Ginger and Turmeric Essential Oils for Weed Control and Food Crop Protection
Abstract
:1. Introduction
2. Results and Discussion
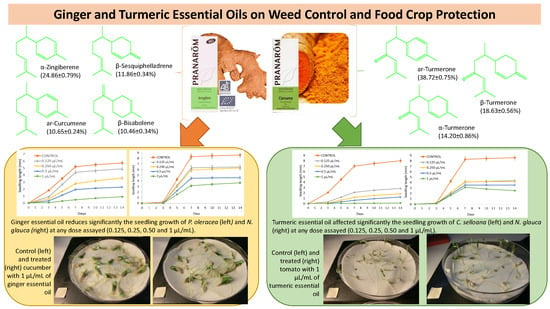
2.1. Chemical Composition of Ginger and Turmeric Essential Oils
2.2. Seed Germination and Seedling Growth Inhibition of P. oleracea, L. multiflorum, E. crus-galli, C. selloana, and N. glauca with Ginger and Turmeric Essential Oils
2.3. Seed Germination and Seedling Growth Effect of Ginger and Turmeric Essential Oils in Tomato, Cucumber, and Rice
3. Materials and Methods
3.1. Essential Oils
3.2. Weed and Food Crop Seeds
3.3. Gas Chromatography–Mass Spectrometry Analysis
3.4. In Vitro Assays: P. oleracea, L. multiflorum, E. crus-galli, C. selloana, N. glauca, Tomato, and Rice Seed Germination and Seedling Growth with Essential Oils
3.5. Statistics
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Xie, Z.; Finley, J.F. Herbs and Spices. In Principles of Food Chemistry; Springer: Berlin, Germany, 2018; pp. 457–481. ISBN 9783319636078. [Google Scholar]
- Székács, A.; Wilkinson, M.G.; Mader, A.; Appel, B. Environmental and food safety of spices and herbs along global food chains. Food Control 2018, 83, 1–6. [Google Scholar] [CrossRef]
- Food and Agriculture Organization: Crops. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 22 May 2018).
- Carney, E.M.; Stein, W.M.; Reigh, N.A.; Gater, F.M.; Bakke, A.J.; Hayes, J.E.; Keller, K.L. Increasing flavor variety with herbs and spices improves relative vegetable intake in children who are propylthiouracil (PROP) tasters relative to nontasters. Physiol. Behav. 2018, 188, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Embuscado, M.E. Spices and herbs: Natural sources of antioxidants—A mini review. J. Funct. Foods 2015, 18, 811–819. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef] [PubMed]
- Ginger. Post-Harvest Operations. Available online: http://www.fao.org/3/a-av003e.pdf (accessed on 22 May 2018).
- Turmeric. Post-Harvest Operations. Available online: http://www.fao.org/fileadmin/user_upload/inpho/docs/Post_Harvest_Compendium_-_Turmeric.pdf (accessed on 22 May 2018).
- Tohma, H.; Gülçin, İ.; Bursal, E.; Gören, A.C.; Alwasel, S.H.; Köksal, E. Antioxidant activity and phenolic compounds of ginger (Zingiber officinale Rosc.) determined by HPLC-MS/MS. J. Food Meas. Charact. 2017, 11, 556–566. [Google Scholar] [CrossRef]
- Srinivasan, K. Spices as influencers of body metabolism: An overview of three decades of research. Food Res. Int. 2005, 38, 77–86. [Google Scholar] [CrossRef]
- Prasad, S.; Tyagi, A.K. Ginger and its constituents: Role in prevention and treatment of gastrointestinal cancer. Gastroenterol. Res. Pract. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shidfar, F.; Rajab, A.; Rahideh, T.; Khandouzi, N.; Hosseini, S.; Shidfar, S. The effect of ginger (Zingiber officinale) on glycemic markers in patients with type 2 diabetes. J. Complement. Integr. Med. 2015, 12, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Kocaadam, B.; Şanlier, N. Curcumin, an active component of turmeric (Curcuma longa), and its effects on health. Crit. Rev. Food Sci. Nutr. 2017, 57, 2889–2895. [Google Scholar] [CrossRef] [PubMed]
- Devassy, J.G.; Nwachukwu, I.D.; Jones, P.J.H. Curcumin and cancer: Barriers to obtaining a health claim. Nutr. Rev. 2015, 73, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shang, Y.; Li, M.; Han, X.; Wang, J.; Wang, J. Curcumin ameliorates asthmatic airway inflammation by activating Nrf2/HO-1 signalling pathway. Clin. Exp. Pharm. Physiol. 2015, 42, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Maiti, P.; Ma, Q.; Zuo, X.; Jones, M.R.; Cole, G.M.; Frautschy, S.A. Clinical development of curcumin in neurodegenerative disease. Expert Rev. Neurother. 2015, 15, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Jeena, K.; Liju, V.B.; Viswanathan, R.; Kuttan, R. Antimutagenic potential and modulation of carcinogen-metabolizing enzymes by ginger essential oil. Phyther. Res. 2014, 28, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.-S.; Lee, W.-C.; Lin, Y.-E.; Ho, C.-T.; Lu, K.-H.; Lin, S.-H.; Panyod, S.; Chu, Y.-L.; Sheen, L.-Y. Ginger essential oil ameliorates hepatic injury and lipid accumulation in high fat diet-induced nonalcoholic fatty liver disease. J. Agric. Food Chem. 2016, 64, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.H.; Yusof, N.M.; Jai, J.; Hamzah, F. Effect of coating adhesion on turmeric essential oil incorporated into chitosan-based edible coating. Mater. Sci. Forum 2017, 890, 204–208. [Google Scholar] [CrossRef]
- Noori, S.; Zeynali, F.; Almasi, H. Antimicrobial and antioxidant efficiency of nanoemulsion-based edible coating containing ginger (Zingiber officinale) essential oil and its effect on safety and quality attributes of chicken breast fillets. Food Control 2018, 84, 312–320. [Google Scholar] [CrossRef]
- Gangwar, P.; Tiwari, S.N. Insecticidal activity of Curcuma longa essential oil and its fractions against Sitophilus oryzae L. and Rhyzopertha dominica F. (Coleoptera). Int. J. Pure Appl. Biosci. 2017, 5, 912–921. [Google Scholar]
- Brado Avanço, G.; Dias Ferreira, F.; Silva Bomfim, N.; De Souza Rodrigues dos Santos, P.A.; Peralta, R.M.; Brugnari, T.; Mallmann, C.A.; de Abreu Filho, B.A.; Graton Mikcha, J.M.; Machinski, M., Jr. Curcuma longa L. essential oil composition, antioxidant effect, and effect on Fusarium verticillioides and fumonisin production. Food Control 2017, 73, 806–813. [Google Scholar] [CrossRef]
- Kumar, N.; Reddy, J.; Mudili, V. Effect of high pressure processing on growth and mycotoxin production of Fusarium graminearum in maize. Food Biosci. 2018, 21, 53–59. [Google Scholar]
- Nerilo, S.B.; Rocha, G.H.O.; Tomoike, C.; Mossini, S.A.G.; Grespan, R.; Mikcha, J.M.G.; Machinski, M. Antifungal properties and inhibitory effects upon aflatoxin production by Zingiber officinale essential oil in Aspergillus flavus. Int. J. Food Sci. Technol. 2016, 51, 286–292. [Google Scholar] [CrossRef]
- Hussein, K.; Joo, J. Antifungal activity and chemical composition of ginger essential oil against ginseng pathogenic fungi. Curr. Res. Environ. Appl. Mycol. 2018, 8, 194–203. [Google Scholar] [CrossRef]
- Javed, S.; Shoaib, A. Herbicidal activity of some medicinal plants extracts against Parthenium hysterophorus L. Pakistan J. Weed Sci. Res. 2014, 20, 279–291. [Google Scholar]
- Sah, D.; Heisnam, P.; Mahato, N.K.; Pandey, A.K. Weed management in ginger (Zingiber officinale Roscoe) through integrated approaches. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1839–1845. [Google Scholar] [CrossRef]
- de Melo, S.; de Sa, L.; de Oliveira, H.; Trettel, J.; da Silva, P.; Goncalves, J.; Gazim, Z.; Magalhaes, H. Chemical constitution and allelopathic effects of “Curcuma zedoaria” essential oil on lettuce achenes and tomato seeds. Aust. J. Crop Sci. 2017, 11, 906–916. [Google Scholar] [CrossRef]
- Akter, J.; Islam, Z.; Takara, K.; Hossain, A. Plant growth inhibitors in turmeric (Curcuma longa) and their effects on Bidens pilosa. Weed Biol. Manag. 2018, 18, 136–145. [Google Scholar] [CrossRef]
- König, W.A.; Krüger, A.; Icheln, D.; Runge, T. Enantiomeric composition of the chiral constituents in essentials oils Part I: Monoterpe hydrocarbons. J. High Resolut. Chromatogr. 1992, 15, 184–189. [Google Scholar] [CrossRef]
- Şener, N.; Özkinali, S.; Gür, M.; Güney, K.; Özkan, O.E.; Khalifa, M.M. Determination of antimicrobial activity and chemical composition of pimento & ginger essential oil. Indian J. Pharm. Educ. Res. 2017, 51, s230–s233. [Google Scholar]
- Höferl, M.; Stoilova, I.; Wanner, J.; Schmidt, E.; Jirovetz, L.; Trifonova, D.; Stanchev, V.; Krastanov, A. Composition and comprehensive antioxidant activity of ginger (Zingiber officinale) essential oil from Ecuador. Nat. Prod. Commun. 2015, 10, 1085–1090. [Google Scholar] [PubMed]
- Sasidharan, I.; Menon, A.N. Comparative chemical composition and antimicrobial activity fresh & dry ginger oils (Zingiber officinale Roscoe). Int. J. Curr. Pharm. Res. 2010, 2, 4–7. [Google Scholar]
- Togar, B.; Türkez, H.; Stefano, A.D.; Tatar, A.; Cetin, D. Zingiberene attenuates hydrogen peroxide-induced toxicity in neuronal cells. Hum. Exp. Toxicol. 2015, 34, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Türkez, H.; Toğar, B.; Çelik, K. In vitro study of human lymphocytes cytological and biochemical effects by zingiberene. J. Essent. Oil Res. 2014, 26, 367–371. [Google Scholar] [CrossRef]
- Lima, I.P.; Resende, J.T.; Oliveira, J.R.; Faria, M.V.; Dias, D.M.; Resende, N.C.; Lima, I.P.; Resende, J.T.; Oliveira, J.R.; Faria, M.V.; et al. Selection of tomato genotypes for processing with high zingiberene content, resistant to pests. Hortic. Bras. 2016, 34, 387–391. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Du, A.L.; Du, A.Q. Isolation of zingiberene from ginger essential oil by two-step intermittent silica gel column chromatography. Adv. Mater. Res. 2012, 550–553, 1666–1670. [Google Scholar] [CrossRef]
- Tyagi, A.K.; Prasad, S.; Yuan, W.; Li, S.; Aggarwal, B.B. Identification of a novel compound (β-sesquiphellandrene) from turmeric (Curcuma longa) with anticancer potential: Comparison with curcumin. Investig. New Drugs 2015, 33, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- Singha, P.; Muthukumarappan, K. Quality changes and freezing time prediction during freezing and thawing of ginger. Food Sci. Nutr. 2016, 4, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Shiyou, L.; Wei, Y.; Guangrui, D.; Ping, W.; Peiying, Y.; Bharat, A. Chemical composition and product quality control of turmeric (Curcuma longa L.). Pharm. Crop. 2011, 2, 28–54. [Google Scholar]
- Singh, S.; Rajesh, B.S.S.; Sahoo, K.; Subudhi, E.; Nayak, S. Chemical composition of turmeric oil (Curcuma longa L. cv. Roma) and its antimicrobial activity against eye infecting pathogens. J. Essent. Oil Res. 2011, 23, 11–18. [Google Scholar] [CrossRef]
- Priya, R.; Prathapan, A.; Raghu, K.G.; Menon, A.N. Chemical composition and in vitro antioxidative potential of essential oil isolated from Curcuma longa L. leaves. Asian Pac. J. Trop. Biomed. 2012, 2, S695–S699. [Google Scholar] [CrossRef]
- Hu, Y.; Kong, W.; Yang, X.; Xie, L.; Wen, J.; Yang, M. GC-MS combined with chemometric techniques for the quality control and original discrimination of Curcumae longae rhizome: Analysis of essential oils. J. Sep. Sci. 2014, 37, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Hucklenbroich, J.; Klein, R.; Neumaier, B.; Graf, R.; Fink, G.; Schroeter, M.; Rueger, M. Aromatic-turmerone induces neural stem cell proliferation in vitro and in vivo. Stem Cell Res. Ther. 2014, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Jin, M.L.; Kim, Y.H.; Kim, Y.; Lee, S.J. Anti-inflammatory effects of aromatic-turmerone through blocking of NF-κB, JNK, and p38 MAPK signaling pathways in amyloid β-stimulated microglia. Int. Immunopharmacol. 2012, 14, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shlomo Navarro, H.; Simcha Finkelman, S.; Dov Zehavi, R.; Refael Dias, H.; Sam Angel, R.; Fadel Mansur, I.; Miriam Rindner, R. Pest-impervious packaging material and pest-control composition. U.S. Patent 7,749,525 B2, 6 July 2010. [Google Scholar]
- De Souza Tavares, W.; de Sousa Freitas, S.; Grazziotti, G.H.; Parente, L.M.L.; Lião, L.M.; Zanuncio, J.C. Ar-turmerone from Curcuma longa (Zingiberaceae) rhizomes and effects on Sitophilus zeamais (Coleoptera: Curculionidae) and Spodoptera frugiperda (Lepidoptera: Noctuidae). Ind. Crops Prod. 2013, 46, 158–164. [Google Scholar]
- Neoob, K.; Castro, D.C.; Canuto, K.M.; Brito, E.D.S.; Andrade, I.M. In vitro efficacy of essential oils with different concentrations of 1,8-cineole against Rhipicephalus (Boophilus) microplus. Braz. J. Vet. Parasitol. 2018, 2961, 1–8. [Google Scholar]
- Liao, P.C.; Yang, T.S.; Chou, J.C.; Chen, J.; Lee, S.C.; Kuo, Y.H.; Ho, C.L.; Chao, L.K.P. Anti-inflammatory activity of neral and geranial isolated from fruits of Litsea cubeba Lour. J. Funct. Foods 2015, 19, 248–258. [Google Scholar] [CrossRef]
- Blázquez, M.A. Role of natural essential oils in sustainable agriculture and food preservation. J. Sci. Res. Rep. 2014, 3, 1843–1860. [Google Scholar] [CrossRef]
- Ibáñez, M.D.; Blázquez, M.A. Phytotoxicity of essential oils on selected weeds: Potential hazard on food crops. Plants 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.S.; Ahluwalia, V.; Shakil, N.A.; Prasad, L. Essential oil composition, antifungal, and seedling growth inhibitory effects of zerumbone from Zingiber zerumbet Smith. J. Essent. Oil Res. 2017, 29, 320–329. [Google Scholar] [CrossRef]
- Ibáñez, M.; Blázquez, M. Herbicidal value of essential oils from oregano-like flavour species. Food Agric. Immunol. 2017, 28, 1168–1180. [Google Scholar] [CrossRef] [Green Version]
- Tei, F.; Montemurro, P.; Baumann, D.; Dobrzanski, A.; Giovinazzo, R.; Kleifeld, Y.; Rocha, F.; Rzozi, S.; Sanseovic, T.; Simoncic, A.; et al. Weeds and weed management in processing tomato. Acta Hortic. 2003, 613, 111–121. [Google Scholar] [CrossRef]
- Han, C.M.; Pan, K.W.; Wu, N.; Wang, J.C.; Li, W. Allelopathic effect of ginger on seed germination and seedling growth of soybean and chive. Sci. Hortic. (Amsterdam) 2008, 116, 330–336. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.K.; Raina, A.P.; Dureja, P. Evaluation of the antifungal and phytotoxic effects of various essential oils against Sclerotium rolfsii (Sacc) and Rhizoctonia bataticola (Taub). Arch. Phytopathol. Plant Prot. 2009, 42, 65–72. [Google Scholar] [CrossRef]
- Hazrati, H.; Saharkhiz, M.J.; Moein, M.; Khoshghalb, H. Phytotoxic effects of several essential oils on two weed species and tomato. Biocatal. Agric. Biotechnol. 2018, 13, 204–212. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Blázquez, M.A.; Carbó, E. Control of Portulaca oleracea by boldo and lemon essential oils in different soils. Ind. Crops Prod. 2015, 76, 515–521. [Google Scholar] [CrossRef]




| RICal | RIRef | Compound | Ginger Essential Oil Relative Area (%) | Turmeric Essential Oil Relative Area (%) | Identification |
|---|---|---|---|---|---|
| Monoterpene hydrocarbons | 19.8 ± 0.5 | 5.4 ± 0.7 | |||
| 919 | 926 | Tricyclene | 0.2 ± 0.0 | - | RI, MS |
| 932 | 939 | α-Pinene | 2.7 ± 0.0 | 0.2 ± 0.0 | RI, MS |
| 948 | 954 | Camphene | 11.6 ± 0.3 | - | RI, MS |
| 973 | 979 | β-Pinene | 0.2 ± 0.0 | - | RI, MS |
| 987 | 990 | Myrcene | 1.3 ± 0.04 | 0.1 ± 0.0 | RI, MS |
| 998 | 1002 | α-Phellandrene | 0.2 ± 0.0 | 4.3 ± 0.4 | RI, MS |
| 1004 | 1011 | δ-3-Carene | - | 0.1 ± 0.0 | RI, MS |
| 1013 | 1017 | α-Terpinene | - | 0.1 ± 0.0 | RI, MS |
| 1021 | 1024 | p-Cymene | - | 0.5 ± 0.1 | RI, MS |
| 1026 | 1029 | Limonene | 3.2 ± 0.1 | 0.2 ± 0.0 | RI, MS |
| 1056 | 1059 | γ-Terpinene | - | 0.2 ± 0.0 | RI, MS |
| 1083 | 1088 | Terpinolene | 0.3 ± 0.0 | 0.2 ± 0.0 | RI, MS |
| Oxygenated monoterpenes | 11.8 ± 0.2 | 1.0 ± 0.0 | |||
| 1029 | 1031 | 1,8-Cineole | 3.0 ± 0.1 | 1.0 ± 0.0 | RI, MS |
| 1095 | 1094 | Linalool | 0.8 ± 0.0 | - | RI, MS |
| 1137 | 1146 | Camphor | 0.2 ± 0.0 | - | RI, MS |
| 1149 | 1153 | Citronellal | 0.2 ± 0.0 | - | RI, MS |
| 1171 | 1177 | Terpinen-4-ol | 0.2 ± 0.0 | - | RI, MS |
| 1188 | 1188 | α-Terpineol | 0.7 ± 0.1 | - | RI, MS |
| 1236 | 1238 | Neral | 2.1 ± 0.1 | - | RI, MS |
| 1267 | 1267 | Geranial | 3.2 ± 0.0 | - | RI, MS |
| 1279 | 1288 | Bornyl Acetate | 0.9 ± 0.0 | - | RI, MS |
| 1378 | 1381 | Geranyl Acetate | 0.6 ± 0.0 | - | RI, MS |
| Sesquiterpene hydrocarbons | 59.6 ± 0.1 | 7.2 ± 0.0 | |||
| 1383 | 1390 | β-Elemene | 0.6 ± 0.1 | - | RI, MS |
| 1414 | 1419 | β-Caryophyllene | - | 0.3 ± 0.0 | RI, MS |
| 1427 | 1434 | α-trans-Bergamotene | 0.2 ± 0.1 | - | RI, MS |
| 1450 | 1456 | (E)-β-Farnesene | 1.0 ± 0.1 | - | RI, MS |
| 1479 | 1480 | ar-Curcumene | 10.7 ± 0.2 | 1.4 ± 0.1 | RI, MS |
| 1492 | 1493 | α-Zingiberene | 24.9 ± 0.8 | 2.6 ± 0.1 | RI, MS |
| 1502 | 1505 | β-Bisabolene | 10.5 ± 0.3 | 0.6 ± 0.0 | RI, MS |
| 1523 | 1522 | β-Sesquiphelladrene | 11.9 ± 0.3 | 2.2 ± 0.0 | RI, MS |
| Oxygenated sesquiterpenes | 1.0 ± 0.2 | 73.9 ± 1.4 | |||
| 1576 | 1583 | ar-Turmerol | - | 0.9 ± 0.0 | RI, MS |
| 1629 | 1628 | 1-epi-Cubenol | 0.9 ± 0.2 | - | RI, MS |
| 1649 | 1646 | Cubenol | 0.2 ± 0.0 | - | RI, MS |
| 1677 | 1669 | ar-Turmerone | - | 38.7 ± 0.8 | RI, MS |
| 1681 | - | α-Turmerone | - | 14.2 ± 0.9 | MS |
| 1709 | - | β-Turmerone | - | 18.6 ± 0.6 | MS |
| 1742 | 1742 | Bisabolone | - | 0.7 ± 0.0 | RI, MS |
| 1778 | 1778 | E-α-Atlantone | - | 0.7 ± 0.0 | RI, MS |
| Others | 2.4 ± 0.1 | - | |||
| 984 | 984 | 6-Methyl-5-Hepten-2-one | 2.1 ± 0.1 | - | RI, MS |
| 1087 | 1087 | 2-Nonanone | 0.1 ± 0.0 | - | RI, MS |
| 1287 | 1287 | 2-Undecanone | 0.2 ± 0.0 | - | RI, MS |
| Total | 94.6 ± 2.0 | 87.7 ± 0.7 | |||
| Seed Germination (% ± S.E.) | |||||
|---|---|---|---|---|---|
| Dose * | Ginger essential oil | ||||
| P. oleracea | L. multiflorum | E. crus-galli | C. selloana | N. glauca | |
| Control | 86.00 ± 2.92 a | 60.00 ± 2.74 a | 86.00 ± 6.00 a | 82.00 ± 3.74 a | 94.00 ± 4.00 a |
| 0.125 | 81.00 ± 4.30 a | 50.00 ± 2.74 a,b | 79.00 ± 3.67 a | 85.00 ± 2.74 a | 85.00 ± 5.48 a |
| 0.25 | 77.00 ± 5.15 a | 47.00 ± 5.61 a,b | 73.00 ± 4.90 a | 81.00 ± 3.32 a | 83.00 ± 6.63 a |
| 0.5 | 82.00 ± 2.55 a | 47.00 ± 4.64 a,b | 69.00 ± 5.79 a | 67.00 ± 6.04 a | 79.00 ± 11.34 a |
| 1 | 47.00 ± 2.55 b | 32.00 ± 8.89 b | 68.00 ± 6.63 a | 46.00 ± 6.21 b | 73.00 ± 2.55 a |
| Dose | Turmeric essential oil | ||||
| Control | 86.00 ± 2.92 a | 60.00 ± 2.74 a | 75.00 ± 7.01 a | 82.00 ± 3.74 a | 94.00 ± 4.00 a |
| 0.125 | 75.00 ± 5.00 a | 50.00 ± 3.87 a | 74.00 ± 3.67 a | 46.00 ± 15.12 a,b | 85.00 ± 6.52 a |
| 0.25 | 71.00 ± 2.45 a | 49.00 ± 4.30 a | 71.00 ± 2.92 a | 43.00 ± 10.68 b | 86.00 ± 2.92 a |
| 0.5 | 70.00 ± 5.24 a | 55.00 ± 3.54 a | 71.00 ± 1.87 a | 32.00 ± 6.82 b | 87.00 ± 2.55 a |
| 1 | 73.00 ± 4.06 a | 49.00 ± 6.40 a | 68.00 ± 2.55 a | 15.00 ± 2.24 b | 85.00 ± 2.24 a |
| *Dose | Control | 0.125 | 0.25 | 0.5 | 1 | ||
|---|---|---|---|---|---|---|---|
| G | PO | Hyp | 3.65 ± 0.22 a | 2.80 ± 0.28 b | 2.01 ± 0.12 c | 1.39 ± 0.16 c,d | 0.63 ± 0.09 d |
| Rad | 2.08 ± 0.26 a | 2.07 ± 0.11 a | 1.57 ± 0.21 a | 0.89 ± 0.13 b | 0.29 ± 0.09 b | ||
| LM | Hyp | 25.76 ± 0.90 a | 19.65 ± 1.52 a,b | 16.39 ± 3.58 b,c | 12.46 ± 2.79 b,c | 8.54 ± 3.16 c | |
| Rad | 16.82 ± 1.93 a | 10.67 ± 1.51 a,b | 10.13 ± 2.12 a,b | 6.69 ± 1.33 b | 4.65 ± 1.85 b | ||
| ECG | Hyp | 16.96 ± 1.22 a | 12.91 ± 0.33 a | 12.88 ± 0.97 a | 12.33 ± 1.82 a | 12.27 ± 1.66 a | |
| Rad | 13.24 ± 0.92 a | 10.47 ± 0.89 a,b | 9.01 ± 0.75 b | 7.95 ± 1.30 b | 6.54 ± 0.90 b | ||
| CS | Hyp | 4.14 ± 0.56 a | 3.92 ± 0.70 a | 2.74 ± 0.52 a,b | 1.59 ± 0.71 b | 1.09 ± 0.78 b | |
| Rad | 3.88 ± 0.36 a | 3.68 ± 0.50 a | 2.63 ± 0.31 a,b | 1.56 ± 0.21 b,c | 0.96 ± 0.26 c | ||
| NG | Hyp | 4.72 ± 0.30 a | 3.26 ± 0.40 a,b | 2.99 ± 0.48 a,b | 1.86 ± 0.57 b | 1.71 ± 0.22 b | |
| Rad | 3.87 ± 0.23 a | 3.22 ± 0.24 a,b | 3.37 ± 0.53 a,b | 2.74 ± 0.70 a,b | 2.00 ± 0.15 b | ||
| T | PO | Hyp | 3.65 ± 0.22 a | 1.97 ± 0.21 b | 1.76 ± 0.13 b | 1.51 ± 0.06 b | 1.59 ± 0.04 b |
| Rad | 2.09 ± 0.26 a | 2.32 ± 0.20 a | 1.62 ± 0.18 a | 1.53 ± 0.29 a | 1.44 ± 0.12 a | ||
| LM | Hyp | 25.76 ± 0.90 a | 15.34 ± 2.96 b | 16.99 ± 1.41 b | 16.85 ± 1.01 b | 17.20 ± 1.62 b | |
| Rad | 16.82 ± 1.93 a | 11.60 ± 1.62 b | 10.31 ± 1.14 b | 10.70 ± 1.10 b | 10.640.64 b | ||
| ECG | Hyp | 16.96 ± 1.22 a | 11.35 ± 1.42 b | 11.19 ± 1.01 b | 10.37 ± 0.58 b | 10.29 ± 0.86 b | |
| Rad | 13.24 ± 0.92 a | 9.80 ± 0.97 b | 9.62 ± 0.60 b | 8.27 ± 0.50 b | 7.36 ± 0.82 b | ||
| CS | Hyp | 4.14 ± 0.56 a | 1.57 ± 0.65 b | 1.12 ± 0.47 b | 0.69 ± 0.23 b | 0.09 ± 0.05 b | |
| Rad | 3.88 ± 0.36 a | 0.88 ± 0.48 b | 0.72 ± 0.29 b | 0.54 ± 0.16 b | 0.01 ± 0.01 b | ||
| NG | Hyp | 4.72 ± 0.30 a | 1.82 ± 0.48 b | 1.31 ± 0.24 b | 1.15 ± 0.16 b | 0.65 ± 0.17 b | |
| Rad | 3.87 ± 0.23 a | 2.55 ± 0.34 b | 2.86 ± 0.09 b,c | 2.40 ± 0.16 b,c | 1.88 ± 0.12 c | ||
| * Dose | Control | 0.125 | 0.25 | 0.5 | 1 | ||
|---|---|---|---|---|---|---|---|
| G | To | Ger | 70.00 ± 5.48 a | 69.00 ± 6.60 a | 66.00 ± 7.97 a | 56.00 ± 5.79 a | 54.00 ± 3.32 a |
| Hyp | 12.13 ± 0.80 a | 8.76 ± 1.19 a,b | 7.60 ± 1.37 b | 3.32 ± 0.40 c | 2.85 ± 0.57 c | ||
| Rad | 13.64 ± 1.41 a | 10.88 ± 1.04 a,b | 8.67 ± 1.56 b,c | 6.12 ± 0.94 c,d | 3.41 ± 0.37 d | ||
| C | Ger | 98.00 ± 1.23 a | 95.00 ± 2.74 a | 97.00 ± 2.00 a | 96.00 ± 2.45 a | 91.00 ± 2.45 a | |
| Hyp | 10.34 ± 0.33 a | 10.48 ± 0.17 a | 10.10 ± 0.52 a | 11.23 ± 0.78 a | 11.75 ± 1.09 a | ||
| Rad | 18.61 ± 0.29 a | 16.16 ± 0.54 a,b | 16.57 ± 0.85 a,b | 14.77 ± 0.74 b | 14.62 ± 1.19 b | ||
| R | Ger | 97.00 ± 2.00 a | 91.00 ± 1.87 a | 94.00 ± 2.45 a | 92.00 ± 1.23 a | 91.00 ± 1.87 a | |
| Hyp | 19.75 ± 2.58 a | 21.78 ± 1.99 a | 25.07 ± 1.31 a | 20.05 ± 1.05 a | 19.01 ± 1.02 a | ||
| Dose | Control | 0.125 | 0.25 | 0.5 | 1 | ||
| T | To | Ger | 93.00 ± 1.23 a | 85.00 ± 5.24 a | 85.00 ± 5.24 a | 78.00 ± 5.39 a | 78.00 ± 5.15 a |
| Hyp | 12.64 ± 1.58 a | 9.91 ± 1.92 a | 8.62 ± 0.58 a | 7.03 ± 0.93 a | 8.77 ± 1.61 a | ||
| Rad | 18.13 ± 1.01 a | 14.52 ± 1.81 a | 14.35 ± 0.26 a | 15.66 ± 3.23 a | 10.11 ± 1.77 a | ||
| C | Ger | 98.00 ± 1.23 a | 92.00 ± 2.55 a | 96.00 ± 1.87 a | 100.00 ± 0.00 a | 97.00 ± 2.00 a | |
| Hyp | 10.34 ± 0.33 a | 10.38 ± 0.55 a | 10.42 ± 0.71 a | 9.57 ± 0.76 a | 9.67 ± 0.08 a | ||
| Rad | 18.61 ± 0.29 a | 17.61 ± 0.94 a | 17.67 ± 0.28 a | 17.00 ± 0.83 a | 16.12 ± 0.51 a | ||
| R | Ger | 97.00 ± 2.00 a | 92.00 ± 1.23 a | 94.00 ± 2.92 a | 94.00 ± 2.45 a | 96.00 ± 1.87 a | |
| Hyp | 19.75 ± 2.58 a | 25.18 ± 1.12 a | 26.83 ± 1.64 a | 22.15 ± 1.92 a | 21.19 ± 2.06 a | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibáñez, M.D.; Blázquez, M.A. Ginger and Turmeric Essential Oils for Weed Control and Food Crop Protection. Plants 2019, 8, 59. https://doi.org/10.3390/plants8030059
Ibáñez MD, Blázquez MA. Ginger and Turmeric Essential Oils for Weed Control and Food Crop Protection. Plants. 2019; 8(3):59. https://doi.org/10.3390/plants8030059
Chicago/Turabian StyleIbáñez, María Dolores, and María Amparo Blázquez. 2019. "Ginger and Turmeric Essential Oils for Weed Control and Food Crop Protection" Plants 8, no. 3: 59. https://doi.org/10.3390/plants8030059