Development of a Micropropagation Protocol for the Ex Situ Conservation of Nuttall’s Scrub Oak (Quercus dumosa)
Abstract
:1. Introduction
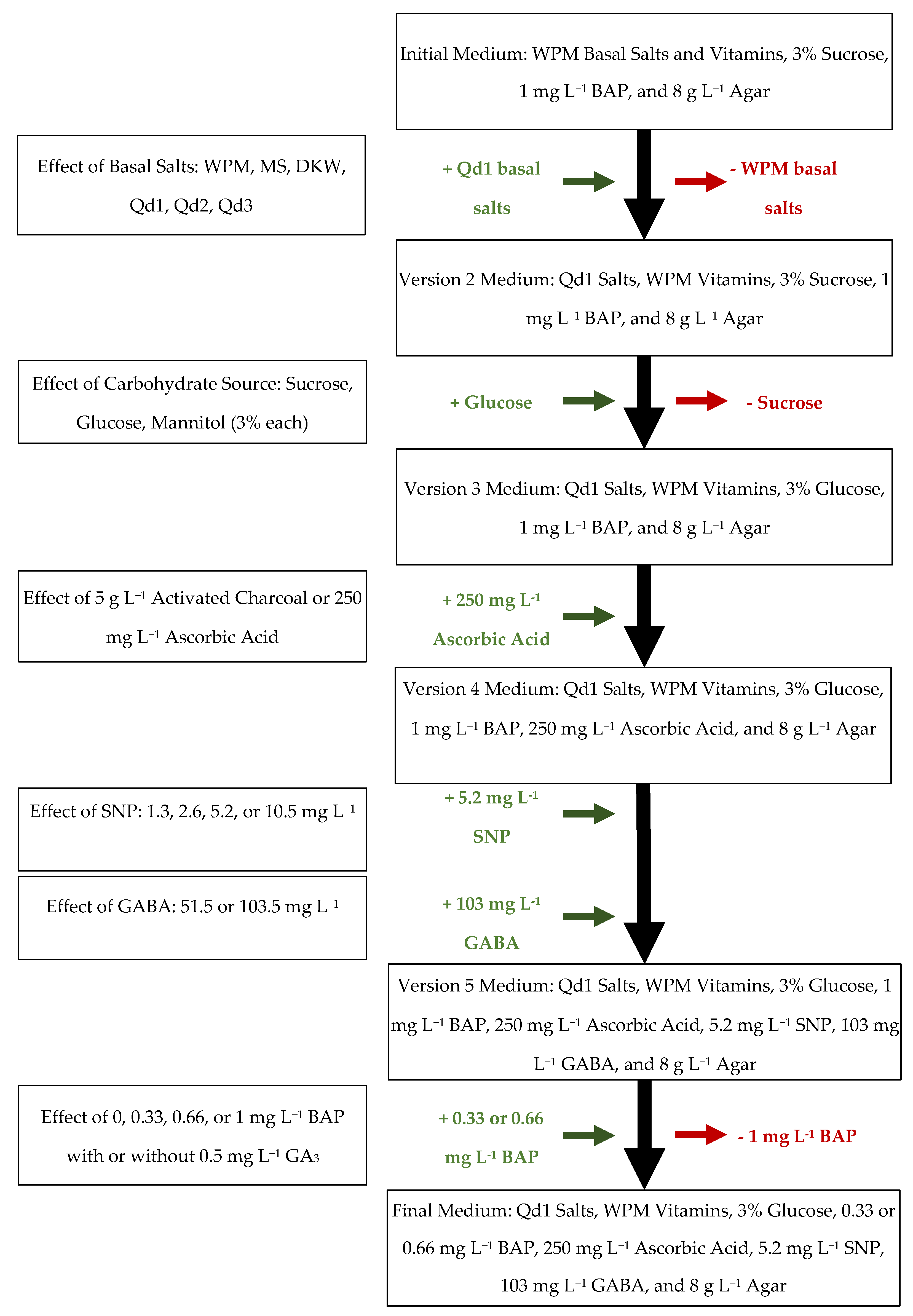
2. Results and Discussion
2.1. Germination and Line Establishment
2.2. Development of a New Basal Medium
2.3. The Effect of Carbohydrate Source
2.4. The Effect of Activated Charcoal and Ascorbic Acid
2.5. The Effect of Sodium Nitroprusside
2.6. The Effect of γ-Aminobutyric Acid
2.7. The Effect of 6-Benzylaminopurine and Gibberellic Acid
2.8. Comparison between Initial and Final Medium Compositions
2.9. Rooting
2.10. Considerations for Working with Slow-Growing and Largely Unstudied Species for Conservation
3. Materials and Methods
3.1. Plant Material
3.2. Development of a New Basal Medium
3.3. The Effect of Carbohydrate Source
3.4. The Effect of Activated Charcoal and Ascorbic Acid
3.5. The Effect of Stress-Mitigating Additives
3.6. The Effect of 6-Benzylaminopurine and Gibberellic Acid
3.7. Comparison between Initial and Final Medium Composition
3.8. Rooting
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrero, C.; Jerome, D.; Beckman, E.; Byrne, A.; Coombes, A.; Deng, M.; González-Rodríguez, A.; Sam, H.V.; Khoo, E.; Nguyen, N.; et al. The Red List of Oaks 2020; The Morton Arboretum: Lisle, IL, USA, 2020; ISBN 978-0-9992656-2-8. [Google Scholar]
- Anderson, M.K.; Keeley, J.E. Native Peoples’ Relationship to the California Chaparral. In Valuing Chaparral: Ecological, Socio-Economic, and Management Perspectives; Springer: Cham, Switzerland, 2018; pp. 79–121. [Google Scholar] [CrossRef]
- Mensing, S. The Paleohistory of California Oaks. In General Technical Report PSW-GTR-251; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA, 2015; Volume 251, pp. 35–47. [Google Scholar]
- Syphard, A.D.; Brennan, T.J.; Keeley, J.E. Chaparral Landscape Conversion in Southern California. In Valuing Chaparral: Ecological, Socio-Economic, and Management Perspectives; Underwood, E.C., Safford, H.D., Molinari, N.A., Keeley, J.E., Eds.; Springer Series on Environmental Management; Springer International Publishing: Cham, Switzerland, 2018; pp. 323–346. ISBN 978-3-319-68303-4. [Google Scholar]
- Beckman, E. IUCN Red List of Threatened Species: Quercus dumosa. In IUCN Red List of Threatened Species; IUCN: Gland, Switzerland, 2016. [Google Scholar]
- Pence, V.C. The Possibilities and Challenges of in Vitro Methods for Plant Conservation. Kew Bull. 2010, 65, 539–547. [Google Scholar] [CrossRef]
- Reed, B.M.; Wada, S.; DeNoma, J.; Niedz, R.P. Mineral Nutrition Influences Physiological Responses of Pear in Vitro. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 699–709. [Google Scholar] [CrossRef]
- McCown, B.H.; Lloyd, G. Woody Plant Medium (WPM)—A Mineral Nutrient Formulation for Microculture for Woody Plant Species. Hort. Sci. 1981, 16, 453. [Google Scholar]
- Kumar, R.; Sharma, K.; Agrawal, V. In Vitro Clonal Propagation of Holarrhena antidysenterica (L.) Wall. through Nodal Explants from Mature Trees. Vitr. Cell. Dev. Biol.-Plant 2005, 41, 137–144. [Google Scholar] [CrossRef]
- Sedlák, J.; Paprštein, F. Micropropagation of Cranberry (Vaccinium macrocarpon) through Shoot Tip Cultures—Short Communication. HortScience 2011, 38, 159–162. [Google Scholar] [CrossRef]
- Brennan, A.N.; Pence, V.C.; Taylor, M.D.; Trader, B.W.; Westwood, M. Tissue Culture Using Mature Material for the Conservation of Oaks. HortTechnology 2017, 27, 644–649. [Google Scholar] [CrossRef]
- Monteiro, A.C.B.d.A.; Higashi, E.N.; Gonçalves, A.N.; Rodriguez, A.P.M. A Novel Approach for the Definition of the Inorganic Medium Components for Micropropagation of Yellow Passionfruit (Passiflora edulis Sims. F. flavicarpa Deg.). Vitr. Cell. Dev. Biol.-Plant 2000, 36, 527–531. [Google Scholar] [CrossRef]
- Correia, D. Otimização da Fase de Multiplicação de Gemas in Vitro de Eucalyptus spp. In Mestrado, Escola Superior de Agricultura Luiz de Queiroz; Universidade de São Paulo: Piracicaba, Brazil, 1992; Volume 109. [Google Scholar]
- Terrer, C.; Tomás, F. Determination of Macronutrients to Be Included in in Vitro Culture Media According to Leaf Concentrations. J. Hortic. Sci. Biotechnol. 2001, 76, 484–488. [Google Scholar] [CrossRef]
- Lozzi, A.; Abdelwahd, R.; Mentag, R.; Abousalim, A. Development of a New Culture Medium and Efficient Protocol for in Vitro Micropropagation of Ceratonia siliqua L. Vitr. Cell. Dev. Biol.-Plant 2019, 55, 615–624. [Google Scholar] [CrossRef]
- Staikidou, I.; Selby, C.; Hanks, G.R. Development of a Medium for in Vitro Culture of Galanthus Species Based on the Mineral Composition of Bulbs. J. Hortic. Sci. Biotechnol. 2006, 81, 537–545. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Tóth, K.; Haapala, T.; Hohtola, A. Alleviation of Browning in Oak Explants by Chemical Pretreatments. Biol. Plant. 1994, 36, 511–517. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Corredoira, E.; Ballester, A.; Muñoz, F.; Durán, J.; Ibarra, M. In Vitro Regeneration of the Important North American Oak Species Quercus alba, Quercus bicolor and Quercus rubra. Plant Cell Tissue Organ Cult. 2009, 98, 135–145. [Google Scholar] [CrossRef]
- Cernadas, M.J.; Martínez, M.T.; Corredoira, E.; San José, M.C. Conservation of Holm Oak (Quercus ilex L.) by in Vitro Culture. Mediterr. Bot. 2018, 39, 97–104. [Google Scholar] [CrossRef]
- Sax, M.S.; Bassuk, N.; Bridgen, M. Tissue Culture Clonal Propagation of Hybrid White Oaks for the Urban Environment. HortScience 2019, 54, 2214–2223. [Google Scholar] [CrossRef]
- Eeuwens, C.J. Mineral Requirements for Growth and Callus Initiation of Tissue Explants Excised from Mature Coconut Palms (Cocos nucifera) and Cultured in Vitro. Physiol. Plant. 1976, 36, 23–28. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In Vitro Propagation of Paradox Walnut Rootstock. HortScience 1984, 19, 507–509. [Google Scholar] [CrossRef]
- McGranahan, G.H.; Driver, J.A.; Tulecke, W. Tissue Culture of Juglans. In Cell and Tissue Culture in Forestry: Case Histories: Gymnosperms, Angiosperms and Palms; Bonga, J.M., Durzan, D.J., Eds.; Forestry Sciences; Springer: Dordrecht, The Netherlands, 1987; pp. 261–271. ISBN 978-94-017-0992-7. [Google Scholar]
- Teixeira da Silva, J.A.; Nezami-Alanagh, E.; Barreal, M.E.; Kher, M.M.; Wicaksono, A.; Gulyás, A.; Hidvégi, N.; Magyar-Tábori, K.; Mendler-Drienyovszki, N.; Márton, L.; et al. Shoot Tip Necrosis of in Vitro Plant Cultures: A Reappraisal of Possible Causes and Solutions. Planta 2020, 252, 47. [Google Scholar] [CrossRef] [PubMed]
- Di Santo, P.; Cocozza, C.; Tognetti, R.; Palumbo, G.; Iorio, E.D.; Paura, B. A Quick Screening to Assess the Phytoextraction Potential of Cadmium and Copper in Quercus Pubescens Plantlets. iforest-Biogeosciences For. 2016, 10, 93. [Google Scholar] [CrossRef]
- Rugini, E. In Vitro Propagation of Some Olive (Olea europaea sativa L.) Cultivars with Different Root-Ability, and Medium Development Using Analytical Data from Developing Shoots and Embryos. Sci. Hortic. 1984, 24, 123–134. [Google Scholar] [CrossRef]
- García, J.L.; Troncoso, J.; Sarmiento, R.; Troncoso, A. Influence of Carbon Source and Concentration on the in Vitro Development of Olive Zygotic Embryos and Explants Raised from Them. Plant Cell Tissue Organ Cult. 2002, 69, 95–100. [Google Scholar] [CrossRef]
- Fadhaladeen, L.H.; Toma, R.S. Effect of Carbon Source in Woody Plant Medium with Different Salt Strengths on Oak (Quercus aegilops L.) Micropropagation. J. Plant Prod. 2019, 10, 751–756. [Google Scholar] [CrossRef]
- Pierik, R.L.M.; Oosterkamp, J.; Ebbing, M.A.C. Factors Controlling Adventitious Root Formation of Explants from Juvenile and Adult Quercus robur ’Fastigiata’. Sci. Hortic. 1997, 71, 87–92. [Google Scholar] [CrossRef]
- Erland, L.A.E.; Mahmoud, S.S. An Efficient Method for Regeneration of Lavandin (Lavandula x intermedia Cv. ‘Grosso’). Vitr. Cell. Dev. Biol.-Plant 2014, 50, 646–654. [Google Scholar] [CrossRef]
- Kadota, M.; Imizu, K.; Hirano, T. Double-Phase in Vitro Culture Using Sorbitol Increases Shoot Proliferation and Reduces Hyperhydricity in Japanese Pear. Sci. Hortic. 2001, 89, 207–215. [Google Scholar] [CrossRef]
- Ndakidemi, C.F.; Mneney, E.; Ndakidemi, P.A. Effects of Ascorbic Acid in Controlling Lethal Browning in in Vitro Culture of Brahylaena huillensis Using Nodal Segments. Am. J. Plant Sci. 2014, 5, 51024. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Oku, H.; Nahar, K.; Bhuyan, M.H.M.B.; Mahmud, J.A.; Baluska, F.; Fujita, M. Nitric Oxide-Induced Salt Stress Tolerance in Plants: ROS Metabolism, Signaling, and Molecular Interactions. Plant Biotechnol. Rep. 2018, 12, 77–92. [Google Scholar] [CrossRef]
- Ghassemi-Golezani, K.; Farhadi, N.; Nikpour-Rashidabad, N. Responses of in Vitro-Cultured Allium hirtifolium to Exogenous Sodium Nitroprusside under PEG-Imposed Drought Stress. Plant Cell Tissue Organ Cult. 2018, 133, 237–248. [Google Scholar] [CrossRef]
- Karthik, S.; Pavan, G.; Krishnan, V.; Sathish, S.; Manickavasagam, M. Sodium Nitroprusside Enhances Regeneration and Alleviates Salinity Stress in Soybean [Glycine max (L.) Merrill]. Biocatal. Agric. Biotechnol. 2019, 19, 101173. [Google Scholar] [CrossRef]
- Hesami, M.; Tohidfar, M.; Alizadeh, M.; Daneshvar, M.H. Effects of Sodium Nitroprusside on Callus Browning of Ficus Religiosa: An Important Medicinal Plant. J. For. Res. 2020, 31, 789–796. [Google Scholar] [CrossRef]
- Tan, B.C.; Chin, C.F.; Alderson, P. Effects of Sodium Nitroprusside on Shoot Multiplication and Regeneration of Vanilla planifolia Andrews. Vitr. Cell. Dev. Biol.-Plant 2013, 49, 626–630. [Google Scholar] [CrossRef]
- Seifikalhor, M.; Aliniaeifard, S.; Hassani, B.; Niknam, V.; Lastochkina, O. Diverse Role of γ-Aminobutyric Acid in Dynamic Plant Cell Responses. Plant Cell Rep. 2019, 38, 847–867. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Zeng, Q.; Ren, Z.; Li, P.; Xu, X. Effect of Exogenous γ-Aminobutyric Acid Treatment on the Enzymatic Browning of Fresh-Cut Potato during Storage. J. Food Sci. Technol. 2018, 55, 5035–5044. [Google Scholar] [CrossRef] [PubMed]
- Booz, M.R.; Kerbauy, G.B.; Guerra, M.P.; Pescador, R. The Role of γ-Aminobutyric acid (Gaba) in Somatic Embryogenesis of Acca sellowiana Berg. (Myrtaceae). Braz. J. Plant Physiol. 2009, 21, 271–280. [Google Scholar] [CrossRef]
- Shah, S.T.; Zamir, R.; Ahmad, J.; Ali, H.; Lutfullah, G. In Vitro Regeneration of Plantlets from Seedlings Explants of Guava (Psidium guajava L.) Cv. Safeda. Pak. J. Bot. 2008, 40, 1195–1200. [Google Scholar]
- Purohit, V.K.; Palni, L.M.S.; Nandi, S.K.; Rikhari, H.C. In Vitro Regeneration of Quercus floribunda Lindl. through Cotyledonary Nodes: An Important Tree of Central Himalaya. Curr. Sci. 2002, 83, 312–316. [Google Scholar]
- Vila, I.; Sales, E.; Ollero, J.; Muñoz-Bertomeu, J.; Segura, J.; Arrillaga, I. Micropropagation of Oleander (Nerium oleander L.). HortScience 2010, 45, 98–102. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Zhang, C.; Wang, Z. In Vitro Shoot Culture of Rhododendron fortunei: An Important Plant for Bioactive Phytochemicals. Ind. Crops Prod. 2018, 126, 459–465. [Google Scholar] [CrossRef]
- Delgadillo-Díaz de León, J.S.; Morales-Domínguez, J.F.; Santos-Díaz, M.d.S.; Pérez-Molphe-Balch, E. In Vitro Propagation of Mexican Oaks (Quercus spp.). Polibotánica 2013, 35, 85–97. [Google Scholar]
- Abdullah, T.A.; Abdul Aziz, M.; Abdul Rashid, A.; Saleh, G.; Elhory, S.M.A.; Panjaitan, S.B.; Sajili, M.H.; Mohamad, N.M. Comparison on the Effect of Treatment and Subculturing on Shoot Regeneration from Shoot Tip Seedlings of Psidium guajava L. Var. Beaumont. Pak. J. Biotechnol. 2009, 6, 21–26. [Google Scholar]
- Smulders, M.J.M.; de Klerk, G.J. Epigenetics in Plant Tissue Culture. Plant Growth Regul. 2011, 63, 137–146. [Google Scholar] [CrossRef]
- Reed, B.M. Screening Pyrus Germplasm for in Vitro Rooting Response. HortScience 1995, 30, 1292–1294. [Google Scholar] [CrossRef]
- Vieitez, A.M.; Concepclón Sánchez, M.; Amo-Marco, J.B.; Ballester, A. Forced Flushing of Branch Segments as a Method for Obtaining Reactive Explants of Mature Quercus robur Trees for Micropropagation. Plant Cell Tissue Organ Cult. 1994, 37, 287–295. [Google Scholar] [CrossRef]
- Sanchez, M.C.; San-Jose, M.C.; Ballester, A.; Vieitez, A.M. Requirements for in Vitro Rooting of Quercus robur and Q. rubra Shoots Derived from Mature Trees. Tree Physiol. 1996, 16, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.F. How Oaks Respond to Water Limitation. In General Technical Report PSW-GTR-251; U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station: Berkeley, CA, USA, 2015; Volume 251, pp. 13–22. [Google Scholar]
- Martins, A. In Vitro Mycorrhization of Micropropagated Plants: Studies on Castanea sativa Mill. In Mycorrhizae: Sustainable Agriculture and Forestry; Siddiqui, Z.A., Akhtar, M.S., Futai, K., Eds.; Springer: Dordrecht, The Netherlands, 2008; pp. 321–336. ISBN 978-1-4020-8770-7. [Google Scholar]
- Díez, J.; Manjón, J.L.; Kovács, G.M.; Celestino, C.; Toribio, M. Mycorrhization of Vitroplants Raised from Somatic Embryos of Cork Oak (Quercus suber L.). Appl. Soil Ecol. 2000, 15, 119–123. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]

| Element | % of Total Mass |
|---|---|
| N | 1.41 |
| K | 1.2 |
| Ca | 0.45 |
| Mg | 0.17 |
| P | 0.16 |
| S | 0.080 |
| Mn | 0.038 |
| Fe | 0.00386 |
| B | 0.0022 |
| Zn | 0.0022 |
| Cu | 0.00089 |
| Compound | WPM 1 | MS 2 | DKW 3 | Qd1 4 | Qd2 5 | Qd3 6 |
|---|---|---|---|---|---|---|
| NH4NO3 | 400 | 1650 | 1416 | 240 | 431 | 50 |
| H3BO3 | 6.2 | 6.2 | 4.8 | 1.89 | 4.11 | 1.42 |
| CaCl2 | 72.5 | 332 | 112.5 | 60 | 0 | 250 |
| Ca(NO3)2•4H2O | 386 | 0 | 1367 | 276.5 | 603 | 402.5 |
| CuSO4•5H2O | 0.25 | 0.025 | 0.25 | 0.527 | 1.15 | 2.33 |
| Na2EDTA•2H2O | 37.3 | 37.3 | 45.4 | 37.3 | 37.3 | 37.3 |
| FeSO4•7H2O | 27.85 | 27.8 | 33.8 | 2.88 | 6.28 | 11.2 |
| MgSO4 | 180.7 | 180.7 | 361.5 | 0 | 0 | 0 |
| MnSO4•H2O | 22.3 | 16.9 | 33.5 | 0 | 0 | 0 |
| Na2MoO4•2H2O | 0.25 | 0.25 | 0.39 | 0.25 | 0.25 | 0.25 |
| KH2PO4 | 170 | 170 | 265 | 0 | 0 | 227 |
| K2SO4 | 990 | 0 | 1559 | 62.5 | 136 | 132.7 |
| ZnSO4•7H2O | 8.6 | 8.6 | 17 | 1.42 | 3.09 | 6.45 |
| KNO3 | 0 | 1900 | 0 | 393 | 860 | 505 |
| KI | 0 | 0.83 | 0 | 0 | 0 | 0 |
| KCl | 0 | 0 | 0 | 0 | 0 | 322 |
| NH4H2PO4 | 0 | 0 | 0 | 0.089 | 0.195 | 0 |
| MnCl2 | 0 | 0 | 0 | 0.013 | 0.028 | 49.02 |
| Mg(NO3)2•6 H2O | 0 | 0 | 0 | 0.269 | 0.59 | 455 |
| NiSO4•6H2O | 0 | 0 | 0.005 | 0 | 0 | 0 |
| CoCl2•6H2O | 0 | 0.025 | 0 | 0 | 0 | 0 |
| Basal Salt Mixture 3 | Necrosis (% of Explants) | # Average Shoots Per Explant 4 | Shoots > 1 cm in Length (%) |
|---|---|---|---|
| Shoot Tip Explants | |||
| WPM | 15.6 ± 3.2 A 5 | 1.0 ± 0.1 A | 36.6 ± 4.6 AB |
| DKW | 41.8 ± 7.1 B | 0.5 ± 0.1 C | 14.8 ± 5.6 AB |
| MS | 45.2 ± 5.5 B | 0.5 ± 01 BC | 10.1 ± 3.1 B |
| Qd1 | 2.1 ± 1.6 A | 1.1 ± 0.1 A | 41.2 ± 6.2 A |
| Qd2 | 4.0 ± 1.8 A | 0.9 ± 0.2 A | 28.8 ± 9.1 AB |
| Qd3 | 1.9 ± 1.3 A | 0.9 ± 0.1 AB | 21.3 ± 8.0 AB |
| Nodal Segment | |||
| WPM | 39.6 ± 7.3 AB | 0.78 ± 0.1 A | 31.3 ± 6.3 A |
| DKW | 55.1 ± 6.2 B | 0.21 ± 0.1 B | 9.7 ± 4.1 AB |
| MS | 56.9 ± 8.7 B | 0.17 ± 0.1 B | 4.8 ± 2.1 B |
| Qd1 | 20.5 ± 6.0 A | 0.6 ± 0.2 AB | 24.1 ± 8.6 AB |
| Qd2 | 17.7 ± 7.1 A | 0.4 ± 0.1 AB | 16.0 ± 6.6 AB |
| Qd3 | 21.2 ± 6.5 A | 0.5 ± 0.1 AB | 11.3 ± 8.0 AB |
| Carbohydrate Source 3 | Culture Line Type | Necrosis (% of Explants) | # Average Shoots Per Explant 4 |
|---|---|---|---|
| Sucrose | 15 Total Lines | 9.6 ± 1.3 B 5 | 1.6 ± 0.2 B |
| 11 Core Lines 6 | 4.4 ± 2.8 ab | 1.69 ± 0.2 ab | |
| 4 Additional Lines | 24.0 ± 6.7 b | 1.19 ± 0.1 b | |
| Glucose | 15 Total Lines | 1.8 ± 3.2 A | 1.89 ± 0.2 A |
| 11 Core Lines | 0.4 ± 0.7 a | 2.06 ± 0.1 a | |
| 4 Additional Lines | 5.6 ± 3.9 ab | 1.40 ± 0.1 b | |
| Mannitol | 15 Total Lines | 64.1 ± 10.7 C | 0.39 ± 0.1 C |
| 11 Core Lines | 51.0 ± 12.6 c | 0.52 ± 0.1 c | |
| 4 Additional Lines | 100 ± 0.0 d | 0.00 ± 0.0 c |
| Treatment | Culture Line Type | Necrosis (% of Explants) | # Average Shoots per Explant |
|---|---|---|---|
| Version 3 Medium 2 | 20 Total Lines | 9.6 ± 3.4 B 3 | 1.6 ± 0.1 A |
| 11 Core Lines 4 | 0.0 ± 0.0 a | 1.8 ± 0.2 a | |
| 9 Additional Lines | 21.3 ± 5.6 b | 1.2 ± 0.1 bc | |
| +5 g L−1 Activated Charcoal | 20 Total Lines | 0.0 ± 0.0 A | 1.1 ± 0.1 B |
| 11 Core Lines | 0.0 ± 0.0 a | 1.1 ± 0.2 bc | |
| 9 Additional Lines | 0.0 ± 0.0 a | 1.0 ± 0.0 c | |
| +250 mg L−1 Ascorbic Acid | 20 Total Lines | 1.3 ± 0.9 A | 1.7 ± 0.1 A |
| 11 Core Lines | 0.0 ± 0.0 a | 1.9 ± 0.2 a | |
| 9 Additional Lines | 2.8 ± 2.1 a | 1.4 ± 0.1 b |
| Treatment | Culture Line Type | Necrosis (% of Explants) | # Average Shoots per Explant |
|---|---|---|---|
| Version 4 Medium 2 | 24 Total lines | 7.3 ± 2.4 B 3 | 1.8 ± 0.1 B |
| 11 Core lines 4 | 2.6 ± 2.3 a | 2.2 ± 0.2 abcd | |
| 13 Additional lines | 11.5 ± 3.6 a | 1.5 ± 0.1 d | |
| +1.3 mg L−1 SNP | 24 Total Lines | 4.2 ± 1.9 AB | 1.8 ± 0.2 AB |
| 11 Core Lines | 0.0 ± 0.0 a | 2.3 ± 0.2 bcd | |
| 13 Additional Lines | 7.7 ± 3.3 a | 1.5 ± 0.2 d | |
| +2.6 mg L−1 SNP | 24 Total Lines | 4.2 ± 2.5 AB | 1.9 ± 0.1 AB |
| 11 Core Lines | 0.0 ± 0.0 a | 2.4 ± 0.2 ab | |
| 13 Additional Lines | 7.7 ± 4.4 a | 1.6 ± 0.1 cd | |
| +5.2 mg L−1 SNP | 24 Total Lines | 1.0 ± 1.0 A | 2.0 ± 0.1 A |
| 11 Core Lines | 0.0 ± 0.0 a | 2.3 ± 0.1 abc | |
| 13 Additional Lines | 1.9 ± 1.9 a | 1.8 ± 0.1 bcd | |
| +10.5 mg L−1 SNP | 24 Total Lines | 1.0 ± 1.0 A | 2.0 ± 0.1 AB |
| 11 Core Lines | 0.0 ± 0.0 a | 2.5 ± 0.2 a | |
| 13 Additional Lines | 1.9 ± 1.9 a | 1.7 ± 0.1 abcd |
| Treatment | Culture Line Type | Necrosis (% of Explants) | # Average Shoots Per Explant |
|---|---|---|---|
| Version 4 Medium 2 | 24 Total Lines | 2.1 ± 1.2 A 3 | 2.0 ± 0.1 B 3 |
| 11 Core Lines 4 | 1.5 ± 1.5 a | 2.6 ± 0.2 a | |
| 13 Additional Lines | 2.6 ± 1.7 a | 1.5 ± 0.1 b | |
| +51.5 mg L−1 GABA | 24 Total Lines | 3.5 ± 1.7 A | 2.0 ± 0.1 B |
| 11 Core Lines | 0.0 ± 0.0 a | 2.5 ± 0.2 a | |
| 13 Additional Lines | 6.4 ± 3.0 a | 1.6 ± 0.1 b | |
| +103 mg L−1 GABA | 24 Total Lines | 1.4 ± 1.0 A | 2.2 ± 0.1 A |
| 11 Core Lines | 0.0 ± 0.0 a | 2.6 ± 0.1 a | |
| 13 Additional Lines | 2.6 ± 1.7 a | 1.8 ± 0.1 b |
| BAP (mg L−1) 3 | Culture Type | Necrosis (% of Explants) | # Average Shoots Per Explant | Shoots Greater Than 2 cm (%) |
|---|---|---|---|---|
| 1 | 94 Total Lines | 10.5 ± 3.0 A 4 | 1.8 ± 0.1 A | 14.4 ± 3.4 AB |
| 11 Core Lines 5 | 0.0 ± 0.0 a | 2.6 ± 0.2 ab | 31.3 ± 9.1 ab | |
| 16 Additional Lines | 4.2 ± 2.5 a | 1.8 ± 0.2 abcd | 11.7 ± 6.5 bc | |
| 67 Static Lines | 16.5 ± 4.5 a | 1.6 ± 0.2 cd | 10.7 ± 4.3 bc | |
| 0.66 | 95 Total Lines | 9.0 ± 2.9 A | 1.9 ± 0.1 A | 19.0 ± 4.1 A |
| 11 Core Lines | 0.0 ± 0.0 a | 2.6 ± 0.2 a | 33.8 ± 7.0 ab | |
| 16 Additional Lines | 1.6 ± 1.5 a | 1.9 ± 0.2 abcd | 17.2 ± 8.0 bc | |
| 68 Static Lines | 15.1 ± 4.4 a | 1.8 ± 0.2 bcd | 15.4 ± 5.7 bc | |
| 0.33 | 95 Total Lines | 10.1 ± 2.9 A | 1.7 ± 0.1 A | 21.2 ± 4.2 A |
| 11 Core Lines | 0.0 ± 0.0 a | 2.3 ± 0.2 abc | 49.4 ± 15.0 a | |
| 16 Additional Lines | 3.4 ± 2.3 a | 1.6 ± 0.2 cd | 23.7 ± 8.7 abc | |
| 68 Static Lines | 16.2 ± 4.4 a | 1.6 ± 0.1 cd | 11.8 ± 4.3 bc | |
| 0 | 94 Total Lines | 25.1 ± 4.1 B | 0.9 ± 0.1 B | 4.1 ± 1.7 B |
| 11 Core Lines | 7.5 ± 2.5 a | 1.1 ± 0.1 e | 6.3 ± 9.1 bc | |
| 16 Additional Lines | 9.6 ± 3.9 a | 1.1 ± 0.1 de | 6.5 ± 6.5 bc | |
| 67 Static Lines | 37.7 ± 5.9 b | 0.7 ± 0.1 e | 2.2 ± 4.3 c |
| Medium Type | Culture Line Type | Necrosis (% of Explants) | # Average Shoots Per Explant |
|---|---|---|---|
| Initial 1 | 25 Total Lines 3 | 51.0 ± 6.9 B 4 | 0.9 ± 0.2 B |
| 11 Core Lines 5 | 29.5 ± 8.1 b | 1.3 ± 0.3 b | |
| 14 Additional Lines | 67.9 ± 7.8 c | 0.5 ± 0.1 b | |
| Final 2 | 25 Total Lines | 6.5 ± 1.9 A | 1.9 ± 0.2 A |
| 11 Core Lines | 1.1 ± 1.1 a | 2.4 ± 0.4 a | |
| 14 Additional Lines | 10.7 ± 2.8 ab | 1.5 ± 0.1 ab |
| Method | Activated Charcoal | Average Rooting (%) | # of Culture Lines with at Least One Rooted Plant | Range of Rooting 2 (%) |
|---|---|---|---|---|
| Long Incubation 3 | 5 g | 4.2 ± 2.1 C | 3 | 12.5–12.5 |
| One-Day Pulse 4 | 47.2 ± 10.2 A 6 | 8 | 25–100 | |
| 15-Second Dip 5 | 0.0 ± 0.0 7 | 0 | N/A | |
| Long Incubation | 0 | 16.7 ± 6.9 BC | 5 | 12.5–62.5 |
| One-Day Pulse | 38.9 ± 10.1 AB | 7 | 12.5–75 | |
| 15-Second Dip | 0.0 ± 0.0 7 | 0 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ree, J.F.; Powell, C.; Folgado, R.; Pence, V.C.; Walters, C.; Maschinski, J. Development of a Micropropagation Protocol for the Ex Situ Conservation of Nuttall’s Scrub Oak (Quercus dumosa). Plants 2024, 13, 1148. https://doi.org/10.3390/plants13081148
Ree JF, Powell C, Folgado R, Pence VC, Walters C, Maschinski J. Development of a Micropropagation Protocol for the Ex Situ Conservation of Nuttall’s Scrub Oak (Quercus dumosa). Plants. 2024; 13(8):1148. https://doi.org/10.3390/plants13081148
Chicago/Turabian StyleRee, Joseph Francis, Christy Powell, Raquel Folgado, Valerie C. Pence, Christina Walters, and Joyce Maschinski. 2024. "Development of a Micropropagation Protocol for the Ex Situ Conservation of Nuttall’s Scrub Oak (Quercus dumosa)" Plants 13, no. 8: 1148. https://doi.org/10.3390/plants13081148





