Test Activities on Hybrid Rocket Engines: Combustion Analyses and Green Storable Oxidizers—A Short Review
Abstract
:1. Introduction
2. Hybrid Rocket Combustion
2.1. Fundamentals
2.2. Studies on Combustion Performance Enhancement
2.3. The O/F Shift, Condensed Combustion Products, and Soot
2.4. Environmental Impact
3. Green Storable Oxidizers
3.1. Nitrous Oxide
| Fuel | ar | nr | Ref. | Notes |
|---|---|---|---|---|
| SP-1a (Paraffin Wax) | 0.155 | 0.50 | [136] | |
| 0.178 | 0.50 | [107] | ||
| SP-7 | 0.078 | 0.545 | [117] | |
| Sasol 0907 w/o diaphragm | 0.132 | 0.56 | [72] | Commercial paraffin wax (microcrystalline): congealing point 356–367 K, assumed as C50H102. |
| Sasol 0907 w 1-hole diaphragm, pre-grain | 0.162 | 0.49 | [72] | |
| Sasol 0907 w 1-hole diaphragm, post-grain | 0.539 | 0.36 | [72] | |
| Sasol 0907 w 4-hole diaphragm, pre | 0.145 | 0.54 | [72] | |
| Sasol 0907 w 4-hole diaphragm, post | 0.293 | 0.52 | [72] | |
| Paraffin | 0.159 | 0.784 | [127] | |
| 50P | 0.115 | 0.504 | [124] | Fuel formulation with 50 wt.% Paraffin + 50 wt.% HTPB |
| 65P | 0.088 | 0.395 | [30] | Fuel formulation with 65 wt.% Paraffin + 15 wt.% HTPB + 4 wt.% PE + 5 wt.% Mg + 10 wt.% Al + 1 wt.% Copper Cromite |
| Paraffin + SEBS-MA | 0.072 | 0.77 | [36,130] | Macrocrystalline wax, 15 wt.% SEBS-MA |
| Sorbitol | 0.286 a | 0.310 a | [108] | |
| HTPB | 0.198 a | 0.325 a | [108] | |
| 0.417 a | 0.347 a | [109] | ||
| 0.008 | 0.77 | [95] | ||
| 0.020 | 1.09 | [36] | Vortex flow pancake (VFP) HRE operating in fuel-rich conditions. | |
| HDPE | 0.104 a | 0.352 a | [108] | |
| 0.248 a | 0.331 a | [109] | ||
| 0.013 | 0.875 | [137] | ||
| PMMA | 0.111 a | 0.377 a | [108] | |
| 0.284 a | 0.335 a | [109] | ||
| ABS | 0.007 b | 0.80 b | [95] |
3.2. Hydrogen Peroxide
| H2O2 Conc. | Fuel | ar | nr | Ref. | Notes a |
|---|---|---|---|---|---|
| 84% | Paraffin | 0.0344 | 0.9593 | [149] | |
| 85% | LDPE | 0.0061 | 0.78 | [150] | , |
| 0.0294 | 0.52 | , | |||
| 0.0419 | 0.49 | , | |||
| 87.5% | HDPE | 0.0066 | 0.8159 | [142] | |
| 87.5% | HDPE | 0.0003 | 1.32 | [137] | Axial with discrete divergent–convergent grain |
| 87.5% | PE | 0.0446 | 0.3288 | [151] | Vortex end-burning configuration |
| 88% | PE | 0.0072 | 0.8 | [152] | |
| 90% | PE | - | 0.45 | [153] | Single-port, rod-and-tube, telescope geometry |
| 90% | HTPB | 0.0402 | 0.5623 | [154] | |
| 90% | Paraffin blend | 0.279 a | 0.732 a | [155] | Fuel formulation with 50 wt.% Paraffin + 20 wt.% PE wax + 18 wt.% EVA + 10 wt.% SA + 2 wt.% Carbon |
| 90% | HTPB + 60 wt.% Al | 0.014 b | 0.7 b | [156] | |
| HTPB + 60 wt.% Al-Mg | 0.029 b | 0.6 b | |||
| 90% | DCPD | 0.057 b | 0.49 b | [157] | |
| HTPB | 0.060 b | 0.50 b | |||
| HTPB + 25 wt.% NaBH4 | 0.019 b | 0.73 b | |||
| HTPB + 50 wt.% NaBH4 | 0.008 b | 0.90 b | |||
| HTPB + 25 wt.% AlH3 | 0.037 b | 0.65 b | |||
| 90% | Paraffin | 0.145 | 0.5 | [158] | |
| 90% | HDPE | 0.0320 | 0.54 | [159] | |
| 95% | 0.0074 | 0.75 | |||
| 90% | HTPB | 0.0939 | 0.53 | [160] | |
| 98% | 0.0982 | 0.53 | |||
| 98% | HTPB + 20 wt.% Al | 0.0039 | 1.0433 | [161] | Solid propellant igniter |
| HTPB + 20 wt.% C14H10 + 20 wt.% Al | 0.0043 | 1.0336 | |||
| HTPB + 28 wt.% Al + 10 wt.% Mg + 2 wt.% C | 0.0267 | 0.7249 |
4. Conclusions and Future Developments
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| A-SOFT | Altering-intensity Swirling-Flow-Type |
| ABS | Acrylonitrile Butadiene Styrene |
| AMROC | American Rocket Company |
| AP | Ammonium Perchlorate |
| CCPs | Condensed Combustion Products |
| CFD | Computational Fluid Dynamics |
| CFP | Carbon Footprint |
| DARE | Delft Aerospace Rocket Engineering |
| GOX | Gaseous Oxygen |
| GWP | Global Warming Potential |
| HDPE | High-density Polyethylene |
| HEROS | Hybrid Experimental Rocket Stuttgart |
| HP | Hydrogen Peroxide |
| HRE | Hybrid Rocket Engine |
| HTP | High-Test Peroxide |
| HTPB | Hydroxyl-terminated Polybutadiene |
| iRF | Instantaneous Radiative Force |
| LH2 | Liquid Hydrogen |
| LOX | Liquid Oxygen |
| NA | Nitric Acid |
| NOx | Nitrogen Oxides |
| O/F | Oxidizer-to-fuel Ratio |
| PMMA | Polymethyl Methacrylate |
| RFNA | Red-fuming Nitric Acid |
| SPG | Space Propulsion Group |
| SPLab | Space Propulsion Laboratory |
| UV | Ultra Violet |
| VFP | Vortex Flow Pancake |
References
- Street, J.; Johnston, C.; Mansell, R.; Bloom, S. Environmental Interactions of Hydrazine Fuels in Soil/Water Systems; Final Report, March 1985–September 1987; Technical Report; Department of Soil Science, Florida University: Gainesville, FL, USA, 1988. [Google Scholar]
- Hanning-Lee, M.A.; Brady, B.B.; Martin, L.R.; Syage, J.A. Ozone decomposition on alumina: Implications for solid rocket motor exhaust. Geophys. Res. Lett. 1996, 23, 1961–1964. [Google Scholar] [CrossRef]
- Voigt, C.; Schumann, U.; Graf, K.; Gottschaldt, K.D. Impact of rocket exhaust plumes on atmospheric composition and climate—An overview. In Progress in Propulsion Physics; De Luca, L., Bonnel, K., Eds.; EDP Sciences: Les Ulis, France, 2013; Volume 4, Chapter 7; pp. 657–670. [Google Scholar] [CrossRef] [Green Version]
- Dallas, J.; Raval, S.; Gaitan, J.A.; Saydam, S.; Dempster, A. The environmental impact of emissions from space launches: A comprehensive review. J. Clean. Prod. 2020, 255, 120209:1–120209:12. [Google Scholar] [CrossRef]
- Ross, M.N.; Whitefield, P.D.; Hagen, D.E.; Hopkins, A.R. In situ measurement of the aerosol size distribution in stratospheric solid rocket motor exhaust plumes. Geophys. Res. Lett. 1999, 26, 819–822. [Google Scholar] [CrossRef]
- Haeseler, D.; Bombelli, V.; Vuillermoz, P.; Lo, R.; Marée, T.; Caramelli, F. Green propellant propulsion concepts for space transportation and technology development needs. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion (ESA SP-557), Cagliari, Italy, 7–8 June 2004. [Google Scholar]
- Ross, M.; Toohey, D.; Peinemann, M.; Ross, P. Limits on the space launch market related to stratospheric ozone depletion. Astropolitics 2009, 7, 50–82. [Google Scholar] [CrossRef] [Green Version]
- Chiaverini, M. Review of solid-fuel regression rate behavior in classical and nonclassical hybrid rocket motors. In Fundamentals of Hybrid Rocket Combustion and Propulsion, 1st ed.; Chiaverini, M., Kuo, K., Eds.; AIAA: Reston, VA, USA, 2007; Chapter 2; pp. 37–126. [Google Scholar] [CrossRef]
- Karabeyoglu, M.; Altman, D.; Cantwell, B.J. Combustion of liquefying hybrid propellants: Part 1, general theory. J. Propuls. Power 2002, 18, 610–620. [Google Scholar] [CrossRef]
- Karabeyoglu, M.; Cantwell, B.J. Combustion of liquefying hybrid propellants: Part 2, stability of liquid films. J. Propuls. Power 2002, 18, 621–630. [Google Scholar] [CrossRef]
- Veale, K.; Adali, S.; Pitot, J.; Brooks, M. A review of the performance and structural considerations of paraffin wax hybrid rocket fuels with additives. Acta Astronaut. 2017, 141, 196–208. [Google Scholar] [CrossRef]
- DeLuca, L.; Galfetti, L.; Maggi, F.; Colombo, G.; Merotto, L.; Boiocchi, M.; Paravan, C.; Reina, A.; Tadini, P.; Fanton, L. Characterization of HTPB-based solid fuel formulations: Performance, mechanical properties, and pollution. Acta Astronaut. 2013, 92, 150–162. [Google Scholar] [CrossRef]
- Okninski, A.; Kopacz, W.; Kaniewski, D.; Sobczak, K. Hybrid rocket propulsion technology for space S revisited-propellant solutions and challenges. FirePhysChem 2021, 1, 260–271. [Google Scholar] [CrossRef]
- Altman, D.; Holzman, A. Overview and history of hybrid rocket propulsion. In Fundamentals of Hybrid Rocket Combustion and Propulsion, 1st ed.; Chiaverini, M., Kuo, K., Eds.; AIAA: Reston, VA, USA, 2007; Chapter 1; pp. 1–36. [Google Scholar] [CrossRef]
- Marquardt, T.; Majdalani, J. Review of classical diffusion-limited regression rate models in hybrid rockets. Aerospace 2019, 6, 75. [Google Scholar] [CrossRef] [Green Version]
- Marxman, G.; Woolridge, C. Advances in tactical rocket propulsion. In Proceedings of the AGARD Colloquium on Progress in Tactical Rocket Propulsion, La Jolla, CA, USA, 22–23 April 1965. [Google Scholar]
- Marxman, G.; Wooldridge, C.; Muzzy, R. Fundamentals of hybrid boundary-layer combustion. In Heterogeneous Combustion; Wolfhard, H., Glassman, I., Green, L., Eds.; AIAA: Reston, VA, USA, 1964; Volume 15, pp. 485–521. [Google Scholar] [CrossRef]
- Marxman, G.; Gilbert, M. Turbulent boundary layer combustion in the hybrid rocket. Symp. (Int.) Combust. 1963, 9, 371–383. [Google Scholar] [CrossRef]
- Strand, L.; Jones, M.; Ray, R.; Cohen, N. Characterization of hybrid rocket internal heat flux and HTPB fuel pyrolysis. In Proceedings of the 30th Joint Propulsion Conference and Exhibit, Indianapolis, IN, USA, 27–29 June 1994. [Google Scholar] [CrossRef]
- Chiaverini, M.; Kuo, K.; Peretz, A.; Harting, G.; Chiaverini, M.; Kuo, K.; Peretz, A.; Harting, G. Heat flux and internal ballistic characterization of a hybrid rocket motor analog. In Proceedings of the 33rd Joint Propulsion Conference and Exhibit, Seattle, WA, USA, 6–9 July 1997. [Google Scholar] [CrossRef]
- Estey, P.; Altman, D.; McFarlane, J. An evaluation of scaling effects for hybrid rocket motors. In Proceedings of the 27th Joint Propulsion Conference, Sacramento, CA, USA, 24–26 June 1991. [Google Scholar] [CrossRef]
- Risha, G.; Evans, B.; Boyer, E.; Kuo, K. Metals, energetic additives, and special binders used in solid fuels for hybrid rockets. In Fundamentals of Hybrid Rocket Combustion and Propulsion, 1st ed.; Chiaverini, M., Kuo, K., Eds.; AIAA: Reston, VA, USA, 2007; Chapter 10; pp. 413–456. [Google Scholar] [CrossRef]
- Paravan, C. Nano-sized and mechanically activated composites: Perspectives for enhanced mass burning rate in aluminized solid fuels for hybrid rocket propulsion. Aerospace 2019, 6, 127. [Google Scholar] [CrossRef] [Green Version]
- Pal, Y.; Mahottamananda, S.N.; Palateerdham, S.K.; Subha, S.; Ingenito, A. Review on the regression rate-improvement techniques and mechanical performance of hybrid rocket fuels. FirePhysChem 2021, 1, 272–282. [Google Scholar] [CrossRef]
- Péres de Araújo, E.; José Maschio, L.; Henrique Gouvêa, L.; Gustavo Ferroni Pereira, L.; Vieira, R. Thermal, Viscosimetric and Thermomechanical Combined Assessment of Mixture Modelled Composite Fuels for Hybrid Propulsion. Propellants Explos. Pyrotech. 2022, 47, e202100314. [Google Scholar] [CrossRef]
- Frederick Jr, R.A.; Whitehead, J.J.; Knox, L.R.; Moser, M.D. Regression rates study of mixed hybrid propellants. J. Propuls. Power 2007, 23, 175–180. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Walker, S.D.; Merkley, D.P.; Sobbi, M. High regression rate hybrid rocket fuel grains with helical port structures. J. Propuls. Power 2015, 31, 1727–1738. [Google Scholar] [CrossRef] [Green Version]
- Story, G. Large-scale hybrid motor testing. In Fundamentals of Hybrid Rocket Combustion and Propulsion, 1st ed.; Chiaverini, M., Kuo, K., Eds.; AIAA: Reston, VA, USA, 2007; Chapter 13; pp. 513–552. [Google Scholar] [CrossRef] [Green Version]
- Jens, E.T.; Karp, A.C.; Miller, V.A.; Hubbard, G.S.; Cantwell, B.J. Experimental Visualization of Hybrid Combustion: Results at Elevated Pressures. J. Propuls. Power 2020, 36, 33–46. [Google Scholar] [CrossRef]
- Liu, L.l.; He, X.; Wang, Y.; Chen, Z.b.; Guo, Q. Regression rate of paraffin-based fuels in hybrid rocket motor. Aerosp. Sci. Technol. 2020, 107, 106269. [Google Scholar] [CrossRef]
- Carrick, P.; Larson, C. Lab scale test and evaluation of cryogenic solid hybrid rocket fuels. In Proceedings of the 31st Joint Propulsion Conference and Exhibit, San Diego, CA, USA, 10–12 July 1995. [Google Scholar] [CrossRef]
- Petrarolo, A.; Kobald, M.; Schlechtriem, S. Visualization of Combustion Phenomena in Paraffin-Based Hybrid Rocket Fuels at Super-Critical Pressures. In Proceedings of the 2018 Joint Propulsion Conference, Cincinnati, OH, USA, 9 November 2018. [Google Scholar] [CrossRef]
- Debus, C.; Ruettgers, A.; Petrarolo, A.; Kobald, M.; Siggel, M. High-performance data analytics of hybrid rocket fuel combustion data using different machine learning approaches. In Proceedings of the AIAA Scitech 2020 Forum, Orlando, FL, USA, 6–10 January 2020. [Google Scholar] [CrossRef]
- Kobald, M.; Ciezki, H.K.; Schlechtriem, S.; Toson, E.; De Luca, L. Evaluation of paraffin-based fuels for hybrid rocket engines. In Proceedings of the 50th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014. [Google Scholar] [CrossRef]
- Paravan, C.; Galfetti, L.; Maggi, F. A critical analysis of paraffin-based fuel formulations for hybrid rocket propulsion. In Proceedings of the 53rd AIAA/SAE/ASEE Joint Propulsion Conference, Atlanta, GA, USA, 10–12 July 2017. [Google Scholar] [CrossRef]
- Paravan, C.; Galfetti, L.; Bisin, R.; Piscaglia, F. Combustion processes in hybrid rockets. Int. J. Energ. Mater. Chem. Propuls. 2019, 18, 255–286. [Google Scholar] [CrossRef]
- Kobald, M.; Schmierer, C.; Ciezki, H.; Schlechtriem, S.; Toson, E.; De Luca, L. Viscosity and regression rate of liquefying hybrid rocket fuels. J. Propuls. Power 2017, 33, 1245–1251. [Google Scholar] [CrossRef]
- Tang, Y.; Chen, S.; Zhang, W.; Shen, R.; DeLuca, L.T.; Ye, Y. Mechanical modifications of paraffin-based fuels and the effects on combustion performance. Propellants Explos. Pyrotech. 2017, 42, 1268–1277. [Google Scholar] [CrossRef]
- Battista, F.; Cardillo, D.; Fragiacomo, M.; Di Martino, G.D.; Mungiguerra, S.; Savino, R. Design and testing of a paraffin-based 1000 N HRE breadboard. Aerospace 2019, 6, 89. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, I.; Hikone, S. Study on the regression rate of paraffin-based hybrid rocket fuels. J. Propuls. Power 2011, 27, 1276–1279. [Google Scholar] [CrossRef]
- Kim, S.; Moon, H.; Kim, J.; Cho, J. Evaluation of paraffin–polyethylene blends as novel solid fuel for hybrid rockets. J. Propuls. Power 2015, 31, 1750–1760. [Google Scholar] [CrossRef]
- Kumar, R.; Ramakrishna, P.A. Studies on EVA-Based Wax Fuel for Launch Vehicle Applications. Propellants Explos. Pyrotech. 2016, 41, 295–303. [Google Scholar] [CrossRef]
- Mengu, D.; Kumar, R. Development of EVA-SEBS based wax fuel for hybrid rocket applications. Acta Astronaut. 2018, 152, 325–334. [Google Scholar] [CrossRef]
- Thomas, J.C.; Paravan, C.; Stahl, J.M.; Tykol, A.J.; Rodriguez, F.A.; Galfetti, L.; Petersen, E.L. Experimental evaluation of HTPB/paraffin fuel blends for hybrid rocket applications. Combust. Flame 2021, 229, 111386. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, S.q.; Liu, X.l.; Liu, L.l. Boundary layer combustion of HTPB/paraffin fuels for hybrid propulsion applications. Aerosp. Sci. Technol. 2022, 129, 107850:1–107850:8. [Google Scholar] [CrossRef]
- Bisin, R.; Paravan, C.; Alberti, S.; Galfetti, L. A new strategy for the reinforcement of paraffin-based fuels based on cellular structures: The armored grain—mechanical characterization. Acta Astronaut. 2020, 176, 494–509. [Google Scholar] [CrossRef]
- Bisin, R.; Paravan, C. A new strategy for the reinforcement of paraffin-based fuels based on cellular structures: The armored grain — Ballistic characterization. Acta Astronaut. 2023, 206, 284–298. [Google Scholar] [CrossRef]
- Thomas, J.C.; Rodriguez, F.A.; Petersen, E.L. Metallic Additives for Solid-Fuel Propulsion Applications. Combust. Sci. Technol. 2023, 195, 1279–1298. [Google Scholar] [CrossRef]
- Karabeyoğlu, A. Performance additives for hybrid rockets. In Chemical Rocket Propulsion; De Luca, L.T., Shimada, T., Sinditskii, V.P., Calabro, M., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 139–163. [Google Scholar] [CrossRef]
- Maggi, F.; Gariani, G.; Galfetti, L.; DeLuca, L.T. Theoretical analysis of hydrides in solid and hybrid rocket propulsion. Int. J. Hydrogen Energy 2012, 37, 1760–1769. [Google Scholar] [CrossRef]
- Paravan, C.; Verga, A.; Maggi, F.; Galfetti, L. Accelerated ageing of micron-and nano-sized aluminum powders: Metal content, composition and non-isothermal oxidation reactivity. Acta Astronaut. 2019, 158, 397–406. [Google Scholar] [CrossRef]
- Hermsen, R. Aluminum oxide particle size for solid rocket motor performance prediction. J. Spacecr. Rockets 1981, 18, 483–490. [Google Scholar] [CrossRef]
- Carlotti, S.; Maggi, F. Experimental techniques for characterization of particles in plumes of sub-scale solid rocket motors. Acta Astronaut. 2021, 186, 496–507. [Google Scholar] [CrossRef]
- Marothiya, G.; Ramakrishna, P. Utilization of mechanically activated aluminum in hybrid rockets. J. Propuls. Power 2018, 34, 1206–1213. [Google Scholar] [CrossRef]
- Connell Jr, T.L.; Yetter, R.A.; Risha, G.A.; Huba, Z.J.; Epshteyn, A.; Fisher, B.T. Enhancement of Solid Fuel Combustion in a Hybrid Rocket Motor Using Amorphous Ti–Al–B Nanopowder Additives. J. Propuls. Power 2019, 35, 662–665. [Google Scholar] [CrossRef]
- Connell, T.L., Jr.; Risha, G.A.; Yetter, R.A.; Roberts, C.W.; Young, G. Boron and polytetrafluoroethylene as a fuel composition for hybrid rocket applications. J. Propuls. Power 2015, 31, 373–385. [Google Scholar] [CrossRef]
- Pal, Y.; Kumar, V.R. Physical and Ballistic Characterization of Aluminum-Loaded Paraffin Hybrid Rocket Fuels. Energy Fuels 2017, 31, 10133–10143. [Google Scholar] [CrossRef]
- Lee, C.; Na, Y.; Lee, G. The enhancement of regression rate of hybrid rocket fuel by helical grain configuration and swirl flow. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar] [CrossRef]
- Saburo, Y.; Noriko, S.; Kousuke, H. Controlling parameters for fuel regression rate of swirling-oxidizer-flow-type hybrid rocket engine. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Franco, M.; Barato, F.; Paccagnella, E.; Santi, M.; Battiston, A.; Comazzetto, A.; Pavarin, D. Regression rate design tailoring through vortex injection in hybrid rocket motors. J. Spacecr. Rockets 2020, 57, 278–290. [Google Scholar] [CrossRef]
- Paccagnella, E.; Barato, F.; Pavarin, D.; Karabeyoğlu, A. Scaling parameters of swirling oxidizer injection in hybrid rocket motors. J. Propuls. Power 2017, 33, 1378–1394. [Google Scholar] [CrossRef]
- Kumar, C.P.; Kumar, A. Effect of swirl on the regression rate in hybrid rocket motors. Aerosp. Sci. Technol. 2013, 29, 92–99. [Google Scholar] [CrossRef]
- Paravan, C.; Glowacki, J.; Carlotti, S.; Maggi, F.; Galfetti, L. Vortex combustion in a lab-scale hybrid rocket motor. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016. [Google Scholar] [CrossRef]
- Sakote, R.; Yadav, N.; Karmakar, S.; Joshi, P.C.; Chatterjee, A.K. Regression Rate Studies of Paraffin Wax-HTPB Hybrid Fuels Using Swirl Injectors. Propellants Explos. Pyrotech. 2014, 39, 859–865. [Google Scholar] [CrossRef]
- Haag, G.S. Alternative Geometry Hybrid Rockets for Spacecraft Orbit Transfer; University of Surrey: Guildford, UK, 2001; pp. 1–24. [Google Scholar]
- Saito, D.; Saburo, Y.; Hirata, K.; Sakurai, T.; Shiraishi, N. Combustion characteristics of paraffin-fueled swirling oxidizer-flow-type hybrid rocket engines. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Shinohara, K.; Nakagawa, I. Regression rate characteristics of paraffin-based fuel under swirled oxidizer flow. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Quadros, F.D.; Lacava, P.T. Swirl injection of gaseous oxygen in a lab-scale paraffin hybrid rocket motor. J. Propuls. Power 2019, 35, 896–905. [Google Scholar] [CrossRef]
- Ozawa, K.; Kitagawa, K.; Aso, S.; Shimada, T. Hybrid Rocket Firing Experiments at Various Axial–Tangential Oxidizer-Flow-Rate Ratios. J. Propuls. Power 2019, 35, 94–108. [Google Scholar] [CrossRef]
- Messineo, J.; Shimada, T. Theoretical investigation on feedback control of hybrid rocket engines. Aerospace 2019, 6, 65. [Google Scholar] [CrossRef] [Green Version]
- Ozawa, K.; Shimada, T. Performance of Mixture-Ratio-Controlled Hybrid Rockets for Nominal Fuel Regression. J. Propuls. Power 2020, 36, 400–414. [Google Scholar] [CrossRef]
- Grosse, M. Effect of a diaphragm on performance and fuel regression of a laboratory scale hybrid rocket motor using nitrous oxide and paraffin. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Denver, CO, USA, 2–5 August 2009. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Walker, S.D. Engineering model for hybrid fuel regression rate amplification using helical ports. J. Propuls. Power 2017, 33, 398–407. [Google Scholar] [CrossRef]
- Nagata, H.; Ito, M.; Maeda, T.; Watanabe, M.; Uematsu, T.; Totani, T.; Kudo, I. Development of CAMUI Hybrid Rocket to Create a Market for Small Rocket Experiments. Acta Astronaut. 2006, 59, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Viscor, T.; Kamps, L.; Yonekura, K.; Isochi, H.; Nagata, H. Large-scale CAMUI type hybrid rocket motor scaling, modeling, and test results. Aerospace 2021, 9, 1. [Google Scholar] [CrossRef]
- Karabeyoğlu, A.; Toson, E.; Evans, B. Effect of “O/F Shift” on Combustion Efficiency. In Proceedings of the 50th AIAA/ASME/SAE/ ASEE Joint Propulsion Conference, Cleveland, OH, USA, 28–30 July 2014. [Google Scholar] [CrossRef]
- Teipel, U.; Förter-Barth, U. Rheology of nano-scale aluminum suspensions. Propellants Explos. Pyrotech. 2001, 26, 268–272. [Google Scholar] [CrossRef]
- Aphale, S.S.; Budzinski, K.; Surina III, G.; DesJardin, P.E. Influence of O2/N2 oxidizer blends on soot formation and radiative heat flux in PMMA-air 2D slab burner for understanding hybrid rocket combustion. Comb. Flame 2021, 234, 111628. [Google Scholar] [CrossRef]
- Whitmore, S.A. Plume Contamination Measurements of an Additively Printed, Green-Propellant Hybrid Thruster. J. Propuls. Power 2022, 38, 671–685. [Google Scholar] [CrossRef]
- Wilcox, L.; Shine, K.; Hoskins, B. Radiative forcing due to aviation water vapour emissions. Atmos. Environ. 2012, 63, 1–13. [Google Scholar] [CrossRef]
- IPCC Report AR6. Available online: https://report.ipcc.ch/ar6/wg3/IPCC_AR6_WGIII_Full_Report.pdf (accessed on 20 February 2023).
- Casalino, L.; Ferrero, A.; Masseni, F.; Pastrone, D. Emission-Driven Hybrid Rocket Engine Optimization for Small Launchers. Aerospace 2022, 9, 807. [Google Scholar] [CrossRef]
- Ruijgrok, G.J.; Van Paassen, D. Elements of Aircraft Pollution; Delft University Press: Delft, The Netherlands, 2005; pp. 1–410. [Google Scholar]
- Malkin, M.S. Environmental Impact Statement for the Space Shuttle Program; Final Statement, April 1978; Technical Report for National Aeronautics and Space Administration (NASA): Washington, DC, USA, 1978. [Google Scholar]
- Potter, A.E. Proceedings of the Space Shuttle Environmental Assessment Workshop on Stratospheric Effects; Technical Memorandum, January 1977; Technical Report for National Aeronautics and Space Administration (NASA): Washington, DC, USA, 1977. [Google Scholar]
- Potter, A.E. Environmental effects of the Space Shuttle. J. Environ. Sci. 1978, 21, 15–21. [Google Scholar]
- Leone, D.; Turns, S. Active chlorine and nitric oxide formation from chemical rocket plume afterburning. In Proceedings of the 32nd Aerospace Sciences Meeting and Exhibit, Reno, NV, USA, 10–13 January 1994. [Google Scholar] [CrossRef] [Green Version]
- Ross, M.N.; Sheaffer, P.M. Radiative forcing caused by rocket engine emissions. Earth’s Future 2014, 2, 177–196. [Google Scholar] [CrossRef]
- “HyImpulse” Website. Available online: https://www.hyimpulse.de/en/projects/3-project-hyplox75 (accessed on 6 October 2022).
- “T4i—Technology for Propulsion and Innovation” Website. Available online: https://www.t4innovation.com/teide-hybrid-propellant/ (accessed on 6 October 2022).
- Faenza, M.; Boiron, A.J.; Haemmerli, B.; Verberne, C.J. The nammo nucleus launch: Norwegian hybrid sounding rocket over 100km. In Proceedings of the AIAA Propulsion and Energy 2019 Forum, Indianapolis, IN, USA, 19–22 August 2019. [Google Scholar] [CrossRef]
- Heister, S.; Wernimont, E. Hydrogen peroxide, hydroxyl ammonium nitrate, and other storable oxidizers. In Fundamentals of Hybrid Rocket Combustion and Propulsion, 1st ed.; Chiaverini, M., Kuo, K., Eds.; AIAA: Reston, VA, USA, 2007; Chapter 11; pp. 457–488. [Google Scholar] [CrossRef]
- Nosseir, A.E.; Cervone, A.; Pasini, A. Review of state-of-the-art green monopropellants: For propulsion systems analysts and designers. Aerospace 2021, 8, 20. [Google Scholar] [CrossRef]
- Okninski, A.; Surmacz, P.; Bartkowiak, B.; Mayer, T.; Sobczak, K.; Pakosz, M.; Kaniewski, D.; Matyszewski, J.; Rarata, G.; Wolanski, P. Development of green storable hybrid rocket propulsion technology using 98% hydrogen peroxide as oxidizer. Aerospace 2021, 8, 234. [Google Scholar] [CrossRef]
- Whitmore, S.A. Nytrox as “drop-in” replacement for gaseous oxygen in SmallSat hybrid propulsion systems. Aerospace 2020, 7, 43. [Google Scholar] [CrossRef] [Green Version]
- C.C.O.H. Safety—“Oxidizers”. Available online: https://safety.fsu.edu/safety_manual/oxidizers.pdf (accessed on 6 October 2022).
- Thermophysical Properties of Fluid Systems, NIST Chemistry WebBook. Available online: https://webbook.nist.gov/chemistry/fluid/ (accessed on 21 April 2023).
- Kamps, L.; Sakurai, K.; Saito, Y.; Nagata, H. Comprehensive data reduction for N2O/HDPE hybrid rocket motor performance evaluation. Aerospace 2019, 6, 45. [Google Scholar] [CrossRef] [Green Version]
- U.S. Environmental Protection Agency. “Greenhouse Gas Overview”. Available online: https://www.epa.gov/enviro/greenhouse-gas-overview (accessed on 6 October 2022).
- Zakirov, V.; Sweeting, M.; Goeman, V.; Lawrence, T. Surrey research on nitrous oxide catalytic decomposition for space applications. In Proceedings of the 14th AIAA/USU Conference on Small Satellites, Logan, UT, USA, 21–24 August 2000. [Google Scholar]
- Zakirov, V.; Sweeting, M.; Lawrence, T.; Sellers, J. Nitrous oxide as a rocket propellant. Acta Astronaut. 2001, 48, 353–362. [Google Scholar] [CrossRef]
- Kaiyang, L.; Jie, F.; Wei, S.; Bing, S.; Guobiao, C. Design and experimental research of a sub-Newton N2O monopropellant thruster with inner-heater. Chinese J. Aeronaut. 2022, 35, 309–318. [Google Scholar]
- Wallbank, J.; Sermon, P.; Baker, A.; Courtney, L.; Sambrook, R. Nitrous oxide as a green monopropellant for small satellites. In Proceedings of the 2nd International Conference on Green Propellants for Space Propulsion (ESA SP-557), Cagliari, Italy, 7–8 June 2004. [Google Scholar]
- Carlotti, S.; Maggi, F. Evaluating New Liquid Storable Bipropellants: Safety and Performance Assessments. Aerospace 2022, 9, 561. [Google Scholar] [CrossRef]
- Kobald, M.; Fischer, U.; Tomilin, K.; Petrarolo, A.; Schmierer, C. Hybrid experimental rocket stuttgart: A low-cost technology demonstrator. J. Spacecr. Rockets 2018, 55, 484–500. [Google Scholar] [CrossRef]
- Sportillo, A. N2O Thermal Decomposition: Reaction Kinetics and Design of a Dedicated Experimental Setup. Master’s Thesis, Politecnico di Milano, Milan, Italy, 3 October 2018. [Google Scholar]
- VanPelt, D.; Skinner, M.; Buchanan, A.; Gulman, R.; Chan, H.; Hopkins, J.; Karabeyoglu, M.A. Overview of a 4-inch OD paraffin-based hybrid sounding rocket program. In Proceedings of the 40th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Fort Lauderdale, FL, USA, 11–14 July 2004. [Google Scholar] [CrossRef]
- Lohner, K.; Dyer, J.; Doran, E.; Dunn, Z.; Zilliac, G. Fuel regression rate characterization using a laboratory scale nitrous oxide hybrid propulsion system. In Proceedings of the 42nd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Sacramento, CA, USA, 9–12 July 2006. [Google Scholar] [CrossRef]
- Doran, E.; Dyer, J.; Lohner, K.; Dunn, Z.; Cantwell, B.; Zilliac, G. Nitrous oxide hybrid rocket motor fuel regression rate characterization. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar] [CrossRef]
- McCormick, A.; Hultgren, E.; Lichtman, M.; Smith, J.; Sneed, R.; Azimi, S. Design, Optimization, and Launch of a 3" Diameter N2O/Aluminized Paraffin Rocket. In Proceedings of the 41st AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Tucson, AZ, USA, 10–13 July 2005. [Google Scholar] [CrossRef]
- Dyer, J.; Doran, E.; Dunn, Z.; Lohner, K.; Bayart, C.; Sadhwani, A.; Zilliac, G.; Cantwel, B.; Karabeyoglu, A. Design and development of a 100km nitrous oxide/paraffin hybrid rocket vehicle. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar]
- Dunn, Z.; Dyer, J.; Lohner, K.; Doran, E.; Bayart, C.; Sadhwani, A.; Zilliac, G.; Karabeyoglu, A.; Cantwell, B. Test facility development for the 15,000 lb thrust peregrine hybrid sounding rocket. In Proceedings of the 43rd AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Cincinnati, OH, USA, 8–11 July 2007. [Google Scholar]
- Dyer, J.; Doran, E.; Zilliac, G.; Lohner, K.; Cantwell, B.; Karabeyoglu, A.; Marzona, M.; Karlik, E. Status Update for the Peregrine 100km Sounding Rocket Project. In Proceedings of the 44th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Hartford, CT, USA, 21–23 July 2008. [Google Scholar]
- Doran, E.; Dyer, J.; Marzona, M.; Karabayaglu, A.; Zilliac, G.; Mosher, R.; Cantwell, B. Status update report for the Peregrine sounding rocket project: Part III. In Proceedings of the 45th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Denver, CO, USA, 2–5 August 2009. [Google Scholar] [CrossRef]
- Zilliac, G.; Waxman, B.; Doran, E.; Dyer, J.; Karabeyoglu, A.; Cantwell, B. Peregrine hybrid rocket motor ground test results. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Simurda, L.; Stober, K.; Boiron, A.; Hornstein, K.; Jens, E.; Fletcher, A. Design and development of a thrust vector controlled Paraffin/Nytrox hybrid rocket. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar]
- Kara, O.; Karakaş, H. Hybrid rockets with mixed N2O/CO2 oxidizers for Mars Ascent Vehicles. Acta Astronaut. 2020, 175, 254–267. [Google Scholar] [CrossRef]
- Fraters, A.; Zandbergen, B.; Weustink, A.; Eiche, M.; Gerth, I.; Hermsen, R.; Huijsman, V.; Knop, T.; Powell, S.; Wildvank, R. Development of a Hybrid Rocket Engine for the Stratos II Project. In Proceedings of the 62nd International Astronautical Congress (IAC), Cape Town, South Africa, 3–7 October 2011. [Google Scholar]
- Knop, T.; Zandbergen, B.; Cervone, A.; Huijsman, R.; Powell, S.; Werner, R.; Ehlen, J.; Lindemann, F.; Wink, J.; Becker, C.; et al. Sorbitol-Based Hybrid Fuel Studies with Nitrous Oxide for the Stratos II Sounding Rocket. In Proceedings of the 49th AIAA/ASME/SAE/ASEE Joint Propulsion Conference, San Jose, CA, USA, 14–17 July 2013. [Google Scholar] [CrossRef]
- Fraters, A.; Cervone, A. Combustion Instabilities and Spontaneous Engine Operation Shifting in a High Mass Flux N2O-PMMA Hybrid Rocket Engine. In Proceedings of the AIAA 2014 Space Propulsion Conference and Exhibition, Cologne, Germany, 19–22 May 2014. [Google Scholar]
- Wink, J.; Cervone, A.; Knop, T.; Huijsman, R.; Powell, S.; Samarawickrama, K.; Fraters, A.; Werner, R.; Becker, C. Test Campaign on a 10 kN class sorbitol-based hybrid rocket motor for the Stratos II Sounding Rocket. In Proceedings of the AIAA 2014 Space Propulsion Conference and Exhibition, Cologne, Germany, 19–22 May 2014. [Google Scholar]
- Knop, T.; Wink, J.; Huijsman, R.; Werner, R.; Ehlen, J.; Powell, S.; Zandbergen, B.; Cervone, A. Failure mode investigation of a sorbitol-based hybrid rocket flight motor for the Stratos II sounding rocket. In Proceedings of the 51st AIAA/SAE/ASEE Joint Propulsion Conference, Orlando, FL, USA, 27–29 July 2015. [Google Scholar] [CrossRef]
- Van den Berg, P.; Barreiro, F.; Klop, C.L.; Van Strydonck, D.; Koehler, S. Development of a 25kN Hybrid Rocket Engine for the Stratos III Sounding Rocket. In Proceedings of the 69th International Astronautical Congress (IAC), Bremen, Germany, 1–5 October 2018. [Google Scholar]
- Lee, T.S.; Tsai, H.L. Fuel regression rate in a paraffin-HTPB nitrous oxide hybrid rocket. In Proceedings of the 7th Asia-Pacific Conference on Combustion (ASPACC), Taipei, Taiwan, 24–27 May 2009. [Google Scholar]
- Bettella, A.; Lazzarin, M.; Bellomo, N.; Barato, F.; Pavarin, D.; Grosse, M. Testing and CFD simulation of diaphragm hybrid rocket motors. In Proceedings of the 47th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, San Diego, CA, USA, 31 July–3 August 2011. [Google Scholar] [CrossRef]
- Bouziane, M.; Bertoldi, A.; Milova, P.; Hendrick, P.; Lefebvre, M. Performance comparison of oxidizer injectors in a 1-kN paraffin-fueled hybrid rocket motor. Aerosp. Sci. Technol. 2019, 89, 392–406. [Google Scholar] [CrossRef]
- Bouziane, M.; Bertoldi, A.M.; Hendrick, P.; Lefebvre, M. Experimental investigation of the axial oxidizer injectors geometry on a 1-kN paraffin-fueled hybrid rocket motor. FirePhysChem 2021, 1, 231–243. [Google Scholar] [CrossRef]
- Bellomo, N.; Faenza, M.; Barato, F.; Bettella, A.; Pavarin, D.; Selmo, A. The "Vortex Reloaded" project: Experimental investigation on fully tangential vortex injection in N2O-paraffin hybrid motors. In Proceedings of the 48th AIAA/ASME/SAE/ASEE Joint Propulsion Conference & Exhibit, Atlanta, GA, USA, 30 July–1 August 2012. [Google Scholar] [CrossRef]
- Nardozzo, P.K.; Connell, T.L., Jr.; Boyer, E.; Yetter, R.; Young, G. Diffusion flame studies of solid fuels with nitrous oxide. Int. J. Energetic Mater. Chem. Propuls. 2020, 19, 73–93. [Google Scholar] [CrossRef]
- Carmicino, C.; Scaramuzzino, F.; Sorge, A.R. Trade-off between paraffin-based and aluminium-loaded HTPB fuels to improve performance of hybrid rocket fed with N2O. Aerosp. Sci. Technol. 2014, 37, 81–92. [Google Scholar] [CrossRef]
- Carmicino, C. Acoustics, vortex shedding, and low-frequency dynamics interaction in an unstable hybrid rocket. J. Propuls. Power 2009, 25, 1322–1335. [Google Scholar] [CrossRef]
- Karabeyoglu, M.A. Nitrous oxide and oxygen mixtures (nytrox) as oxidizers for rocket propulsion applications. J. Propuls. Power 2014, 30, 696–706. [Google Scholar] [CrossRef]
- Rhodes, G. Investigation of Decomposition Characteristics of Gaseous and Liquid Nitrous Oxide; Final Report, August–December 1973; Technical Report for Air Force Weapons Laboratory: Kirtland Air Force Base, NM, USA, 1974. [Google Scholar]
- Whitmore, S.A.; Frischkorn, C.I. Analyzing and Reducing Ignition Latency of a Nytrox/ABS Hybrid Propulsion System. In Proceedings of the AIAA Propulsion and Energy 2020 Forum, Virtual, 24–28 August 2020. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Stoddard, R.L. N2O/O2 blends safe and volumetrically efficient oxidizers for small spacecraft hybrid propulsion. Aeron. Aero. Open Access J. 2019, 3, 171–196. [Google Scholar] [CrossRef]
- Karabeyoglu, A.; Zilliac, G.; Castellucci, P.; Urbanczyk, P.; Inalhan, G.; Cantwell, B. Flight Demonstration of the High Burning Rate Hydrocarbon-Based Hybrid Rocket Fuels. In Proceedings of the 39th AIAA/ASME/SAE/ASEE Joint Propulsion Conference and Exhibit, Huntsville, AL, USA, 20–23 July 2003. [Google Scholar] [CrossRef]
- Glaser, C.; Gelain, R.; Bertoldi, A.; Levard, Q.; Hijlkema, J.; Lestrade, J.Y.; Hendrick, P.; Anthoine, J. Experimental regression rate profiles of stepped fuel grains in Hybrid Rocket Engines. Acta Astronaut. 2023, 204, 186–198. [Google Scholar] [CrossRef]
- Whitmore, S.A.; Armstrong, I.W.; Heiner, M.C.; Martinez, C.J. High-performing hydrogen peroxide hybrid rocket with 3-D printed and extruded ABS fuel. In Proceedings of the 2018 Joint Propulsion Conference, Cincinnati, OH, USA, 9–11 July 2018. [Google Scholar] [CrossRef]
- Gilmour Space Technologies. Eris Rocket. Available online: https://www.gspace.com/launch (accessed on 19 April 2023).
- TriasRnD. ILR-33 AMBER 2K Rocket. Available online: https://triasrnd.com/l/358-ilr-33-amber-2k-rocket (accessed on 19 April 2023).
- Nammo Space. Nucleus Sounding Rocket. Available online: https://www.nammo.com/product/nucleus-sounding-rocket/ (accessed on 19 April 2023).
- Granado, E.Q.; Pelenghi, G.; Hijlkema, J.; Anthoine, J.; Lestrade, J.Y. A new System Design Tool for a Hybrid Rocket Engine Application. In Proceedings of the 73rd International Astronautical Congress (IAC), Paris, France, 18–22 September 2022. [Google Scholar]
- Meng, X.; Tian, H.; Zhu, H.; Wang, Z.; Yu, R.; Guo, Z.; Cai, G. Effects of aluminum and aluminum hydride additives on the performance of hybrid rocket motors based on 95% hydrogen peroxide. Aerosp. Sci. Technol. 2022, 130, 107914:1–107914:9. [Google Scholar] [CrossRef]
- Zhao, Z.; Cai, G.; Zhao, B.; Liu, Y.; Yu, N. Experimental investigation of a flow-oriented throttleable injector designed for throttleable hybrid rocket motor. Acta Astronaut. 2022, 192, 122–132. [Google Scholar] [CrossRef]
- Zhu, H.; Xiao, M.; Zhang, J.; Cai, G. Uncertainty design and optimization of a hybrid rocket motor with mixed random-interval uncertainties. Aerosp. Sci. Technol. 2022, 128, 107791:1–107791:12. [Google Scholar] [CrossRef]
- Meng, X.; Tian, H.; Chen, X.; Jiang, X.; Wang, P.; Wei, T.; Cai, G. Numerical simulation of combustion surface regression based on Butterworth filter in hybrid rocket motor. Acta Astronaut. 2023, 202, 400–410. [Google Scholar] [CrossRef]
- Wei, T.; Cai, G.; Tian, H.; Jiang, X. Experiment and numerical research on regression rate of hybrid rocket motor with single-port wagon wheel fuel grain. Acta Astronaut. 2023, 207, 265–282. [Google Scholar] [CrossRef]
- Yun, Y.; Kim, J.; Kwon, S. Parametric Study of Solid Fuel for Hydrogen Peroxide Hybrid Rocket Design. J. Propuls. Power 2022, 38, 229–240. [Google Scholar] [CrossRef]
- Brown, T.R.; Lydon, M.C. Testing of Paraffin-Based Hybrid Rocket Fuel Using Hydrogen Peroxide Oxidizer. In Proceedings of the AIAA Region V Student Conference, Wichita, KS, USA, 15-16 January 2005. [Google Scholar]
- Wernimont, E.; Heister, S. Combustion experiments in hydrogen peroxide/polyethylene hybrid rocket with catalytic ignition. J. Propuls. Power 2000, 16, 318–326. [Google Scholar] [CrossRef]
- Lestrade, J.Y.; Anthoine, J.; Musker, A.J.; Lecossais, A. Experimental demonstration of an end-burning swirling flow hybrid rocket engine. Aerosp. Sci. Technol. 2019, 92, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Wernimont, E.J.; Meyer, S.E. Hydrogen peroxide hybrid rocket engine performance investigation. In Proceedings of the 30th Joint Propulsion Conference and Exhibit, Indianapolis, IN, USA, 27–29 June 1994. [Google Scholar] [CrossRef]
- Moore, G.E.; Berman, K. A solid-liquid rocket propellant system. J. Jet Propuls. 1956, 26, 965–968. [Google Scholar] [CrossRef]
- Cai, G.; Zeng, P.; Li, X.; Tian, H.; Yu, N. Scale effect of fuel regression rate in hybrid rocket motor. Aerosp. Sci. Technol. 2013, 24, 141–146. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, X.; Lin, X.; Li, S.; Wei, X.; Zhu, C.; Wu, L. Experimental investigation of fuel composition and mix-enhancer effects on the performance of paraffin-based hybrid rocket motors. Aerosp. Sci. Technol. 2018, 82, 620–627. [Google Scholar] [CrossRef] [Green Version]
- Farbar, E.; Louwers, J.; Kaya, T. Investigation of metallized and nonmetallized hydroxyl terminated polybutadiene/hydrogen peroxide hybrid rockets. J. Propuls. Power 2007, 23, 476–486. [Google Scholar] [CrossRef]
- Shark, S.; Pourpoint, T.; Son, S.; Heister, S. Performance of dicyclopentadiene/H2O2-based hybrid rocket motors with metal hydride additives. J. Propuls. Power 2013, 29, 1122–1129. [Google Scholar] [CrossRef]
- Paccagnella, E.; Santi, M.; Ruffin, A.; Barato, F.; Pavarin, D.; Misté, G.A.; Venturelli, G.; Bellomo, N. Testing of a long-burning-time paraffin-based hybrid rocket motor. J. Propuls. Power 2019, 35, 432–442. [Google Scholar] [CrossRef]
- Kang, S.; Lee, D.; Lee, E.; Kwon, S. Design and performance evaluation of hybrid rocket using 95 wt.% H2O2. In Proceedings of the 52nd AIAA/SAE/ASEE Joint Propulsion Conference, Salt Lake City, UT, USA, 25–27 July 2016. [Google Scholar] [CrossRef]
- Cai, G.; Zhu, H.; Rao, D.; Tian, H. Optimal design of hybrid rocket motor powered vehicle for suborbital flight. Aerosp. Sci. Technol. 2013, 25, 114–124. [Google Scholar] [CrossRef]
- Li, X.; Tian, H.; Yu, N.; Cai, G. Experimental investigation of fuel regression rate in a HTPB based lab-scale hybrid rocket motor. Acta Astronaut. 2014, 105, 95–100. [Google Scholar] [CrossRef]
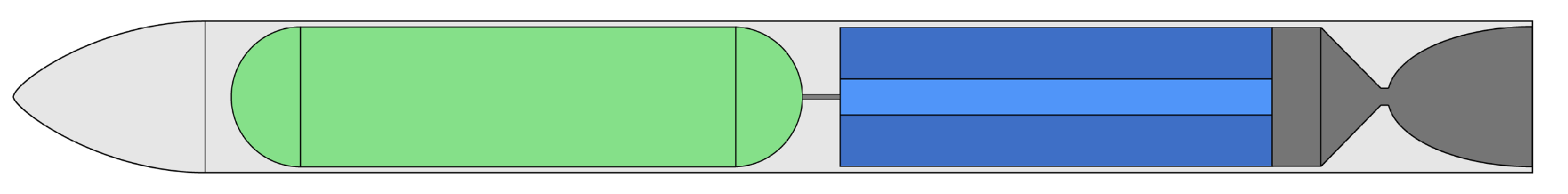
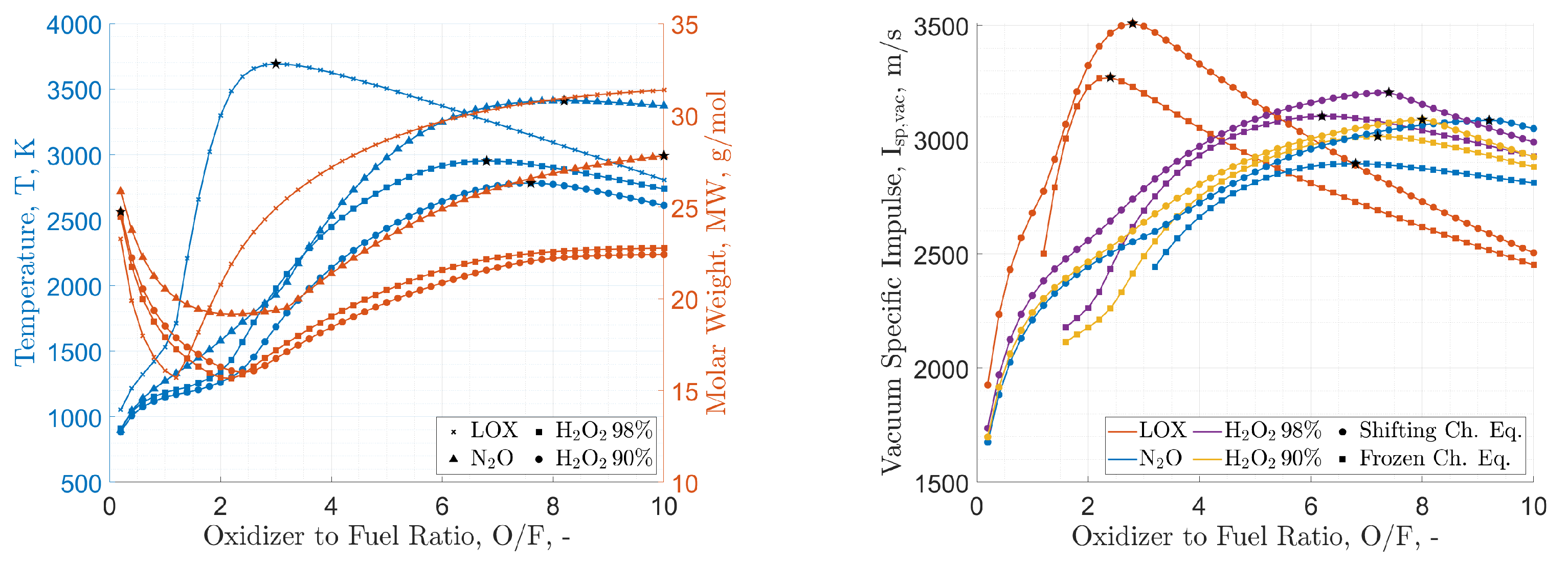
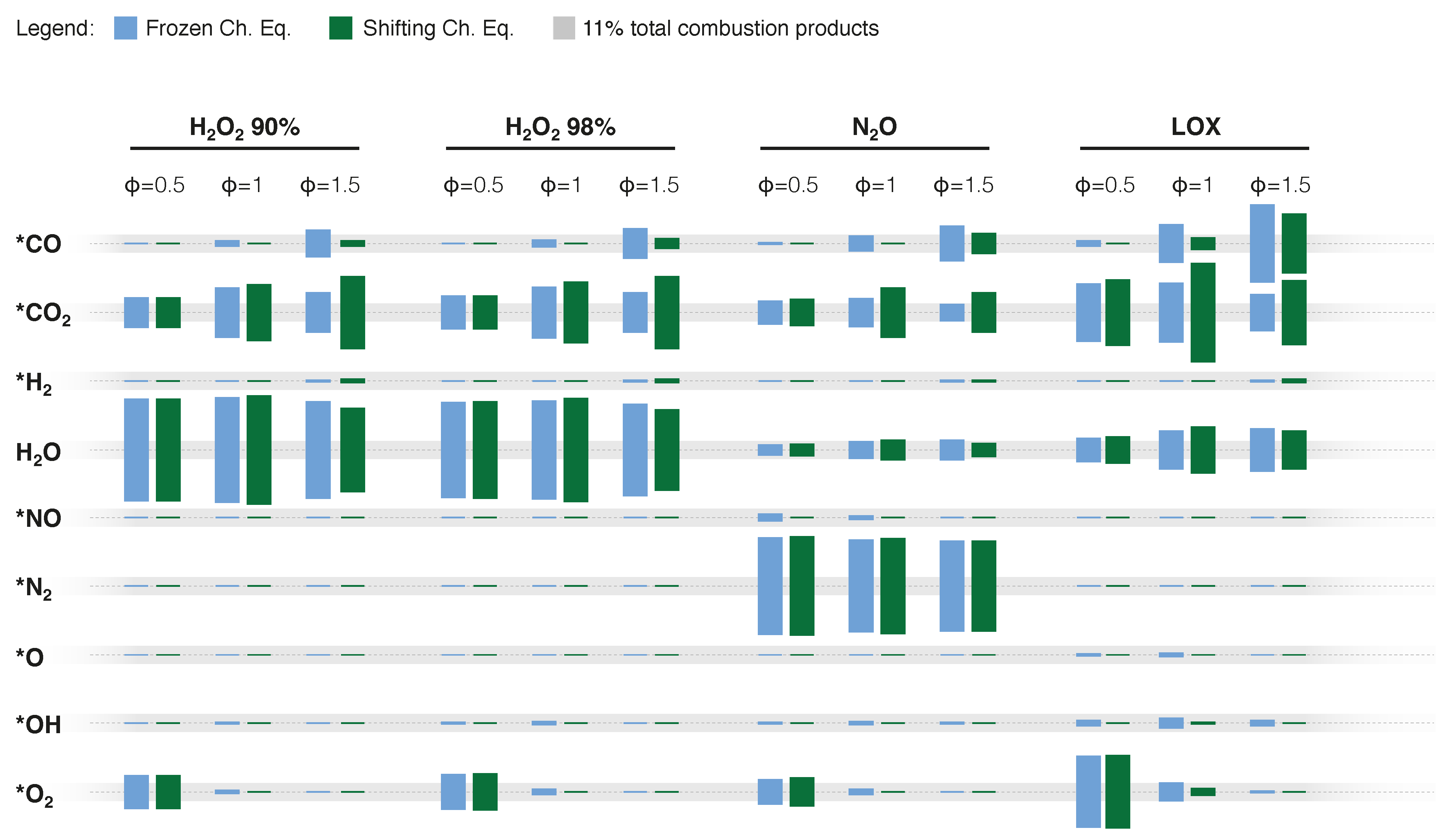
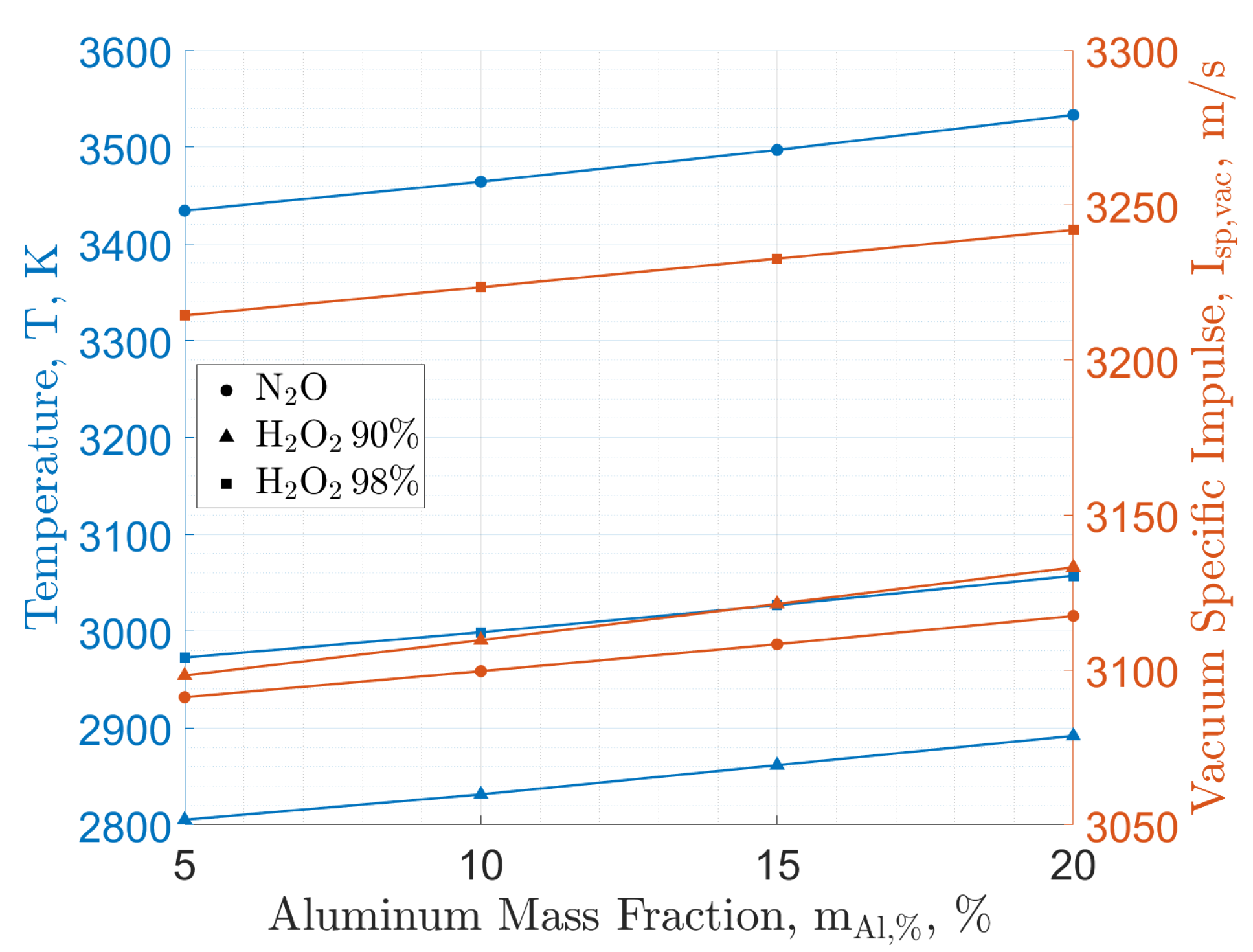
| Property | HP | HP | N2O | LOX |
|---|---|---|---|---|
| 90 wt.% | 98 wt.% | |||
| Active O2 content [%] | 42 | 46 | 36 | 100 |
| Boiling point @ 1 atm [K] | 414 | 422 | 185 | 90 |
| Freezing point @ 1 atm [K] | 261 | 270 | 182 | 54 |
| Density @ a 293 K, 1 atm [g/cm3] | 1.395 | 1.431 | 0.786 b | 1.141 c |
| Vapor pressure @ a 293 K [Pa] | 200 | 133 | 5.0 × 106 | 9.9 × 104 c |
| Molar mass [g/mol] | 32.4 | 33.7 | 44 | 32 |
| Decomposition Temperature @ 2.0 MPa [K] | 1029 | 1225 | 1907 | N.A. |
| Additive | Oxidizer | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| N2O | HP (90 wt.%) | HP (98 wt.%) | |||||||
| O/F | CCPs | O/F | CCPs | O/F | CCPs | ||||
| - | wt.% | - | wt.% | - | wt.% | ||||
| - | 3083 | 9.2 | 0 | 3087 | 8.0 | 0 | 3206 | 7.3 | 0 |
| Al | 3100 | 8.3 | 2.0 | 3110 | 7.4 | 2.2 | 3224 | 6.7 | 2.3 |
| Mg | 3091 | 8.3 | 2.9 | 3102 | 7.4 | 1.5 | 3215 | 6.7 | 1.1 |
| AlH3 | 3106 | 8.5 | 1.7 | 3116 | 7.6 | 1.9 | 3231 | 6.9 | 2.0 |
| MgH2 | 3091 | 8.5 | 1.5 | 3101 | 7.5 | 1.3 | 3215 | 6.8 | 0.9 |
| LiAlH4 | 3099 | 8.5 | 1.4 | 3111 | 7.6 | 1.5 | 3225 | 6.9 | 1.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paravan, C.; Hashish, A.; Santolini, V. Test Activities on Hybrid Rocket Engines: Combustion Analyses and Green Storable Oxidizers—A Short Review. Aerospace 2023, 10, 572. https://doi.org/10.3390/aerospace10070572
Paravan C, Hashish A, Santolini V. Test Activities on Hybrid Rocket Engines: Combustion Analyses and Green Storable Oxidizers—A Short Review. Aerospace. 2023; 10(7):572. https://doi.org/10.3390/aerospace10070572
Chicago/Turabian StyleParavan, Christian, Anwer Hashish, and Valerio Santolini. 2023. "Test Activities on Hybrid Rocket Engines: Combustion Analyses and Green Storable Oxidizers—A Short Review" Aerospace 10, no. 7: 572. https://doi.org/10.3390/aerospace10070572






