Changes in Growth and Heavy Metal and Phenolic Compound Accumulation in Buddleja cordata Cell Suspension Culture under Cu, Fe, Mn, and Zn Enrichment
Abstract
:1. Introduction
2. Results
2.1. Tolerance and Accumulation of HMs in a B. cordata Cell Suspension Culture Grown in a Culture Medium Enriched with Cu, Fe, Mn, and Zn
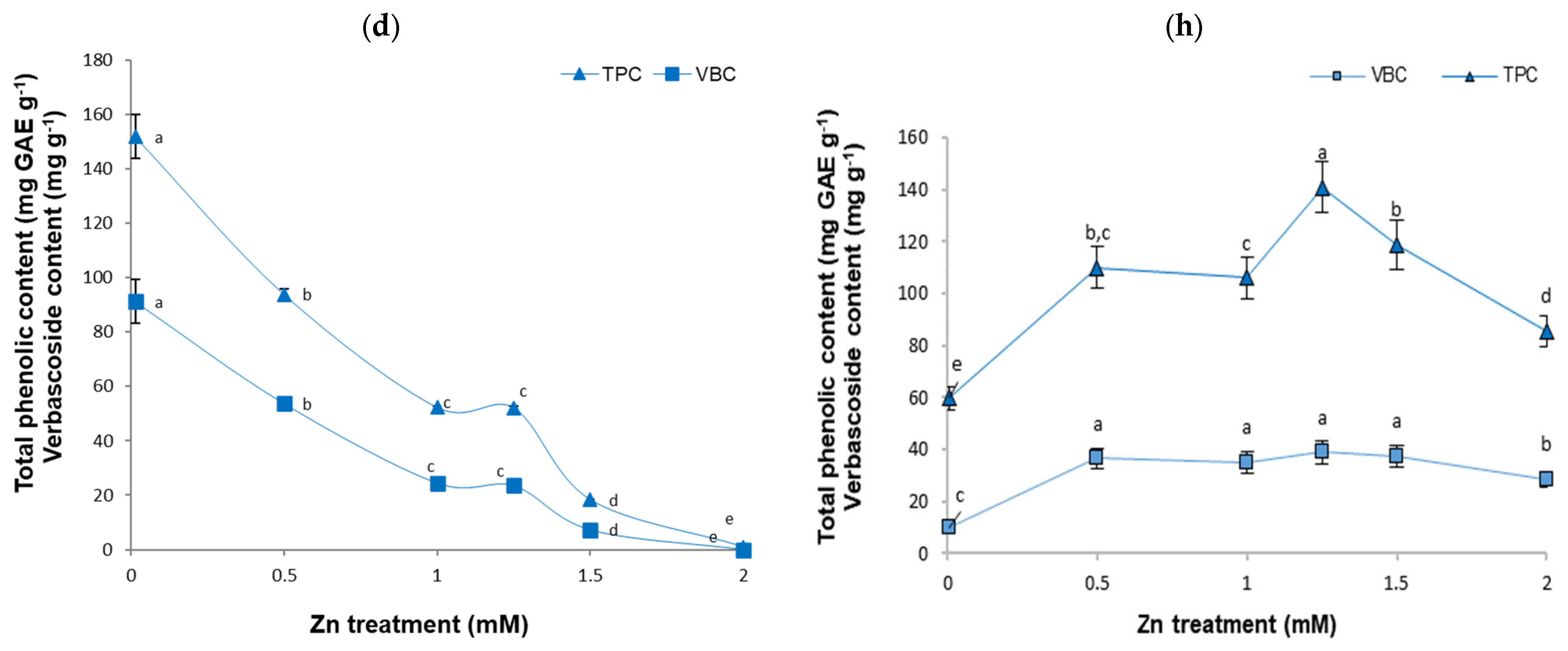
2.2. Total Phenolic and Verbascoside Content Changes in the B. cordata Cell Suspension Culture Grown in a Culture Medium Enriched with Cu, Fe, Mn, and Zn
3. Discussion
4. Materials and Methods
4.1. Cell Suspension Culture
4.2. HM Bioassays
4.3. Determination of the HM Content in Cells
4.4. Effect of HM on the Accumulation of Phenolic Compounds
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kosakivska, I.V.; Babenko, L.M.; Romanenko, K.O.; Korotka, I.Y.; Potters, G. Molecular mechanisms of plant adaptative responses to heavy metals stress. Cell Biol. Int. 2021, 45, 258–272. [Google Scholar] [CrossRef]
- Goyal, D.; Yadav, A.; Prasad, M.; Singh, T.B.; Shrivastav, P.; Ali, A.; Dantu, P.K.; Mishra, S. Effect oh heavy metals on plant growth: An overview. In Contaminants in Agriculture Sources, Impacts and Management, 1st ed.; Naeem, M., Ansari, A.A., Gill, S.S., Eds.; Springer: Cham, Switzerland, 2020; pp. 79–101. [Google Scholar] [CrossRef]
- Ejaz, U.; Khan, S.M.; Ahmad, Z.; Jehangir, S.; Fatima, R.Z.; Lho, L.H.; Han, H.; Raposo, A. Detoxifying the heavy metals: A multipronged study of tolerance strategies against heavy metals toxicity in plants. Front. Plant Sci. 2023, 14, 1154571. [Google Scholar] [CrossRef]
- Viehweger, K. How plants cope with heavy metals. Bot. Stud. 2014, 55, 35. [Google Scholar] [CrossRef]
- Pasricha, S.; Mathur, V.; Garg, A.; Lenka, S.; Verma, K.; Agarwal, S. Molecular mechanisms underlying heavy metal uptake, translocation and tolerance in hyperaccumulators-an analysis Heavy metal tolerance in hyperaccumulators. Environ. Chall. 2021, 4, 100197. [Google Scholar] [CrossRef]
- Wuana, R.A.; Okieimen, F.E. Heavy metals in contaminated soils: A review of sources, chemistry, risks and best available strategies for remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Jutsz, A.M.; Gnida, A. Mechanisms of stress avoidance and tolerance by plants used in phytoremediation of heavy metals. Arch. Environ. Prot. 2015, 41, 104–114. [Google Scholar] [CrossRef]
- Goncharuk, E.A.; Zagoskina, N.V. Heavy metals, their phytotoxicity, and the role of phenolic antioxidants in plant stress responses with focus on cadmium: Review. Molecules 2023, 28, 3921. [Google Scholar] [CrossRef]
- Garnica-Díaz, C.; Berazaín, I.R.; Cabrera, B.; Calderón-Morales, E.; Felipe, F.L.; García, R.; Gómez, H.J.L.; Franklin, G.A.; Medina, E.; Paul, A.L.D.; et al. Global plant ecology of tropical ultramafic ecosystems. Bot. Rev. 2023, 89, 115–157. [Google Scholar] [CrossRef]
- Rotkittikhun, P.; Kruatrachue, M.; Chaiyarat, R.; Ngernsansaruay, C.; Pokethitiyook, P.; Paijitprapaporn, A.; Baker, A.J.M. Uptake and accumulation of lead by plants from the Bo Ngam lead mine area in Thailand. Environ. Pollut. 2006, 144, 681–688. [Google Scholar] [CrossRef]
- Waranusantigul, P.; Kruatrachue, M.; Pokethitiyook, P. Evaluation of Pb phytoremediation potential in Buddleja asiatica and B. paniculata. Water Air Soil Pollut. 2008, 193, 79–90. [Google Scholar] [CrossRef]
- Salas-Luévano, M.A.; Manzanares-Acuña, E.; Letechipía-de León, C.; Vega-Carrillo, H.R. Tolerant and hyperaccumulators autochthonous plant species from mine tailing disposal sites. Asian J. Exp. Sci. 2009, 23, 27–32. [Google Scholar]
- Alvarado-López, S.; Gómez-Maqueo, X.; Soriano, D.; Orozco-Segovia, A.; Gamboa-Debuen, A. Mobilization and synthesis of seed storage and LEA proteins during natural priming of Buddleja cordata and Opuntia tomentosa. Bot. Sci. 2018, 96, 76–83. [Google Scholar] [CrossRef]
- Navarrete, G.D.M.; Pons, M.N.; Cuevas, S.J.A.; Echeverria, G. Is metal hyperaccumulation ocurring in ultramafic vegetation of central and southern Mexico? Ecol. Res. 2018, 33, 641–649. [Google Scholar] [CrossRef]
- Quiroz, A.; Espinosa-García, F.; Ilangovan, K. Effects of natural hydrosoluble chelates of three plant species on the mobilization of heavy metals. Bull. Environ. Contam. Toxicol. 2002, 68, 862–869. [Google Scholar] [CrossRef]
- Awa, S.H.; Hadibarata, T. Removal of heavy metals in contaminated soil by phytoremediation mechanism: A review. Water Air Soil Pollut. 2020, 231, 47. [Google Scholar] [CrossRef]
- Jaskulak, M.; Grobelak, A.; Vandenbulcke, F. Modelling assisted phytoremediation of soils contaminated with heavy metals: Main opportunities, limitations, decision making and future prospects. Chemosphere 2020, 249, 126196. [Google Scholar] [CrossRef]
- Majumder, A. Plant in vitro cultures for phytoremediation of heavy metals. In Back to Basics: A Multidisciplinary Approach to Organic Living and Waste Management, 1st ed.; Ghosh, K.K., Mandal, P., Neogi, S., Eds.; Jogamaya Devi College Interdisciplinary: Calcutta, India, 2022; pp. 24–44. [Google Scholar]
- Estrada-Zúñiga, M.E.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Verde-Calvo, J.R.; Vernon-Carter, E.J. Phenylpropanoid production in callus and cell suspension cultures of Buddleja cordata Kunth. Plant Cell Tissue Organ Cult. 2009, 97, 39–47. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Gruissem, W.; Jones, R.L. Biochemistry and Molecular Biology of Plants, 2nd ed.; Wiley Blackwell: Hoboken, NJ, USA, 2015; pp. 1–1280. [Google Scholar]
- Tang, Z.; Wan, H.Q.; Chen, J.D.; Zhao, F.J. Molecular mechanisms underlying the toxicity and detoxification of trace metals and metalloids in plants. J. Integr. Plant Biol. 2023, 65, 570–593. [Google Scholar] [CrossRef]
- Rajput, V.; Minkina, T.; Semenkov, I.; Klink, G.; Tarigholizadeh, S.; Sushkova, S. Phylogenetic analysis of hyperaccumulator plant species for heavy metals and polycyclic aromatic hydrocarbons. Environ. Geochem. Health 2021, 43, 1629–1654. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef]
- Anjitha, K.S.; Sameena, P.P.; Puthur, S.T. Functional aspects of plant secondary metabolites in metal stress tolerance and their importance in pharmacology. Plant Stress 2021, 2, 100038. [Google Scholar] [CrossRef]
- Thangavel, P.; Long, S. Changes in phytochelatins and their biosynthetic intermediates in red spruce (Picea rubens Sarg.) cell suspension cultures under cadmium and zinc stress. Plant Cell Tissue Organ Cult. 2007, 88, 201–216. [Google Scholar] [CrossRef]
- Tennstedt, P.; Peisker, D.; Böttcher, C.; Trampczynska, A.; Clemens, S. Phytochelatin synthesis is essential for the detoxification of excess zinc and contributes significantly to the accumulation of zinc. Plant Physiol. 2009, 149, 938–948. [Google Scholar] [CrossRef]
- Siwek, M. Plants in postindustrial sites, contaminated with heavy metals. Part I. Uptake, transport and toxicity of heavy (trace) metals. Wiad. Bot. 2008, 1, 7–22. [Google Scholar]
- Ahmad, P.; Jaleel, C.A.; Salem, M.A.; Nabi, G.; Sharma, S. Roles of enzymatic and nonenzymatic antioxidants in plants during abiotic stress. Crit. Rev. Biotechnol. 2010, 30, 161–175. [Google Scholar] [CrossRef]
- Valadez-Villareal, A.; Maldonado-Magaña, A.; Bernabé-Antonio, A.; Estrada-Zúñiga, M.E.; Román-Guerrero, A.; Cruz-Sosa, F. Effect of Cr and Pb on the activity of antioxidant enzymes in a cell suspension culture of Jatropa curcas. Rev. Mex. Ing. Quim. 2015, 14, 681–689. [Google Scholar]
- Michalak, A. Phenolic compounds and their antioxidant activity in plants growing under heavy metal stress. Pol. J. Environ. Stud. 2006, 15, 523–530. [Google Scholar]
- Curie, C.; Briat, J.F. Iron transport and signaling in plants. Annu. Rev. Plant Biol. 2003, 54, 183–206. [Google Scholar] [CrossRef]
- Jin, C.W.; You, G.Y.; He, Y.F.; Tang, C.; Wu, P.; Zhen, S.J. Iron deficiency-induced secretion of phenolics facilitates the reutilization of root apoplastic iron in red clover. Plant Physiol. 2007, 144, 278–285. [Google Scholar] [CrossRef]
- Masarovičová, E.; Kráľová, K.; Kummerová, M. Principles of classification of medicinal plants as hyperaccumulators or excluders. Acta Physiol. Plant 2010, 32, 823–829. [Google Scholar] [CrossRef]
- Alipieva, K.; Korkina, L.; Orhan, I.E.; Georgiev, M.I. Verbascoside—A review of its occurrence, (bio) synthesis and pharmacological significance. Biotechnol. Adv. 2014, 32, 1065–1076. [Google Scholar] [CrossRef]
- Zhao, C.; Dodin, G.; Yuan, C.; Chen, H.; Zhen, R.; Jia, Z.; Fan, B.T. “In vitro” protection of DNA from Fenton reaction by plant polyphenol verbascoside. BBA-Gen. Subj. 2005, 1723, 114–123. [Google Scholar] [CrossRef]
- Rout, G.R.; Samantaray, S.; Das, P. In vitro selection and biochemical characterization of zinc and manganese adapted callus lines in Brassica spp. Plant Sci. 1999, 137, 89–100. [Google Scholar] [CrossRef]
- Sharma, N.C.; Sahi, S.V.; Jain, J.C. Sesbania drummondii cell cultures: ICP-MS determination of the accumulation of Pb and Cu. Microchem. J. 2005, 81, 163–169. [Google Scholar] [CrossRef]
- Watmough, S.A.; Dickinson, N.M. Multiple metal resistance and co-resistance in Acer pseudoplatanus L. (Sycamore) callus culture. Annu. Bot. 1995, 76, 465–472. [Google Scholar] [CrossRef]
- Klein, M.A.; Sekimoto, H.; Milner, M.J.; Kochian, L.V. Investigation of heavy metal hyperaccumulation at the cellular level: Development and characterization of Thlaspi caerulescens suspension cell lines. Plant Physiol. 2008, 147, 2006–2016. [Google Scholar] [CrossRef]
- Santos-Díaz, M.S.; Barrón-Cruz, M.C.; Alfaro-De la Torre, M.C. Induction of in vitro roots cultures of Thypha latifolia and Scirpus americanus and study of their capacity to remove heavy metals. Electron. J. Biotechnol. 2007, 10, 417–424. [Google Scholar] [CrossRef]
- Mandal, A.B.; Roy, B. In vitro selection of mature seed derived calli for increased tolerance towards Fe-toxicity in rice and their isozyme profiling. J. Genet. Breed 2003, 57, 325–340. [Google Scholar]
- Bernabé-Antonio, A.; Álvarez, L.; Buendía-González, L.; Maldonado-Magaña, A.; Cruz-Sosa, F. Accumulation and tolerance of Cr and Pb using a cell suspension culture system of Jatropha curcas. Plant Cell Tissue Organ Cult. 2015, 120, 221–228. [Google Scholar] [CrossRef]
- Sinha, S. Accumulation of Cu, Cd, Cr, Mn and Pb from artificially contamined soil by Bacopa monnieri. Environ. Monit. Assess. 1999, 57, 253–264. [Google Scholar] [CrossRef]
- Buendía-González, L.; Orozco-Villafuerte, J.; Cruz-Sosa, F.; Barrera-Díaz, C.E.; Vernon-Carter, E.J. Prosopis laevigata a potential chromium (VI) and cadmium (II) hyperaccumulator desert plant. Bioresour. Technol. 2010, 10, 5862–5867. [Google Scholar] [CrossRef] [PubMed]
- Buendía-González, L.; Orozco-Villafuerte, J.; Estrada-Zúñiga, M.E.; Barrera-Díaz, C.E.; Vernon-Carter, E.J.; Cruz-Sosa, F. In vitro lead and nickel accumulation in mesquite (Prosopis laevigata) seedlings. Rev. Mex. Ing. Quim. 2010, 9, 1–9. [Google Scholar]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Fones, H.N.; Preston, G.M. Trade-offs between metal hyperaccumulation and induced disease resistance in metal hyperaccumulator plants. Plant Pathol. 2013, 62, 63–71. [Google Scholar] [CrossRef]
- Nasim, S.A.; Dhir, B. Heavy metals alter the potency of medicinal plants. Rev. Environ. Contam. Toxicol. 2010, 203, 139–149. [Google Scholar] [CrossRef]
- De, B. Cell signaling and elicitation of secondary plant metabolite production. J. Med. Arom. Plant Sci. 2021, 23, 413–428. [Google Scholar]
- Ali, M.B.; Hahn, E.J.; Paek, K.Y. Copper-induced changes in the growth, oxidative metabolism, and saponin production in suspension culture roots of Panax ginseng in bioreactors. Plant Cell Rep. 2006, 25, 1122–1132. [Google Scholar] [CrossRef]
- Kasparova, M.; Siatka, T.; Dusek, J. Abiotic elicitation of Trifolium pratense L. suspension culture. Ceska Slov. Farm. 2007, 56, 225–229. [Google Scholar]
- Arano-Varela, H.; Fernández, F.J.; Estrada-Zúñiga, M.E.; Cruz-Sosa, F. Verbascoside production in long-term Buddleja cordata Kunth cell suspension cultures. 3 Biotech 2020, 10, 245. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Zhong, J.J. Biochemical engineering of the production of plant-specific secondary metabolites by cell suspension cultures. In Plant Cells, Advances in Biochemical Engineering/Biotechnology; Scheper, T., Zhong, J.J., Eds.; Springer: Berlin, Germany, 2001; Volume 72, pp. 1–26. [Google Scholar] [CrossRef]
- Bernabé-Antonio, A.; Maldonado-Magaña, A.; Estrada-Zúñiga, M.E.; Buendía-González, L.; Cruz-Sosa, F. Procedure for estimating the tolerance and accumulation of heavy metals using plant cell cultures. In Plant Cell Culture Protocols, Methods in Molecular Biology; Loyola-Vargas, V., Ochoa-Alejo, N., Eds.; Humana Press: New York, NY, USA, 2018; Volume 1815, pp. 333–337. [Google Scholar] [CrossRef]
- Vazquez-Marquez, A.M.; Zepeda-Gómez, C.; Burrola-Aguilar, C.; Bernabé-Antonio, A.; Nieto-Trujillo, A.; Cruz-Sosa, F.; Rodríguez-Monroy, M.; Estrada-Zúñiga, M.E. Effect of stirring speed on the production of phenolic secondary metabolites and growth of Buddleja cordata cells cultured in mechanically agitated bioreactor. Plant Cell Tissue Organ Cult. 2019, 139, 155–166. [Google Scholar] [CrossRef]
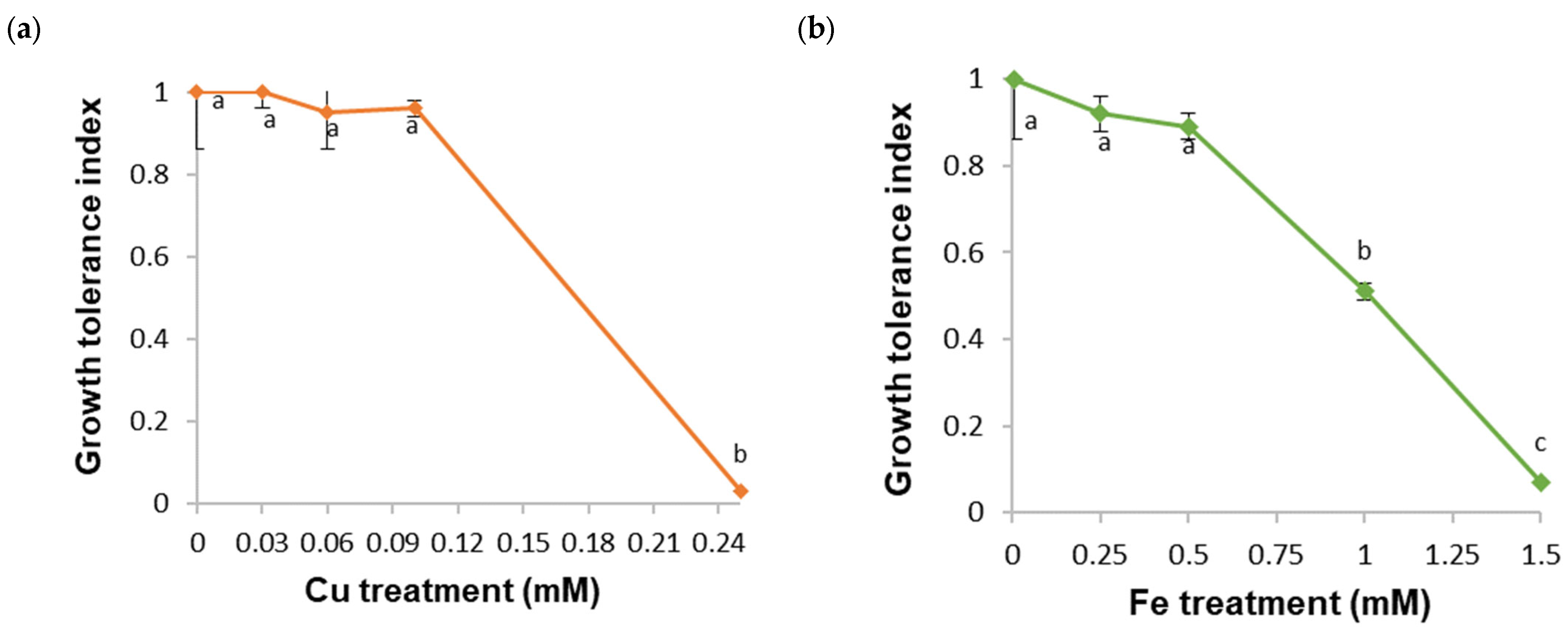
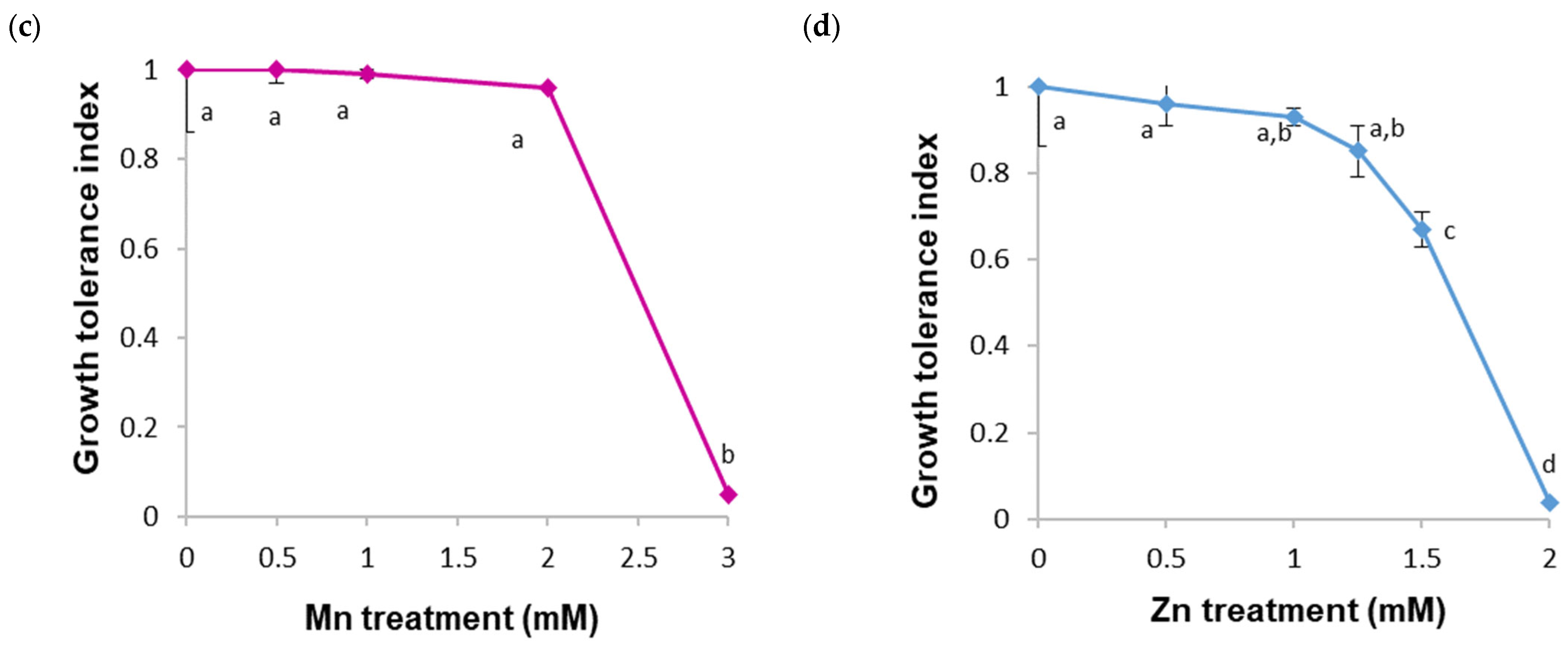
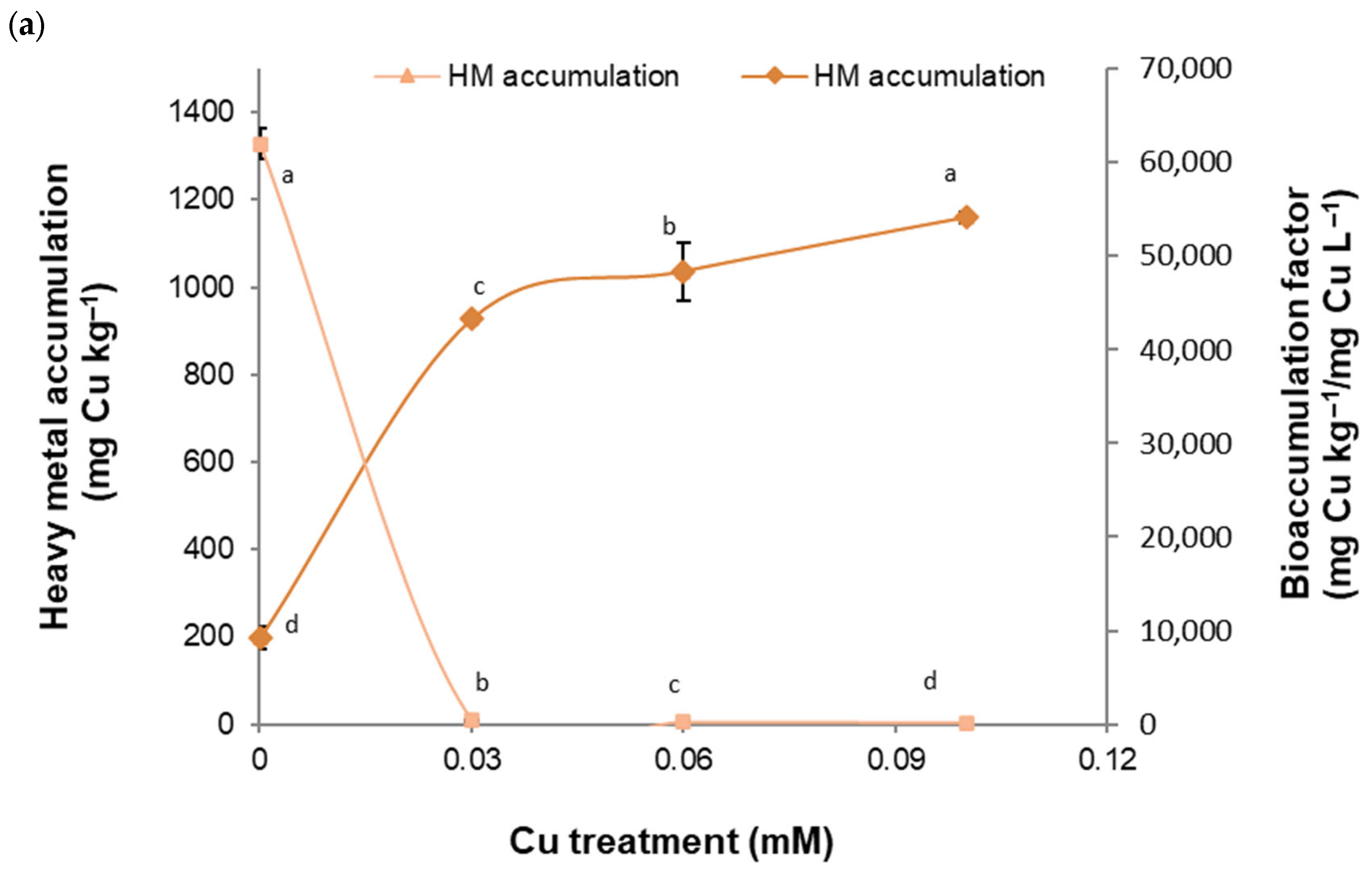
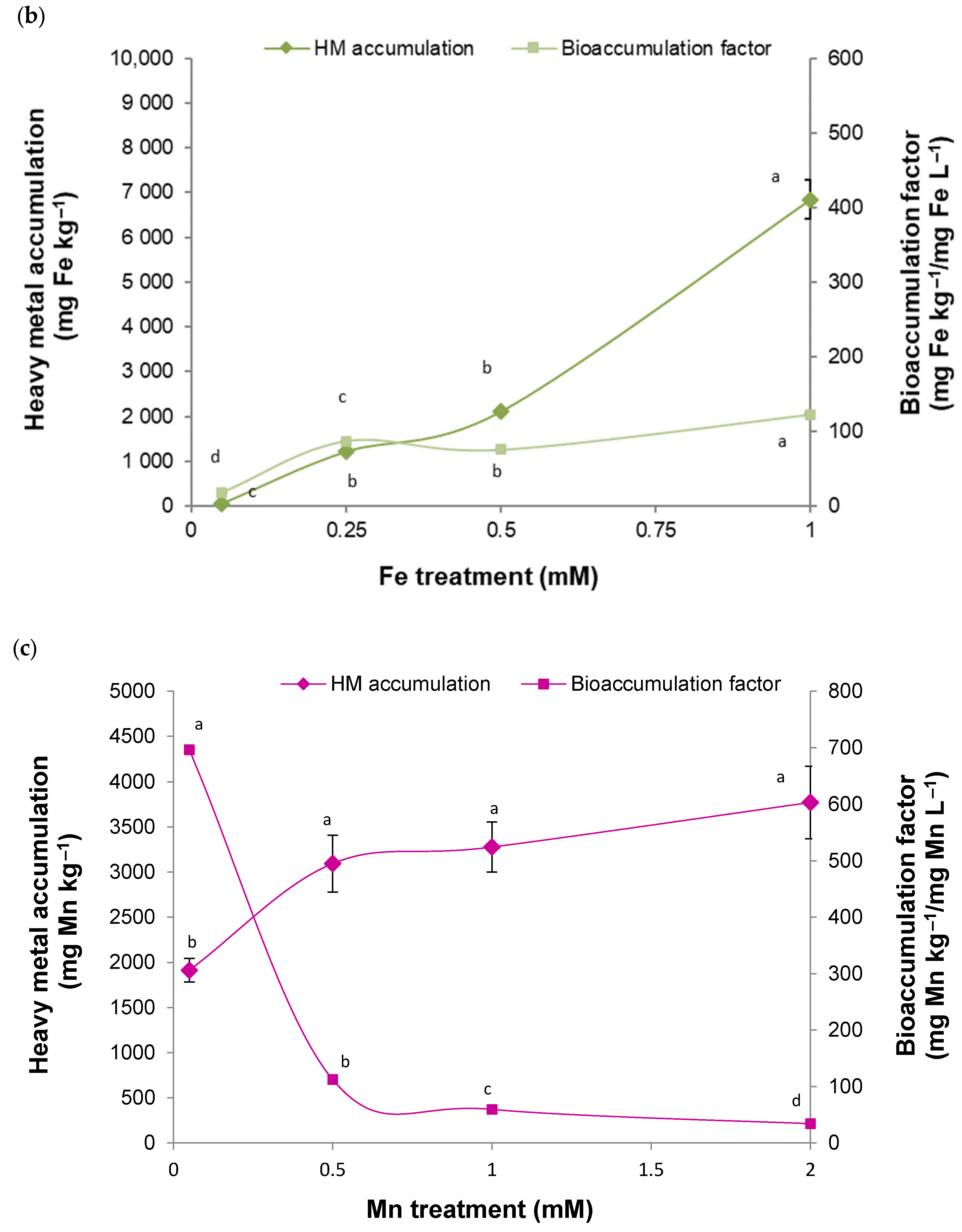


| Heavy Metal Treatment (mM) | Growth | Culture Medium | ||
|---|---|---|---|---|
| Cell Viability (%) | Growth Index | pH | Total Sugar Content (g L−1) | |
| Copper | ||||
| Control (0.00005) | 81.33 ± 1.58 a | 6.46 ± 0.88 a | 6.38 ± 0.05 b | 5.57 ± 0.40 b |
| 0.03 | 77.00 ± 3.54 a | 6.43 ± 0.27 a | 6.44 ± 0.05 b | 5.21 ± 0.72 b |
| 0.06 | 73.25 ± 0.65 b | 6.11 ± 0.58 a | 6.66 ± 0.02 a | 4.34 ± 0.61 b |
| 0.10 | 72.40 ± 1.15 b | 6.23 ± 0.15 a | 6.63 ± 0.10 a | 4.44 ± 0.54 b |
| 0.25 | 20.50 ± 0.78 c | 0.21 ± 0.01 b | 4.22 ± 0.03 c | 28.13 ± 1.11 a |
| Iron | ||||
| Control (0.05) | 81.33 ± 1.58 a | 6.46 ± 0.88 a | 6.38 ± 0.05 a,b | 5.57 ± 0.40 c |
| 0.25 | 75.97 ± 2.10 b | 5.97 ± 0.24 a | 6.64 ± 0.05 a | 3.91 ± 0.15 c |
| 0.50 | 71.94 ± 1.82 b | 5.77 ± 0.18 a | 6.55 ± 0.10 a | 5.94 ± 0.02 c |
| 1.00 | 66.99 ± 0.84 c | 3.29 ± 0.14 b | 5.69 ± 0.01 c | 16.22 ± 2.01 b |
| 1.50 | 12.86 ± 1.26 c | 0.44 ± 0.02 c | 4.43 ± 0.01 d | 27.56 ± 0.40 a |
| Manganese | ||||
| Control (0.05) | 81.33 ± 1.58 a | 6.46 ± 0.88 a | 6.38 ± 0.05 a | 5.57 ± 0.40 b |
| 0.50 | 78.41 ± 1.85 a | 6.48 ± 0.19 a | 6.49 ± 0.04 a | 1.71 ± 0.22 c |
| 1.00 | 67.71 ± 2.53 b | 6.38 ± 0.09 a | 6.42 ± 0.09 a | 1.58 ± 0.29 c |
| 2.00 | 66.75 ± 2.25 b | 6.17 ± 0.01 a | 6.26 ± 0.04 b | 1.38 ± 0.33 c |
| 3.00 | 10.34 ± 0.25 c | 0.34 ± 0.04 b | 4.11 ± 0.04 b | 27.88 ± 1.26 a |
| Zinc | ||||
| Control (0.015) | 81.33 ± 1.58 a | 6.46 ± 0.88 a | 6.38 ± 0.05 a | 5.57 ± 0.40 d |
| 0.50 | 72.32 ± 2.44 b | 6.22 ± 0.35 a | 6.37 ± 0.06 a | 4.34 ± 0.46 d |
| 1.00 | 70.77 ± 1.83 b | 5.99 ± 0.13 a,b | 5.84 ± 0.06 b | 13.04 ± 1.79 c |
| 1.25 | 54.56 ± 0.41 c | 5.46 ± 0.41 a,b | 5.22 ± 0.03 c | 17.70 ± 0.80 b |
| 1.50 | 26.06 ± 1.40 d | 4.36 ± 0.24 b | 4.98 ± 0.04 d | 24.35 ± 1.66 a |
| 2.00 | 12.20 ± 2.54 e | 0.24 ± 0.02 c | 4.34 ± 0.02 e | 28.31 ± 2.48 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vazquez-Marquez, A.M.; Bernabé-Antonio, A.; Correa-Basurto, J.; Burrola-Aguilar, C.; Zepeda-Gómez, C.; Cruz-Sosa, F.; Nieto-Trujillo, A.; Estrada-Zúñiga, M.E. Changes in Growth and Heavy Metal and Phenolic Compound Accumulation in Buddleja cordata Cell Suspension Culture under Cu, Fe, Mn, and Zn Enrichment. Plants 2024, 13, 1147. https://doi.org/10.3390/plants13081147
Vazquez-Marquez AM, Bernabé-Antonio A, Correa-Basurto J, Burrola-Aguilar C, Zepeda-Gómez C, Cruz-Sosa F, Nieto-Trujillo A, Estrada-Zúñiga ME. Changes in Growth and Heavy Metal and Phenolic Compound Accumulation in Buddleja cordata Cell Suspension Culture under Cu, Fe, Mn, and Zn Enrichment. Plants. 2024; 13(8):1147. https://doi.org/10.3390/plants13081147
Chicago/Turabian StyleVazquez-Marquez, Alicia Monserrat, Antonio Bernabé-Antonio, José Correa-Basurto, Cristina Burrola-Aguilar, Carmen Zepeda-Gómez, Francisco Cruz-Sosa, Aurelio Nieto-Trujillo, and María Elena Estrada-Zúñiga. 2024. "Changes in Growth and Heavy Metal and Phenolic Compound Accumulation in Buddleja cordata Cell Suspension Culture under Cu, Fe, Mn, and Zn Enrichment" Plants 13, no. 8: 1147. https://doi.org/10.3390/plants13081147









