Systematic Screening of Associations between Medication Use and Risk of Neurodegenerative Diseases Using a Mendelian Randomization Approach
Abstract
:1. Introduction
2. Materials and Methods
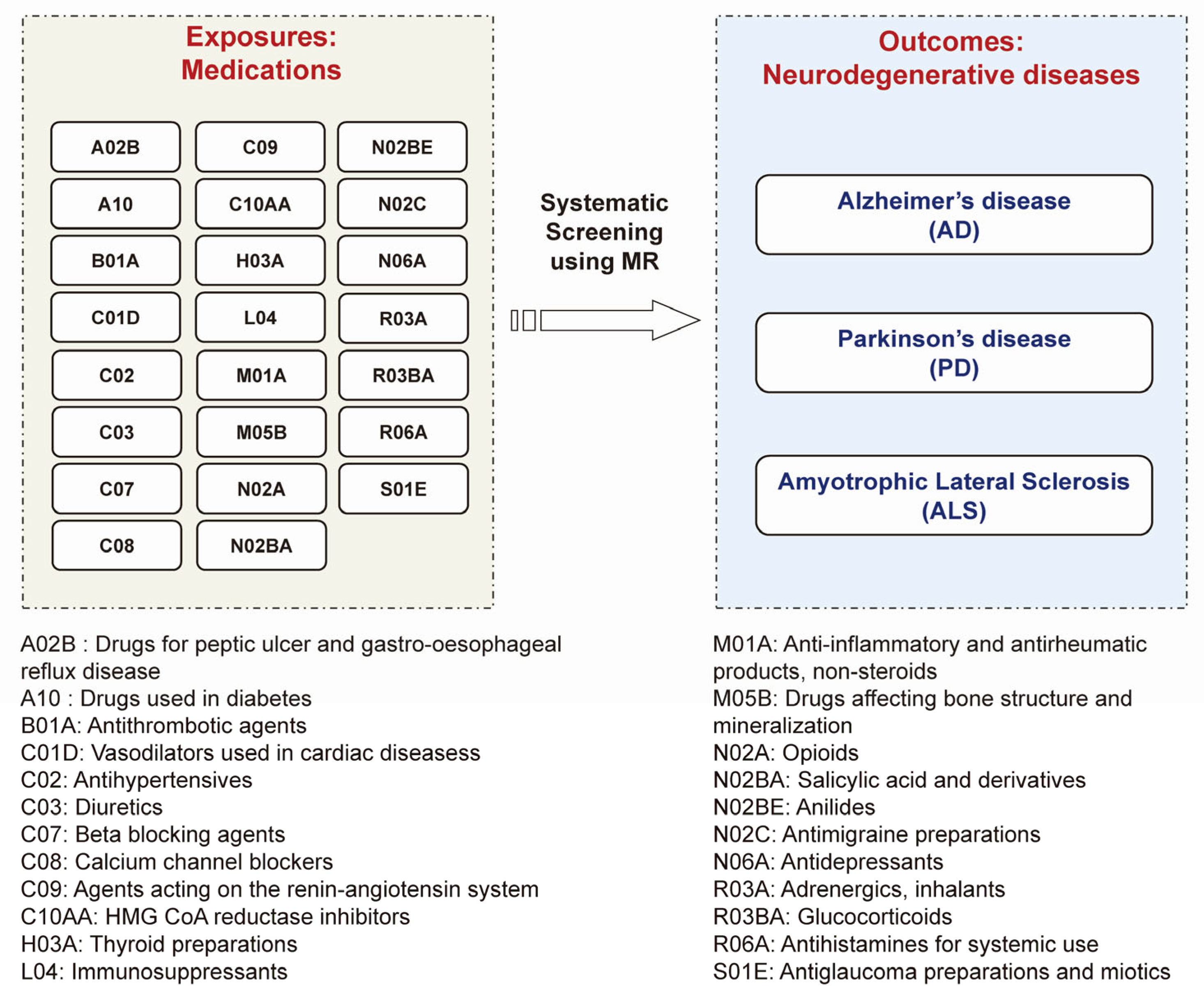
2.1. Exposure Data: GWAS Summary Statistics for 23 Medication Categories
2.2. Outcome Data: GWAS Summary Statistics for Three Major Neurodegenerative Diseases
2.3. MR Analysis
2.4. Further Analysis for Identified Associations between Medications and NDs
3. Results
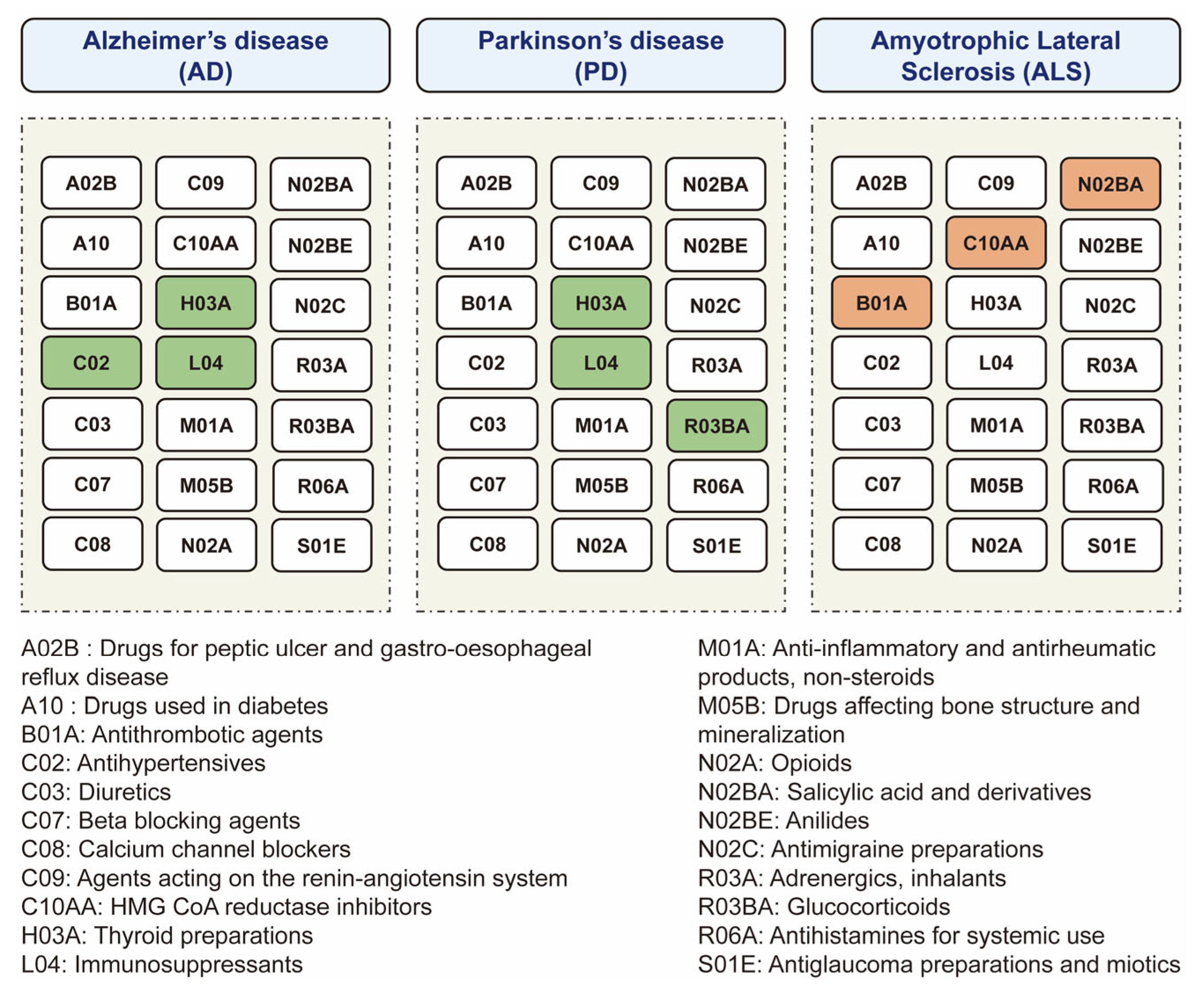
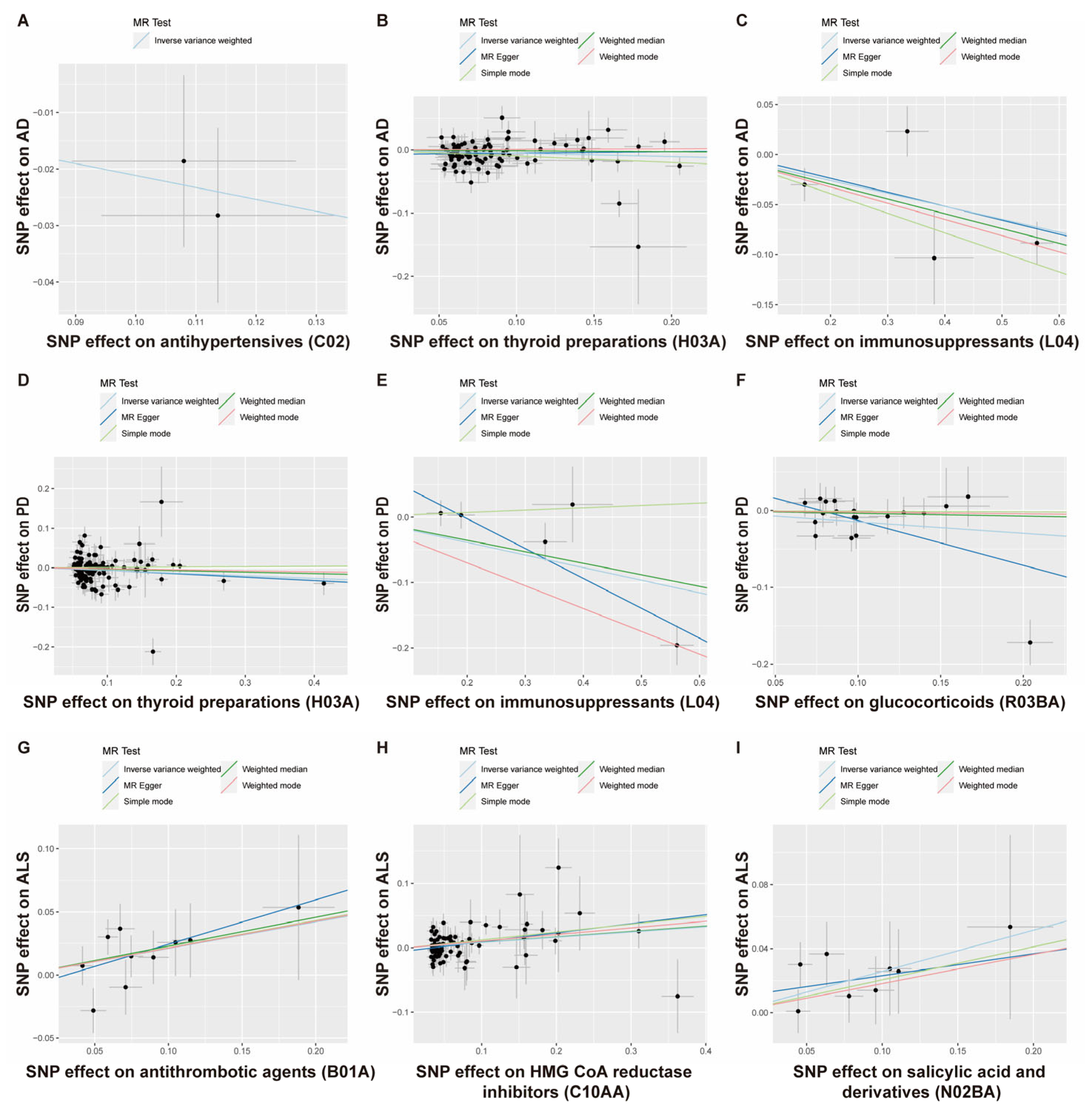
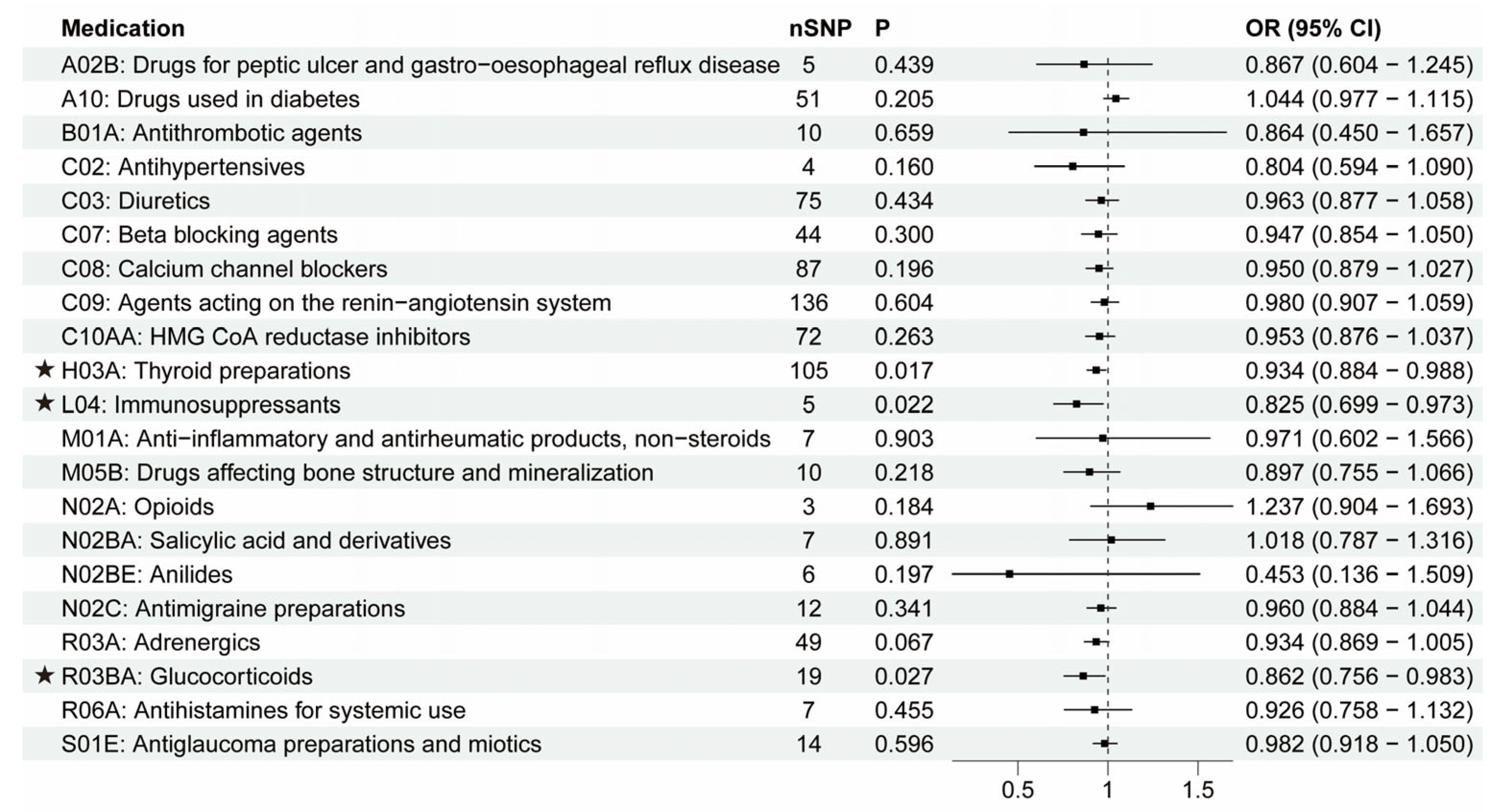
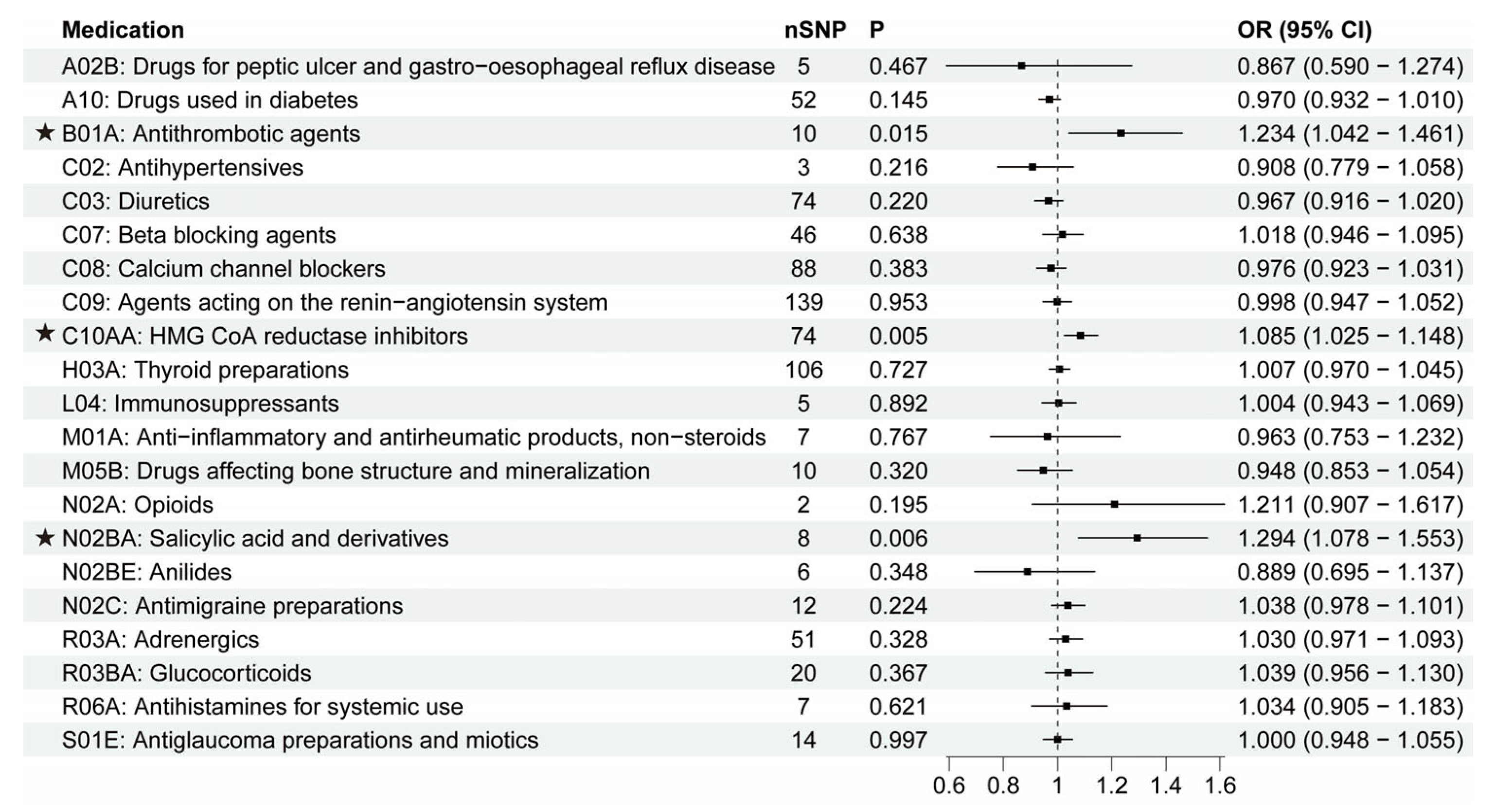
3.1. The Putative Causal Relationship between Medication-Taking Traits and AD
3.2. The Putative Causal Relationship between Medication-Taking Traits and PD
3.3. The Putative Causal Relationship between Medication-Taking Traits and ALS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jucker, M.; Walker, L.C. Propagation and spread of pathogenic protein assemblies in neurodegenerative diseases. Nat. Neurosci. 2018, 21, 1341–1349. [Google Scholar] [CrossRef]
- Gammon, K. Neurodegenerative disease: Brain windfall. Nature 2014, 515, 299–300. [Google Scholar] [CrossRef] [Green Version]
- Durães, F.; Pinto, M.; Sousa, E. Old drugs as new treatments for neurodegenerative diseases. Pharmaceuticals 2018, 11, 44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novac, N. Challenges and opportunities of drug repositioning. Trends Pharmacol. Sci. 2013, 34, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 2014, 14, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Wang, X.; Zhang, L.; Fang, Y.; Zheng, Q.; Liu, X.; Yu, W.; Chen, S.; Ying, J.; Hua, F. Lipid metabolism and storage in neuroglia: Role in brain development and neurodegenerative diseases. Cell Biosci. 2022, 12, 106. [Google Scholar] [CrossRef] [PubMed]
- Barnham, K.J.; Masters, C.L.; Bush, A.I. Neurodegenerative diseases and oxidative stress. Nat. Rev. Drug Discov. 2004, 3, 205–214. [Google Scholar] [CrossRef]
- Leiter, O.; Walker, T.L. Platelets in neurodegenerative conditions—Friend or foe? Front. Immunol. 2020, 11, 747. [Google Scholar] [CrossRef]
- Chong, C.R.; Sullivan, D.J. New uses for old drugs. Nature 2007, 448, 645–646. [Google Scholar] [CrossRef]
- Sanson-Fisher, R.W.; Bonevski, B.; Green, L.W.; D’Este, C. Limitations of the randomized controlled trial in evaluating population-based health interventions. Am. J. Prev. Med. 2007, 33, 155–161. [Google Scholar] [CrossRef]
- Jepsen, P.; Johnsen, S.P.; Gillman, M.; Sørensen, H. Interpretation of observational studies. Heart 2004, 90, 956–960. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Byrne, E.M.; Zheng, Z.; Kemper, K.E.; Yengo, L.; Mallett, A.J.; Yang, J.; Visscher, P.M.; Wray, N.R. Genome-wide association study of medication-use and associated disease in the uk biobank. Nat. Commun. 2019, 10, 1891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emdin, C.A.; Khera, A.V.; Kathiresan, S. Mendelian randomization. JAMA 2017, 318, 1925–1926. [Google Scholar] [CrossRef] [PubMed]
- Hartwig, F.P.; Davies, N.M.; Hemani, G.; Davey Smith, G. Two-sample mendelian randomization: Avoiding the downsides of a powerful, widely applicable but potentially fallible technique. Int. J. Epidemiol. 2017, 45, 1717–1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosoff, D.B.; Smith, G.D.; Lohoff, F.W. Prescription opioid use and risk for major depressive disorder and anxiety and stress-related disorders: A multivariable mendelian randomization analysis. JAMA Psychiatry 2021, 78, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; He, L.; Wang, H.; Rong, X.; Chen, M.; Shen, Q.; Li, X.; Li, M.; Peng, Y. Genetic liability for prescription opioid use and risk of cardiovascular diseases: A multivariable mendelian randomization study. Addiction 2022, 117, 1382–1391. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Chong, L.; Zhang, X.; Li, R. Immunosuppressants contribute to a reduced risk of parkinson’s disease in rheumatoid arthritis. Int. J. Epidemiol. 2022, 51, 1328–1338. [Google Scholar] [CrossRef]
- Lawlor, D.A. Commentary: Two-sample mendelian randomization: Opportunities and challenges. Int. J. Epidemiol. 2016, 45, 908–915. [Google Scholar] [CrossRef] [Green Version]
- Santos, R.; Ursu, O.; Gaulton, A.; Bento, A.P.; Donadi, R.S.; Bologa, C.G.; Karlsson, A.; Al-Lazikani, B.; Hersey, A.; Oprea, T.I. A comprehensive map of molecular drug targets. Nat. Rev. Drug Discov. 2017, 16, 19–34. [Google Scholar] [CrossRef]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; Van Der Lee, S.J.; Amlie-Wolf, A. Genetic meta-analysis of diagnosed alzheimer’s disease identifies new risk loci and implicates aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [Green Version]
- Nalls, M.A.; Blauwendraat, C.; Vallerga, C.L.; Heilbron, K.; Bandres-Ciga, S.; Chang, D.; Tan, M.; Kia, D.A.; Noyce, A.J.; Xue, A. Identification of novel risk loci, causal insights, and heritable risk for parkinson’s disease: A meta-analysis of genome-wide association studies. Lancet Neurol. 2019, 18, 1091–1102. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, A.; Kenna, K.P.; Renton, A.E.; Ticozzi, N.; Faghri, F.; Chia, R.; Dominov, J.A.; Kenna, B.J.; Nalls, M.A.; Keagle, P.; et al. Genome-wide analyses identify kif5a as a novel als gene. Neuron 2018, 97, 1268–1283.e1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hemani, G.; Zheng, J.; Elsworth, B.; Wade, K.H.; Haberland, V.; Baird, D.; Laurin, C.; Burgess, S.; Bowden, J.; Langdon, R.; et al. The mr-base platform supports systematic causal inference across the human phenome. eLife 2018, 7, e34408. [Google Scholar] [CrossRef] [PubMed]
- Burgess, S.; Bowden, J.; Fall, T.; Ingelsson, E.; Thompson, S.G. Sensitivity analyses for robust causal inference from mendelian randomization analyses with multiple genetic variants. Epidemiology 2017, 28, 30–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bowden, J.; Davey Smith, G.; Haycock, P.C.; Burgess, S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet. Epidemiol. 2016, 40, 304–314. [Google Scholar] [CrossRef] [Green Version]
- Burgess, S.; Thompson, S.G. Interpreting findings from mendelian randomization using the mr-egger method. Eur. J. Epidemiol. 2017, 32, 377–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verbanck, M.; Chen, C.-Y.; Neale, B.; Do, R. Detection of widespread horizontal pleiotropy in causal relationships inferred from mendelian randomization between complex traits and diseases. Nat. Genet. 2018, 50, 693–698. [Google Scholar] [CrossRef]
- Hemani, G.; Bowden, J.; Davey Smith, G. Evaluating the potential role of pleiotropy in mendelian randomization studies. Hum. Mol. Genet. 2018, 27, R195–R208. [Google Scholar] [CrossRef] [Green Version]
- Khachaturian, A.S.; Zandi, P.P.; Lyketsos, C.G.; Hayden, K.M.; Skoog, I.; Norton, M.C.; Tschanz, J.T.; Mayer, L.S.; Welsh-Bohmer, K.A.; Breitner, J.C. Antihypertensive medication use and incident alzheimer disease: The cache county study. Arch. Neurol. 2006, 63, 686–692. [Google Scholar] [CrossRef] [Green Version]
- Fu, A.L.; Zhou, C.Y.; Chen, X. Thyroid hormone prevents cognitive deficit in a mouse model of alzheimer’s disease. Neuropharmacology 2010, 58, 722–729. [Google Scholar] [CrossRef]
- Van Osch, L.A.; Hogervorst, E.; Combrinck, M.; Smith, A.D. Low thyroid-stimulating hormone as an independent risk factor for alzheimer disease. Neurology 2004, 62, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Meier-Stephenson, F.S.; Meier-Stephenson, V.C.; Carter, M.D.; Meek, A.R.; Wang, Y.; Pan, L.; Chen, Q.; Jacobo, S.; Wu, F.; Lu, E. Alzheimer’s disease as an autoimmune disorder of innate immunity endogenously modulated by tryptophan metabolites. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2022, 8, e12283. [Google Scholar] [CrossRef] [PubMed]
- Charoenngam, N.; Rittiphairoj, T.; Ponvilawan, B.; Prasongdee, K. Thyroid dysfunction and risk of parkinson’s disease: A systematic review and meta-analysis. Front. Endocrinol. 2022, 13, 863281. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-F.; Yang, Y.-C.; Hsu, C.-Y.; Shen, Y.-C. Risk of parkinson’s disease in patients with hypothyroidism: A nationwide population-based cohort study. Parkinsonism Relat. Disord. 2020, 74, 28–32. [Google Scholar] [CrossRef]
- Herrero, M.-T.; Estrada, C.; Maatouk, L.; Vyas, S. Inflammation in parkinson’s disease: Role of glucocorticoids. Front. Neuroanat. 2015, 9, 32. [Google Scholar] [CrossRef] [Green Version]
- Diekmann, K.; Kuzma-Kozakiewicz, M.; Piotrkiewicz, M.; Gromicho, M.; Grosskreutz, J.; Andersen, P.M.; de Carvalho, M.; Uysal, H.; Osmanovic, A.; Schreiber-Katz, O. Impact of comorbidities and co-medication on disease onset and progression in a large german als patient group. J. Neurol. 2020, 267, 2130–2141. [Google Scholar] [CrossRef]
- Colman, E.; Szarfman, A.; Wyeth, J.; Mosholder, A.; Jillapalli, D.; Levine, J.; Avigan, M. An evaluation of a data mining signal for amyotrophic lateral sclerosis and statins detected in fda’s spontaneous adverse event reporting system. Pharmacoepidemiol. Drug Saf. 2008, 17, 1068–1076. [Google Scholar] [CrossRef]
- Schmidt, A.F.; Finan, C.; Gordillo-Marañón, M.; Asselbergs, F.W.; Freitag, D.F.; Patel, R.S.; Tyl, B.; Chopade, S.; Faraway, R.; Zwierzyna, M. Genetic drug target validation using mendelian randomisation. Nat. Commun. 2020, 11, 3255. [Google Scholar] [CrossRef]
- Storm, C.S.; Kia, D.A.; Almramhi, M.M.; Bandres-Ciga, S.; Finan, C.; Hingorani, A.D.; Wood, N.W. Finding genetically-supported drug targets for parkinson’s disease using mendelian randomization of the druggable genome. Nat. Commun. 2021, 12, 7342. [Google Scholar] [CrossRef]
- Ou, Y.-N.; Yang, Y.-X.; Deng, Y.-T.; Zhang, C.; Hu, H.; Wu, B.-S.; Liu, Y.; Wang, Y.-J.; Zhu, Y.; Suckling, J. Identification of novel drug targets for alzheimer’s disease by integrating genetics and proteomes from brain and blood. Mol. Psychiatry 2021, 26, 6065–6073. [Google Scholar] [CrossRef]
- Daneman, R.; Prat, A. The blood–brain barrier. Cold Spring Harb. Perspect. Biol. 2015, 7, a020412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Ratka, A. Opioid system and alzheimer’s disease. Neuromol. Med. 2012, 14, 91–111. [Google Scholar] [CrossRef] [PubMed]
- D’Ovidio, F.; d’Errico, A.; Farina, E.; Calvo, A.; Costa, G.; Chiò, A. Amyotrophic lateral sclerosis incidence and previous prescriptions of drugs for the nervous system. Neuroepidemiology 2016, 47, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Arnold, S.E. Repurposing diabetes drugs for brain insulin resistance in alzheimer disease. Diabetes 2014, 63, 2253–2261. [Google Scholar] [CrossRef] [Green Version]
- Gebre, A.K.; Altaye, B.M.; Atey, T.M.; Tuem, K.B.; Berhe, D.F. Targeting renin–angiotensin system against alzheimer’s disease. Front. Pharmacol. 2018, 9, 440. [Google Scholar] [CrossRef] [Green Version]
- Walker, V.M.; Kehoe, P.G.; Martin, R.M.; Davies, N.M. Repurposing antihypertensive drugs for the prevention of alzheimer’s disease: A mendelian randomization study. Int. J. Epidemiol. 2020, 49, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Bae, S.-C.; Lee, Y. Causal association between rheumatoid arthritis and a decreased risk of alzheimer’s disease: A mendelian randomization study. Z. Rheumatol. 2019, 78, 359–364. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Amrhein, V.; Greenland, S.; McShane, B. Scientists rise up against statistical significance. Nature 2019, 567, 305–307. [Google Scholar] [CrossRef] [Green Version]
- Power, M.C.; Weuve, J.; Sharrett, A.R.; Blacker, D.; Gottesman, R.F. Statins, cognition, and dementia—Systematic review and methodological commentary. Nat. Rev. Neurol. 2015, 11, 220–229. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zhang, L.; Xia, K.; Huang, T.; Fan, D. Mendelian randomization analysis reveals statins potentially increase amyotrophic lateral sclerosis risk independent of peripheral cholesterol-lowering effects. Biomedicines 2023, 11, 1359. [Google Scholar] [CrossRef] [PubMed]

| Medication | Primary Disease | NDs | Associations between Primary Disease and ND (before Removing Medication Use Associated SNPs) | Associations between Primary Disease and ND (after Removing Medication Use Associated SNPs) | ||||
|---|---|---|---|---|---|---|---|---|
| p | OR before | SNPs (N) | p | OR | SNPs (N) | |||
| Antihypertensives | Systolic blood pressure (SBP) | AD | 0.399 | 0.997 (0.990–1.004) | -- | -- | -- | -- |
| Thyroid preparations | Hypothyroidism | AD | 0.701 | 1.585 (0.150–16.706) | -- | -- | -- | -- |
| Immunosuppressants | Rheumatoid arthritis (RA) | AD | 0.032 | 0.958 (0.922–0.996) | 51 | 0.173 | 0.968 (0.923–1.015) | 48 |
| Thyroid preparations | Hypothyroidism | PD | 0.172 | 0.088 (0.003–2.872) | -- | -- | -- | -- |
| Immunosuppressants | Rheumatoid arthritis (RA) | PD | 0.001 | 0.926 (0.886–0.969) | 50 | 0.071 | 0.947 (0.893–1.005) | 47 |
| Glucocorticoids | Asthma | PD | 0.686 | 0.981 (0.894–1.077) | -- | -- | -- | -- |
| Antithrombotic agents/Salicylic acid and derivatives | Coronary artery disease (CAD) | ALS | 0.285 | 1.034 (0.972–1.100) | -- | -- | -- | -- |
| HMG CoA reductase inhibitors | Low density lipoprotein cholesterol (LDL-C) | ALS | 0.017 | 1.075 (1.013–1.141) | 310 | 0.432 | 1.036 (0.949–1.131) | 266 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Zhang, L.; Cao, W.; Xia, K.; Huo, J.; Huang, T.; Fan, D. Systematic Screening of Associations between Medication Use and Risk of Neurodegenerative Diseases Using a Mendelian Randomization Approach. Biomedicines 2023, 11, 1930. https://doi.org/10.3390/biomedicines11071930
Wang W, Zhang L, Cao W, Xia K, Huo J, Huang T, Fan D. Systematic Screening of Associations between Medication Use and Risk of Neurodegenerative Diseases Using a Mendelian Randomization Approach. Biomedicines. 2023; 11(7):1930. https://doi.org/10.3390/biomedicines11071930
Chicago/Turabian StyleWang, Wenjing, Linjing Zhang, Wen Cao, Kailin Xia, Junyan Huo, Tao Huang, and Dongsheng Fan. 2023. "Systematic Screening of Associations between Medication Use and Risk of Neurodegenerative Diseases Using a Mendelian Randomization Approach" Biomedicines 11, no. 7: 1930. https://doi.org/10.3390/biomedicines11071930








