) | 14.7 (±0.7) | 6.2 (±0.1) | 14.7 (±0.7) | 8.0 (±0.3) | 14.7 (±0.7) | 8.9 (±0.3) |
| K+ [mg/L] | 120.0 (±0.0) | 23.7 (±0.6) | 19.3 (±0.6) | 6.4 (±0.4) | 19.3 (±0.6) | 6.1 (±0.2) | 19.3 (±0.6) | 6.1 (±0.2) |
| Ca2+ [mg/L] | 45 (±4) | 25 (±3) | 130 (±5) | 88 (±3) | 130 (±5) | 97 (±3) | 130 (±5) | 83 (±8) |
| Mg2+ [mg/L] | 48 (±1) | 35 (±1) | 29 (±1) | 20 (±1) | 29 (±1) | 22 (±1) | 29 (±1) | 21 (±1) |
| SO42− [mg/L] | 210 (±0) | 203 (±3) | 119 (±3) | 90 (±0) | 119 (±3) | 98 (±1) | 119 (±3) | 90 (±4) |
| Na+ [mg/L] | 45.8 (±2.9) | 38.7 (±1.0) | 49.5 (±3.6) | 43.6 (±1.4) | 49.5 (±3.6) | 43.4 (±1.7) | 49.5 (±3.6) | 29.6 (±1.4) |
| Cl− [mg/L] | 71 (±5) | 60 (±2) | 76 (±6) | 67 (±2) | 76 (±6) | 67 (±3) | 76 (±6) | 46 (±2) |
| Total Mn [mg/L] | 2.27 (±0.18) | 2.15 (±0.09) | 0.28 (±0.07) | 0.04 (±0.01) | 0.28 (±0.07) | 0.13 (±0.08) | 0.28 (±0.07) | 0.14 (±0.06) |
| F− [mg/L] | 60.72 (±5.45) | 46.86 (±5.45) | 2.80 (±0.40) | 0.68 (±0.32) | 2.80 (±0.40) | 0.59 (±0.06) | 2.80 (±0.40) | 0.73 (±0.06) |
| Parameter Change T0–T1 | Variant of Nutrient Solution | |||
|---|---|---|---|---|
| HYDRO | AQP | AQP + TricH | AQP + BM | |
| pH | 0.03 (±0.06) a | −0.17 (±0.15) a | −0.63 (±0.15) b | −0.73 (±0.12) b |
| EC [mS/cm] | 0.6 (±0.0) A | 0.3 (±0.0) AB | 0.2 (±0.0) B | 0.3 (±0.0) AB |
| NO3− [mg/L] | 211 (±12) a | 177 (±15) b | 143 (±12) c | 207 (±12) ab |
| Total ammonia N [mg/L] | 6.61 (±0.36) A | 0.00 (±0.00) AB | −0.16 (±0.02) AB | −0.29 (±0.11) B |
| PO42− [mg/L] | 0.8 (±1.4) c | 8.5 (±0.6) a | 6.6 (±0.4) ab | 5.8 (±0.6) b |
| K+ [mg/L] | 96.3 (±0.6) A | 12.9 (±0.7) A | 13.2 (±0.7) A | 13.2 (±0.4) A |
| Ca2+ [mg/L] | 21 (±1) c | 42 (±6) ab | 33 (±6) bc | 47 (±6) a |
| Mg2+ [mg/L] | 13 (±1) A | 9 (±1) A | 7 (±1) A | 7 (±1) A |
| SO42− [mg/L] | 7 (±3) b | 29 (±3) a | 21 (±2) a | 28 (±6) a |
| Na+ [mg/L] | 7.1 (±2.3) b | 5.8 (±4.5) b | 6.1 (±2.9) b | 19.9 (±2.9) a |
| Cl− [mg/L] | 11 (±4) b | 9 (±7) b | 9 (±5) b | 31 (±5) a |
| Total Mn [mg/L] | 0.12 (±0.09) a | 0.24 (±0.06) a | 0.15 (±0.10) a | 0.14 (±0.08) a |
| F− [mg/L] | 13.86 (±7.86) A | 2.12 (±0.71) A | 2.21 (±0.46) A | 2.07 (±0.46) A |
| Crop | Nutritional Parameter | Variant of Nutrient Solution | |||
|---|---|---|---|---|---|
| HYDRO | AQP | AQP + TricH | AQP + BM | ||
| ‘Barlach’ lettuce | K [mg/kg] | 3565.1 (±192.54) A | 1220.7 (±212.10) AB | 1089.6 (±66.01) B | 1174.6 (±143.02) AB |
| Na [mg/kg] | 39.7 (±6.02) B | 1352.5 (±149.04) A | 1198.4 (±73.63) AB | 1162.7 (±120.01) AB | |
| Ca [mg/kg] | 0.0 (±0.00) B | 176.0 (±50.50) AB | 418.1 (±78.53) A | 167.7 (±252.83) AB | |
| Mg [mg/kg] | 0.0 (±0.00) A | 209.7 (±117.56) A | 82.5 (±6.93) A | 111.5 (±164.50) A | |
| P [mg/kg] | 35.6 (±1.97) A | 21.0 (±1.98) AB | 20.4 (±1.06) B | 21.8 (±0.90) AB | |
| Chlorophyll a [mg/kg] | 162.1 (±5.41) a | 185.0 (±33.52) a | 183.0 (±32.12) a | 203.4 (±14.61) a | |
| Chlorophyll b [mg/kg] | 76.8 (±2.52) a | 83.6 (±15.16) a | 88.8 (±17.14) a | 98.7 (±6.51) a | |
| Carotenoids [mg/kg] | 41.1 (±1.21) a | 56.7 (±10.49) a | 48.3 (±11.17) a | 61.8 (±3.14) a | |
| Vitamin C [mg/kg] | 50.6 (±5.59) d | 91.2 (±6.20) bc | 70.0 (±2.45) cd | 100.9 (±7.89) ab | |
| Nitrates [mg/kg] | 3924.4 (±388.5) A | 2156.2 (±195.41) AB | 2310.8 (±291.67) AB | 1557.6 (±81.38) B | |
| Basil | K [mg/kg] | 4647.6 (±640.86) A | 1353.9 (±104.40) AB | 1458.2 (±219.66) AB | 1226.6 (±191.66) B |
| Na [mg/kg] | 61.0 (±8.42) a | 63.9 (±12.26) a | 80.8 (±11.26) a | 60.8 (±21.56) a | |
| Ca [mg/kg] | 622.2 (±36.54) A | 160.3 (±12.59) B | 200.1 (±42.66) AB | 189.7 (±19.29) AB | |
| Mg [mg/kg] | 131.2 (±15.61) B | 819.3 (±61.14) AB | 1043.2 (±205.27) A | 950.3 (±54.99) A | |
| P [mg/kg] | 92.7 (±17.03) A | 33.2 (±2.52) B | 45.1 (±7.45) AB | 42.8 (±5.56) AB | |
| Chlorophyll a [mg/kg] | 287.4 (±29.93) a | 295.0 (±24.08) a | 319.1 (±62.20) a | 268.4 (±29.10) a | |
| Chlorophyll b [mg/kg] | 125.9 (±9.66) a | 126.6 (±11.86) a | 135.9 (±29.26) a | 112.7 (±14.08) a | |
| Carotenoids [mg/kg] | 73.0 (±9.27) b | 86.0 (±5.88) ab | 106.8 (±18.33) a | 88.5 (±8.84) ab | |
| Vitamin C [mg/kg] | 5.5 (±2.86) b | 23.6 (±4.64) b | 53.4 (±8.58) a | 63.4 (±7.25) a | |
| Nitrates [mg/kg] | 4313.4 (±447.15) ab | 3772.8 (±281.35) b | 5144.8 (±444.29) a | 3651.9 (±391.94) b | |
| ‘Hilbert’ lettuce | K [mg/kg] | 3606.1 (±435.51) A | 1324.3 (±105.02) A | 1194.7 (±160.31) A | 1253.1 (±257.79) A |
| Na [mg/kg] | 32.5 (±7.78) B | 1162.8 (±195.15) A | 970.1 (±118.61) AB | 868.4 (±78.98) AB | |
| Ca [mg/kg] | 281.9 (±192.37) A | 496.1 (±83.80) A | 228.2 (±154.02) A | 351.1 (±239.15) A | |
| Mg [mg/kg] | 60.6 (±41.87) A | 92.4 (±11.19) A | 270.5 (±128.27) A | 65.3 (±44.16) A | |
| P [mg/kg] | 36.3 (±5.80) A | 21.3 (±1.46) A | 21.6 (±2.47) A | 22.2 (±2.49) A | |
| Chlorophyll a [mg/kg] | 242.5 (±39.05) a | 276.9 (±31.30) a | 236.7 (±18.38) a | 278.2 (±40.12) a | |
| Chlorophyll b [mg/kg] | 140.3 (±24.58) a | 164.3 (±22.58) a | 138.5 (±13.43) a | 165.1 (±29.00) a | |
| Carotenoids [mg/kg] | 56.7 (±7.93) a | 67.4 (±4.54) a | 63.9 (±5.03) a | 66.2 (±5.95) a | |
| Vitamin C [mg/kg] | 67.6 (±12.88) c | 87.5 (±20.45) bc | 94.6 (±14.98) b | 126.8 (±17.73) a | |
| Nitrates [mg/kg] | 3569.9 (±148.93) a | 2269.6 (±524.58) b | 2408.0 (±440.33) b | 2302.2 (±171.90) b | |
| Nutrient Use Efficiency [%] | Variant of Nutrient Solution | |||
|---|---|---|---|---|
| HYDRO | AQP | AQP + TricH | AQP + BM | |
| PUE | 2.19 (±0.07) B | 5.37 (±0.24) AB | 5.81 (±0.26) AB | 6.26 (±0.28) A |
| KUE | 48.15 (±0.00) B | 73.88 (±2.17) AB | 70.11 (±2.06) AB | 73.97 (±2.17) A |
| CaUE | 6.83 (±0.60) A | 2.54 (±0.10) AB | 2.84 (±0.11) AB | 2.17 (±0.08) B |
| MgUE | 1.38 (±0.02) B | 10.05 (±0.21) AB | 11.23 (±0.23) A | 9.55 (±0.20) AB |
| N Input (T0)/100 L [g] | N Dynamics (T0 − T1)/100 L [g] | N Incorporated to Plants in the Form of NO3− [g] | N Incorporated in Other Organic Forms/Used by Microbiota [g] | Theoretical NUE [%] in the Case of Utilization of All N from the Solution [%] | |
|---|---|---|---|---|---|
| HYDRO | 12 | 5 | 2.4 | 6.2 | 40.0 |
| AQP | 14 | 4 | 1.1 | 6.1 | 28.2 |
| AQP + TricH | 14 | 3 | 1.3 | 4.6 | 22.9 |
| AQP + BM | 14 | 5 | 1.0 | 7.4 | 33.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patloková, K.; Pokluda, R. Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status. Plants 2024, 13, 291. https://doi.org/10.3390/plants13020291
Patloková K, Pokluda R. Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status. Plants. 2024; 13(2):291. https://doi.org/10.3390/plants13020291
Chicago/Turabian StylePatloková, Kateřina, and Robert Pokluda. 2024. "Optimization of Plant Nutrition in Aquaponics: The Impact of Trichoderma harzianum and Bacillus mojavensis on Lettuce and Basil Yield and Mineral Status" Plants 13, no. 2: 291. https://doi.org/10.3390/plants13020291





