Endophyte Inoculation and Elevated Potassium Supply on Productivity, Growth and Physiological Parameters of Spring Barley (Hordeum vulgare L.) Genotypes over Contrasting Seasons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of the Experimental Field Site and Design
2.2. Inoculum Preparation and Colonization Evaluation
2.3. Analyzed Parameters
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shakoor, U.; Saboor, A.; Ali, I.; Mohsin, A.Q. Impact of Climate Change on Agriculture: Empirical Evidence from Arid Region. Pak. J. Agric. Sci. 2011, 48, 327–333. [Google Scholar]
- Dodd, I.C.; Ryan, A.C. Whole-Plant Physiological Responses to Water-Deficit Stress. In eLS—Encyclopedia of Life Sciences; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–9. [Google Scholar]
- Osakabe, Y.; Osakabe, K.; Shinozaki, K.; Tran, L.-S.P. Response of Plants to Water Stress. Front. Plant Sci. 2014, 5, 86. [Google Scholar] [CrossRef] [PubMed]
- Hermans, K.; McLeman, R. Climate Change, Drought, Land Degradation and Migration: Exploring the Linkages. Curr. Opin. Environ. Sustain. 2021, 50, 236–244. [Google Scholar] [CrossRef]
- Kaur, H.; Kohli, S.K.; Khanna, K.; Bhardwaj, R. Scrutinizing the Impact of Water Deficit in Plants: Transcriptional Regulation, Signaling, Photosynthetic Efficacy, and Management. Physiol. Plant 2021, 172, 935–962. [Google Scholar] [CrossRef] [PubMed]
- Trnka, M.; Semerádová, D.; Novotný, I.; Dumbrovský, M.; Drbal, K.; Pavlík, F.; Vopravil, J.; Štěpánková, P.; Vizina, A.; Balek, J.; et al. Assessing the Combined Hazards of Drought, Soil Erosion and Local Flooding on Agricultural Land: A Czech Case Study. Clim. Res. 2016, 70, 231–249. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil Health in Agricultural Systems. Phil. Trans. R. Soc. B 2008, 363, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Wyka, T.P.; Bagniewska-Zadworna, A.; Kuczyńska, A.; Mikołajczak, K.; Ogrodowicz, P.; Żytkowiak, M.; Surma, M.; Adamski, T. Drought-Induced Anatomical Modifications of Barley (Hordeum vulgare L.) Leaves: An Allometric Perspective. Environ. Exp. Bot. 2019, 166, 103798. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Younis, A.; Riaz, A.; Mansoor, F.; Hameed, M.A.; Akram, N.A.; Abideen, Z. Morpho-anatomical adaptations of two Tagetes erecta L. cultivars with contrasting response to drought stress. Pak. J. Bot. 2020, 52, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.; Ramegowda, V.; Kumar, A.; Pereira, A. Plant Adaptation to Drought Stress. F1000Research 2016, 5, 1554. [Google Scholar] [CrossRef] [PubMed]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding Plant Responses to Drought—From Genes to the Whole Plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef] [PubMed]
- Shankar Naik, B. Functional Roles of Fungal Endophytes in Host Fitness during Stress Conditions. Symbiosis 2019, 79, 99–115. [Google Scholar] [CrossRef]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the Role of Fungal Symbionts in Plant Abiotic Stress Tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, M.R.; Mirzaei, M.; Ghabooli, M.; Khatabi, B.; Wu, Y.; Zabet-Moghaddam, M.; Mohammadi-Nejad, G.; Haynes, P.A.; Hajirezaei, M.R.; Sepehri, M.; et al. Endophytic Fungus Piriformospora Indica Improves Drought Stress Adaptation in Barley by Metabolic and Proteomic Reprogramming. Environ. Exp. Bot. 2019, 157, 197–210. [Google Scholar] [CrossRef]
- Murphy, B.R.; Martin Nieto, L.; Doohan, F.M.; Hodkinson, T.R. Fungal Endophytes Enhance Agronomically Important Traits in Severely Drought-stressed Barley. J. Agron. Crop Sci. 2015, 201, 419–427. [Google Scholar] [CrossRef]
- Bleša, D.; Matušinský, P.; Sedmíková, R.; Baláž, M. The Potential of Rhizoctonia-Like Fungi for the Biological Protection of Cereals against Fungal Pathogens. Plants 2021, 10, 349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yu, X.; Zhang, W.; Lang, D.; Zhang, X.; Cui, G.; Zhang, X. Interactions between Endophytes and Plants: Beneficial Effect of Endophytes to Ameliorate Biotic and Abiotic Stresses in Plants. J. Plant Biol. 2019, 62, 1–13. [Google Scholar] [CrossRef]
- Baron, N.C.; Rigobelo, E.C. Endophytic Fungi: A Tool for Plant Growth Promotion and Sustainable Agriculture. Mycology 2022, 13, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Zhang, G. The Influence of Drought Stress on Malt Quality Traits of the Wild and Cultivated Barleys. J. Integr. Agric. 2020, 19, 2009–2015. [Google Scholar] [CrossRef]
- Tyagi, J.; Chaudhary, P.; Mishra, A.; Khatwani, M.; Dey, S.; Varma, A. Role of Endophytes in Abiotic Stress Tolerance: With Special Emphasis on Serendipita indica. Int. J. Environ. Res. 2022, 16, 62. [Google Scholar] [CrossRef]
- Ghaffari, M.R.; Ghabooli, M.; Khatabi, B.; Hajirezaei, M.R.; Schweizer, P.; Salekdeh, G.H. Metabolic and Transcriptional Response of Central Metabolism Affected by Root Endophytic Fungus Piriformospora indica under Salinity in Barley. Plant Mol. Biol. 2016, 90, 699–717. [Google Scholar] [CrossRef] [PubMed]
- Achatz, B.; Kogel, K.-H.; Franken, P.; Waller, F. Piriformospora indica Mycorrhization Increases Grain Yield by Accelerating Early Development of Barley Plants. Plant Signal. Behav. 2010, 5, 1685–1687. [Google Scholar] [CrossRef] [PubMed]
- Bago, B.; Pfeffer, P.E.; Abubaker, J.; Jun, J.; Allen, J.W.; Brouillette, J.; Douds, D.D.; Lammers, P.J.; Shachar-Hill, Y. Carbon Export from Arbuscular Mycorrhizal Roots Involves the Translocation of Carbohydrate as Well as Lipid. Plant Physiol. 2003, 131, 1496–1507. [Google Scholar] [CrossRef] [PubMed]
- Rozpądek, P.; Wężowicz, K.; Nosek, M.; Ważny, R.; Tokarz, K.; Lembicz, M.; Miszalski, Z.; Turnau, K. The Fungal Endophyte Epichloë Typhina Improves Photosynthesis Efficiency of Its Host Orchard Grass (Dactylis glomerata). Planta 2015, 242, 1025–1035. [Google Scholar] [CrossRef] [PubMed]
- Baum, M.; Von Korff, M.; Guo, P.; Lakew, B.; Hamwieh, A.; Lababidi, S.; Udupa, S.M.; Sayed, H.; Choumane, W.; Grando, S.; et al. Molecular Approaches and Breeding Strategies for Drought Tolerance in Barley. In Genomics-Assisted Crop Improvement: Vol 2: Genomics Applications in Crops; Varshney, R.K., Tuberosa, R., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 51–79. ISBN 978-1-4020-6297-1. [Google Scholar]
- Sabagh, A.E.; Hossain, A.; Islam, M.S.; Barutcular, C.; Hussain, S.; Hasanuzzaman, M.; Akram, T.; Mubeen, M.; Nasim, W.; Fahad, S.; et al. Drought and Salinity Stresses in Barley: Consequences and Mitigation Strategies. Aust. J. Crop Sci. 2019, 13, 810–820. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Hancock, J.T. Hydrogen sulfide in horticulture: Emerging roles in the era of climate change. Plant Physiol. Biochem. 2020, 155, 667–675. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, J.T.; Turkington, T.K.; Edney, M.J.; Clayton, G.W.; McKenzie, R.H.; Juskiw, P.E.; Lafond, G.P.; Grant, C.A.; Brandt, S.; Harker, K.N. Seeding Rate, Nitrogen Rate, and Cultivar Effects on Malting Barley Production. Agron. J. 2011, 103, 709–716. [Google Scholar] [CrossRef]
- Asghar, M.G.; Bashir, A. Protagonist of Mineral Nutrients in Drought Stress Tolerance of Field Crops. In Abiotic Stress in Plants; Fahad, S., Saud, S., Chen, Y., Wu, C., Wang, D., Eds.; IntechOpen: Rijeka, Croatia, 2020; Chapter 9; ISBN 978-1-83881-062-7. [Google Scholar]
- Sehar, S.; Adil, M.F.; Zeeshan, M.; Holford, P.; Cao, F.; Wu, F.; Wang, Y. Mechanistic Insights into Potassium-Conferred Drought Stress Tolerance in Cultivated and Tibetan Wild Barley: Differential Osmoregulation, Nutrient Retention, Secondary Metabolism and Antioxidative Defense Capacity. Int. J. Mol. Sci. 2021, 22, 13100. [Google Scholar] [CrossRef] [PubMed]
- Andersen, M.N.; Jensen, C.R.; Lösch, R. The Interaction Effects of Potassium and Drought in Field-Grown Barley. II. Nutrient Relations, Tissue Water Content and Morphological Development. Acta Agric. Scand. B Soil Plant Sci. 1992, 42, 45–56. [Google Scholar] [CrossRef]
- Jones, C.A.; Jacobsen, J.S.; Wraith, J.M. Response of Malt Barley to Phosphorus Fertilization Under Drought Conditions. J. Plant Nutr. 2005, 28, 1605–1617. [Google Scholar] [CrossRef]
- Tavakol, E.; Jákli, B.; Cakmak, I.; Dittert, K.; Senbayram, M. Optimization of Potassium Supply under Osmotic Stress Mitigates Oxidative Damage in Barley. Plants 2022, 11, 55. [Google Scholar] [CrossRef] [PubMed]
- Askarnejad, M.R.; Soleymani, A.; Javanmard, H.R. Barley (Hordeum vulgare L.) Physiology Including Nutrient Uptake Affected by Plant Growth Regulators under Field Drought Conditions. J. Plant Nutr. 2021, 44, 2201–2217. [Google Scholar] [CrossRef]
- Ragel, P.; Raddatz, N.; Leidi, E.O.; Quintero, F.J.; Pardo, J.M. Regulation of K+ Nutrition in Plants. Front. Plant Sci. 2019, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Nafees, M.; Darras, A.; Shaukat, N.; Chen, J.; Ferrante, A.; Zaid, A.; Latif, N.; Raza, A.; Siddique, K.H. Pre-harvest potassium foliar application improves yield, vase life and overall postharvest quality of cut gladiolus inflorescences. Postharvest Biol. Technol. 2022, 192, 112027. [Google Scholar] [CrossRef]
- Wójcik-Jagła, M.; Rapacz, M.; Tyrka, M.; Kościelniak, J.; Crissy, K.; Żmuda, K. Comparative QTL Analysis of Early Short-Time Drought Tolerance in Polish Fodder and Malting Spring Barleys. Theor. Appl. Genet. 2013, 126, 3021–3034. [Google Scholar] [CrossRef] [PubMed]
- Barnabás, B.; Jäger, K.; Fehér, A. The Effect of Drought and Heat Stress on Reproductive Processes in Cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Kolář, P.; Trnka, M.; Brázdil, R.; Hlavinka, P. Influence of Climatic Factors on the Low Yields of Spring Barley and Winter Wheat in Southern Moravia (Czech Republic) during the 1961–2007 Period. Theor. Appl. Climatol. 2014, 117, 707–721. [Google Scholar] [CrossRef]
- Khosravinejad, F.; Heydari, R.; Farboodnia, T. Effect of Salinity on Organic Solutes Contents in Barley. Pak. J. Biol. Sci. 2009, 12, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Munjonji, L.; Ayisi, K.K.; Vandewalle, B.; Haesaert, G.; Boeckx, P. Carbon Isotope Discrimination as a Surrogate of Grain Yield in Drought Stressed Triticale. In Climate Change Adaptation in Africa. Climate Change Management; Leal Filho, W., Belay, S., Kalangu, J., Menas, W., Munishi, P., Musiyiwa, K., Eds.; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Ubierna, N.; Winter, K.; Holtum, J.A.; Marshall, J.D.; Farquhar, G.D. Environmental and Physiological Determinants of Carbon Isotope Discrimination in Terrestrial Plants. New Phytol. 2013, 200, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chang, S.X.; Anyia, A.O. The Physiology and Stability of Leaf Carbon Isotope Discrimination as a Measure of Water-Use Efficiency in Barley on the Canadian Prairies. J. Agron. Crop Sci. 2011, 197, 1–11. [Google Scholar] [CrossRef]
- Schelling, K.; Born, K.; Weissteiner, C.; Kühbauch, W. Relationships between Yield and Quality Parameters of Malting Barley (Hordeum vulgare L.) and Phenological and Meteorological Data. J. Agron. Crop Sci. 2003, 189, 113–122. [Google Scholar] [CrossRef]
- Bütehorn, B.; Rhody, D.; Franken, P. Isolation and Characterisation of Pitef1 Encoding the Translation Elongation Factor EF-1α of the Root Endophyte Piriformospora indica. Plant Biol. 2000, 2, 687–692. [Google Scholar] [CrossRef]
- Lancashire, P.D.; Bleiholder, H.; Boom, T.V.D.; Langelüddeke, P.; Stauss, R.; Weber, E.; Witzenberger, A. A Uniform Decimal Code for Growth Stages of Crops and Weeds. Ann. Appl. Biol. 1991, 119, 561–601. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- ISO 10520:1997; Native Starch—Determination of Starch Content—Ewers Polarimetric Method. ISO International Organization for Standardization: Geneva, Switzerland, 1997.
- Klem, K.; Rajsnerová, P.; Novotná, K.; Mìša, P.; Křen, J. Changes in Vertical Distribution of Spectral Reflectance within Spring Barley Canopy as an Indicator of Nitrogen Nutrition, Canopy Structure and Yield Parameters. Agriculture (Pol’nohospodárstvo) 2014, 60, 50–59. [Google Scholar] [CrossRef]
- Kaczmarek, M.; Fedorowicz-Strońska, O.; Głowacka, K.; Waśkiewicz, A.; Sadowski, J. CaCl2 treatment improves drought stress tolerance in barley (Hordeum vulgare L.). Acta Physiol. Plant. 2017, 39, 41. [Google Scholar] [CrossRef]
- Sazegari, S.; Zinati, Z.; Tahmasebi, A. Dynamic transcriptomic analysis uncovers key genes and mechanisms involved in seed priming-induced tolerance to drought in barley. Gene Rep. 2020, 21, 100941. [Google Scholar] [CrossRef]
- Daszkowska-Golec, A.; Collin, A.; Sitko, K.; Janiak, A.; Kalaji, H.M.; Szarejko, I. Genetic and physiological dissection of photosynthesis in barley exposed to drought stress. Int. J. Mol. Sci. 2019, 20, 6341. [Google Scholar] [CrossRef] [PubMed]
- de Mezer, M.; Turska-Taraska, A.; Kaczmarek, Z.; Glowacka, K.; Swarcewicz, B.; Rorat, T. Differential physiological and molecular response of barley genotypes to water deficit. Plant Physiol. Biochem. 2014, 80, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Kirby, E.J.M. Analysis of leaf, stem and ear growth in wheat from terminal spikelet stage to anthesis. Field Crops Res. 1988, 18, 127–140. [Google Scholar] [CrossRef]
- Waddington, S.R.; Cartwright, P.M.; Wall, P.C. A quantitative scale of spike initial and pistil development in barley and wheat. Ann. Bot. 1983, 51, 119–130. [Google Scholar] [CrossRef]
- Gómez, J.F.; Wilson, Z.A. Non-destructive staging of barley reproductive development for molecular analysis based upon external morphology. J. Exp. Bot. 2012, 63, 4085–4094. [Google Scholar] [CrossRef] [PubMed]
- Boussora, F.; Allam, M.; Guasmi, F.; Ferchichi, A.; Rutten, T.; Hansson, M.; Helmy, M.Y.; Börner, A. Spike developmental stages and ABA role in spikelet primordia abortion contribute to the final yield in barley (Hordeum vulgare L.). Bot. Stud. 2019, 60, 13. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Barmeier, G.; Schmidhalter, U. Genetic Variation in Grain Yield and Quality Traits of Spring Malting Barley. Agronomy 2021, 11, 1177. [Google Scholar] [CrossRef]
- Hussain, S.; Ali, B.; Saqib, M. Chapter 15—Seed Priming to Enhance Salt and Drought Stress Tolerance in Plants: Advances and Prospects. In Climate Change and Crop Stress; Shanker, A.K., Shanker, C., Anand, A., Maheswari, M., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 441–464. ISBN 978-0-12-816091-6. [Google Scholar]
- Tabassum, T.; Ahmad, R.; Farooq, M.; Basra, S.M.A. Improving the Drought Tolerance in Barley by Osmopriming and Biopriming. Int. J. Agric. Biol. 2018, 20, 1597–1606. [Google Scholar]
- Abideen, Z.; Cardinale, M.; Zulfiqar, F.; Koyro, H.W.; Rasool, S.G.; Hessini, K.; Darbali, W.; Zhao, F.; Siddique, K.H. Seed endophyte bacteria enhance drought stress tolerance in Hordeum vulgare by regulating, physiological characteristics, antioxidants and minerals uptake. Front. Plant Sci. 2022, 13, 980046. [Google Scholar] [CrossRef] [PubMed]
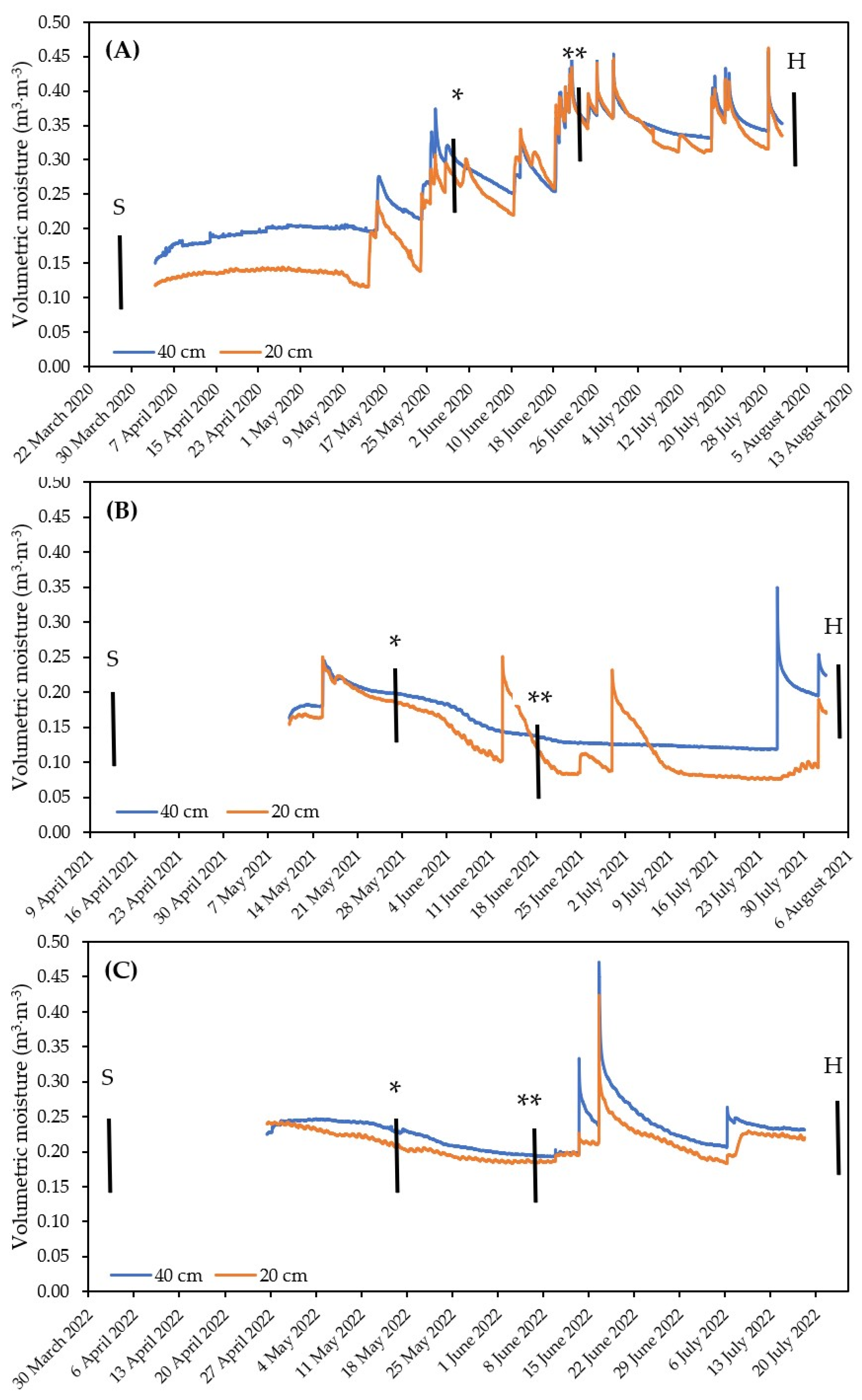


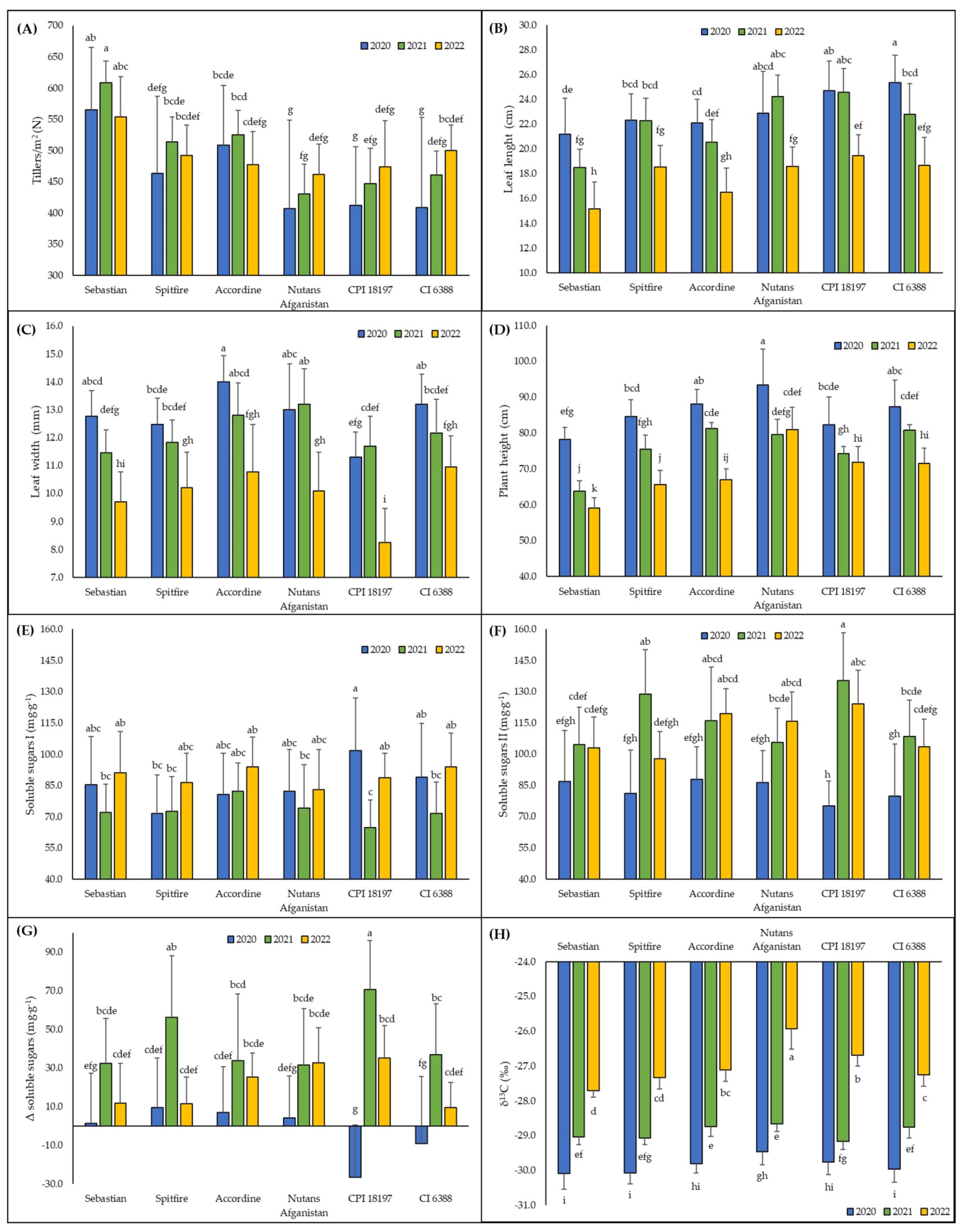
| (A) | Yield per Plot (kg) | TGW (g) | Starch in Grains (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor | F | p | F | p | F | p | |||
| Genotype (GE) | 141.806 | <0.001 * | 358.560 | <0.001 * | 288.541 | <0.001 * | |||
| Fertilization (FE) | 0.236 | 0.790 | 2.148 | 0.120 | 2.803 | 0.063 | |||
| Inoculation (IN) | 0.091 | 0.763 | 0.465 | 0.496 | 0.564 | 0.454 | |||
| Season (SE) | 72.215 | <0.001 * | 3022.513 | <0.001 * | 19.764 | <0.001 * | |||
| GE × FE | 0.515 | 0.878 | 0.871 | 0.562 | 1.652 | 0.095 | |||
| GE × IN | 1.064 | 0.382 | 1.078 | 0.374 | 1.774 | 0.120 | |||
| FE × IN | 0.122 | 0.885 | 1.385 | 0.253 | 0.030 | 0.970 | |||
| GE × SE | 49.682 | <0.001 * | 25.628 | <0.001 * | 18.164 | <0.001 * | |||
| FE × SE | 4.465 | 0.002 * | 3.428 | 0.010 * | 2.876 | 0.024 * | |||
| IN × SE | 0.294 | 0.746 | 0.189 | 0.828 | 0.690 | 0.503 | |||
| GE × FE × IN | 0.738 | 0.688 | 0.663 | 0.758 | 0.597 | 0.815 | |||
| GE × FE × SE | 0.838 | 0.665 | 1.378 | 0.138 | 1.235 | 0.231 | |||
| GE × IN × SE | 0.746 | 0.680 | 0.956 | 0.483 | 0.906 | 0.529 | |||
| FE × IN × SE | 0.390 | 0.816 | 3.686 | 0.007 * | 0.792 | 0.532 | |||
| (B) | Tillers/m2 (N) | Leaf Length (cm) | Leaf Width (mm) | Plant Height (cm) | |||||
| Factor | F | p | F | p | F | p | F | p | |
| Genotype (GE) | 31.389 | <0.001 * | 31.215 | <0.001 * | 19.699 | <0.001 * | 81.148 | <0.001 * | |
| Fertilization (FE) | 21.168 | <0.001 * | 0.959 | 0.385 | 7.113 | 0.001 * | 2.521 | 0.083 | |
| Inoculation (IN) | 0.729 | 0.394 | 0.853 | 0.357 | 0.180 | 0.672 | 0.120 | 0.729 | |
| Season (SE) | 9.395 | <0.001 * | 169.498 | <0.001 * | 139.924 | <0.001 * | 287.943 | <0.001 * | |
| GE × FE | 2.215 | 0.019 * | 1.874 | 0.051 | 0.991 | 0.453 | 0.780 | 0.648 | |
| GE × IN | 1.279 | 0.275 | 2.634 | 0.025 * | 1.355 | 0.244 | 0.730 | 0.602 | |
| FE × IN | 0.214 | 0.807 | 2.653 | 0.073 | 1.369 | 0.257 | 0.182 | 0.834 | |
| GE × SE | 2.305 | 0.014 * | 2.447 | 0.009 * | 2.709 | 0.004 * | 9.021 | <0.001 * | |
| FE × SE | 20.911 | <0.001 * | 1.995 | 0.097 | 1.614 | 0.173 | 1.545 | 0.191 | |
| IN × SE | 0.203 | 0.817 | 1.798 | 0.169 | 1.037 | 0.357 | 0.299 | 0.742 | |
| GE × FE × IN | 2.756 | 0.003 * | 1.609 | 0.107 | 1.019 | 0.429 | 0.287 | 0.984 | |
| GE × FE × SE | 2.030 | 0.008 * | 1.113 | 0.339 | 0.853 | 0.646 | 2.272 | 0.002 * | |
| GE × IN × SE | 1.525 | 0.134 | 1.404 | 0.182 | 0.721 | 0.704 | 0.963 | 0.477 | |
| FE × IN × SE | 0.380 | 0.823 | 0.610 | 0.656 | 0.745 | 0.563 | 2.967 | 0.021 * | |
| (C) | Soluble Sugars I (mg·g−1) | Soluble Sugars II (mg·g−1) | Δ soluble Sugars (mg·g−1) | δ13C (‰) | |||||
| Factor | F | p | F | p | F | p | F | p | |
| Genotype (GE) | 1.763 | 0.123 | 3.386 | 0.006 * | 2.154 | 0.061 | 40.423 | <0.001 * | |
| Fertilization (FE) | 2.624 | 0.075 | 2.753 | 0.066 | 0.680 | 0.508 | 0.050 | 0.951 | |
| Inoculation (IN) | 5.615 | 0.019 * | 0.489 | 0.485 | 5.244 | 0.023 * | 0.779 | 0.379 | |
| Season (SE) | 18.223 | <0.001 * | 82.481 | <0.001 * | 74.047 | <0.001 * | 1673.900 | <0.001 * | |
| GE × FE | 1.321 | 0.222 | 0.488 | 0.896 | 1.342 | 0.211 | 0.852 | 0.579 | |
| GE × IN | 0.660 | 0.654 | 0.417 | 0.836 | 0.230 | 0.949 | 0.940 | 0.456 | |
| FE × IN | 0.244 | 0.784 | 0.219 | 0.803 | 0.476 | 0.622 | 0.085 | 0.918 | |
| GE × SE | 2.282 | 0.015 * | 4.691 | <0.001 * | 5.519 | <0.001 * | 11.492 | <0.001 * | |
| FE × SE | 1.267 | 0.285 | 1.388 | 0.240 | 1.338 | 0.258 | 0.511 | 0.728 | |
| IN × SE | 0.030 | 0.970 | 0.542 | 0.582 | 0.254 | 0.776 | 0.180 | 0.835 | |
| GE × FE × IN | 0.547 | 0.855 | 1.483 | 0.149 | 0.488 | 0.897 | 1.683 | 0.088 | |
| GE × FE × SE | 0.764 | 0.754 | 0.865 | 0.631 | 0.967 | 0.505 | 1.657 | 0.044 * | |
| GE × IN × SE | 0.393 | 0.949 | 0.766 | 0.661 | 0.597 | 0.815 | 0.334 | 0.971 | |
| FE × IN × SE | 0.622 | 0.648 | 0.066 | 0.992 | 0.190 | 0.943 | 0.564 | 0.689 | |
| (A) | Genotype | Yield Per Plot (kg) | TGW (g) | Starch in Grains (%) | Tillers/m2 (N) | Leaf Length (cm) | Leaf Width (mm) | Plant Height (cm) | Soluble Sugars I (mg·g−1) | Soluble Sugars II (mg·g−1) | Δ Soluble Sugars (mg·g−1) | δ13C (‰) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sebastian | 1.87 ± 0.23 a | 47.24 ± 7.77 c | 59.4 ± 1.5 b | 576 ± 74 a | 18.3 ± 3.3 d | 11.3 ± 1.6 c | 67.0 ± 8.7 e | 82.8 ± 20.4 a | 98.1 ± 20.8 b | 15.3 ± 26.2 a | −28.9 ± 1.0 d | |
| Spitfire | 1.68 ± 0.45 b | 54.85 ± 7.64 a | 60.7 ± 1.0 a | 490 ± 81 bc | 21.1 ± 2.6 b | 11.5 ± 1.4 bc | 75.2 ± 8.9 d | 76.8 ± 17.6 a | 102.6 ± 27.1 ab | 25.8 ± 32.7 a | −28.8 ± 1.2 cd | |
| Accordine | 1.81 ± 0.35 a | 53.52 ± 5.12 b | 60.3 ± 1.5 a | 504 ± 68 b | 19.7 ± 3.0 c | 12.5 ± 1.9 a | 78.8 ± 9.4 bc | 85.6 ± 16.8 a | 107.7 ± 23.3 ab | 22.1 ± 27.1 a | −28.5 ± 1.2 b | |
| Nutans Afganistan | 1.31 ± 0.19 c | 46.71 ± 6.48 cd | 54.2 ± 1.9 d | 433 ± 91 d | 21.9 ± 3.4 ab | 12.1 ± 2.0 ab | 84.7 ± 9.5 a | 79.8 ± 20.0 a | 102.6 ± 19.4 ab | 22.8 ± 26.4 a | −28.0 ± 1.6 a | |
| CPI 18197 | 1.21 ± 0.12 d | 46.77 ± 6.68 cd | 54.8 ± 1.3 d | 444 ± 79 d | 22.9 ± 3.2 a | 10.4 ± 1.9 d | 76.1 ± 6.9 cd | 85.1 ± 23.3 a | 111.5 ± 31.5 a | 26.4 ± 46.7 a | −28.5 ± 1.4 b | |
| CI 6388 | 1.68 ± 0.19 b | 46.08 ± 7.03 d | 57.4 ± 1.5 c | 457 ± 95 cd | 22.3 ± 3.6 a | 12.1 ± 1.4 ab | 79.9 ± 8.2 b | 84.8 ± 21.5 a | 97.3 ± 22.6 b | 12.5 ± 31.8 a | −28.7 ± 1.2 bc | |
| (B) | Season | Yield Per Plot (kg) | TGW (g) | Starch in Grains (%) | Tillers/m2 (N) | Leaf Length (cm) | Leaf Width (mm) | Plant Height (cm) | Soluble Sugars I (mg·g−1) | Soluble Sugars II (mg·g−1) | Δ Soluble Sugars (mg·g−1) | δ13C (‰) |
| 2020 | 1.59 ± 0.38 b | 40.48 ± 4.43 c | 57.3 ± 3.5 c | 461 ± 129 b | 23.1 ± 2.9 a | 12.8 ± 1.4 a | 85.6 ± 8.1 a | 85.1 ± 23.5 a | 82.9 ± 19.5 b | −2.2 ± 28.9 c | −29.9 ± 0.4 c | |
| 2021 | 1.73 ± 0.37 a | 51.04 ± 4.11 b | 58.3 ± 2.5 a | 497 ± 74 a | 22.2 ± 2.8 b | 12.2 ± 1.2 b | 75.9 ± 6.7 b | 72.9 ± 16.2 b | 116.4 ± 23.3 a | 43.5 ± 31.5 a | −28.9 ± 0.3 b | |
| 2022 | 1.46 ± 0.29 c | 56.07 ± 3.59 a | 57.8 ± 2.6 b | 493 ± 62 a | 17.8 ± 2.4 c | 10.0 ± 1 0.6 c | 69.3 ± 7.9 c | 89.5 ± 16.1 a | 110.6 ± 16.7 a | 21.1 ± 18.8 b | −27.0 ± 0.7 a | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bleša, D.; Matušinský, P.; Baláž, M.; Nesvadba, Z.; Zavřelová, M. Endophyte Inoculation and Elevated Potassium Supply on Productivity, Growth and Physiological Parameters of Spring Barley (Hordeum vulgare L.) Genotypes over Contrasting Seasons. Plants 2024, 13, 1168. https://doi.org/10.3390/plants13081168
Bleša D, Matušinský P, Baláž M, Nesvadba Z, Zavřelová M. Endophyte Inoculation and Elevated Potassium Supply on Productivity, Growth and Physiological Parameters of Spring Barley (Hordeum vulgare L.) Genotypes over Contrasting Seasons. Plants. 2024; 13(8):1168. https://doi.org/10.3390/plants13081168
Chicago/Turabian StyleBleša, Dominik, Pavel Matušinský, Milan Baláž, Zdeněk Nesvadba, and Marta Zavřelová. 2024. "Endophyte Inoculation and Elevated Potassium Supply on Productivity, Growth and Physiological Parameters of Spring Barley (Hordeum vulgare L.) Genotypes over Contrasting Seasons" Plants 13, no. 8: 1168. https://doi.org/10.3390/plants13081168





