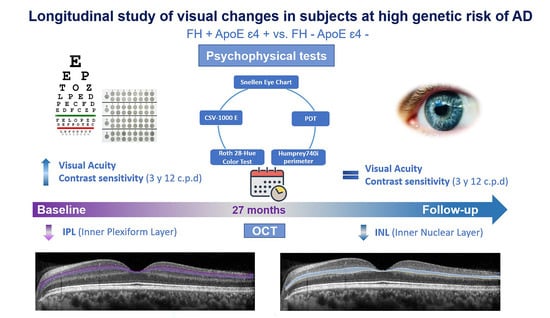
Exploratory Longitudinal Study of Ocular Structural and Visual Functional Changes in Subjects at High Genetic Risk of Developing Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Optical Coherence Tomography (OCT)
2.3. Psychophysical Test
2.3.1. Visual Acuity
2.3.2. Contrast Sensitivity
2.3.3. Color Perception Test
2.3.4. Perception Digital Test
2.3.5. Visual Field
2.4. Allelic Characterization
2.5. Statistical Analysis
2.6. Colorimetric Representation
3. Results
3.1. Demographic Study
3.2. Longitudinal Study in the FH− ApoE ε4− Group
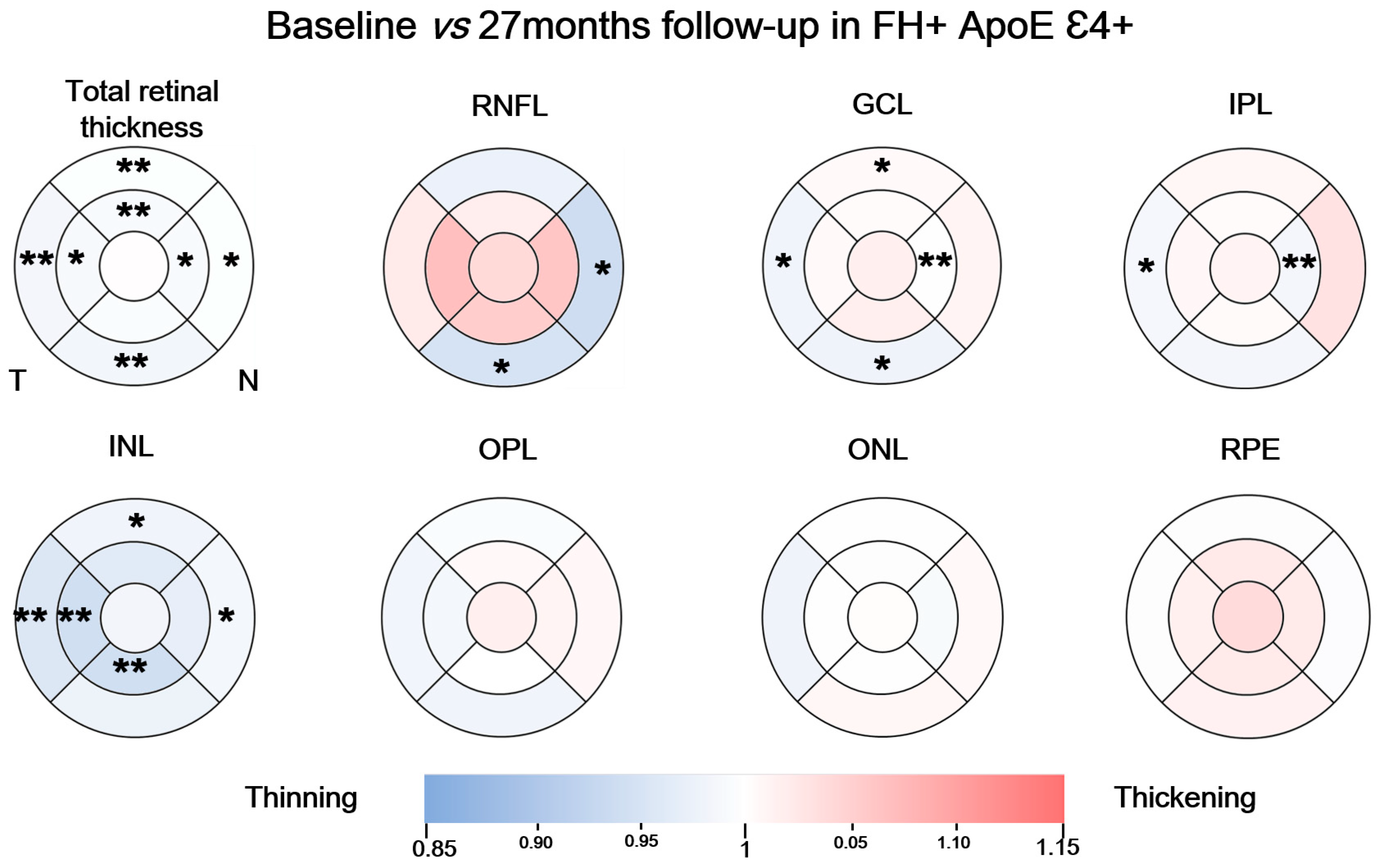
3.3. Longitudinal Study in the FH+ ApoE ε4+ Group
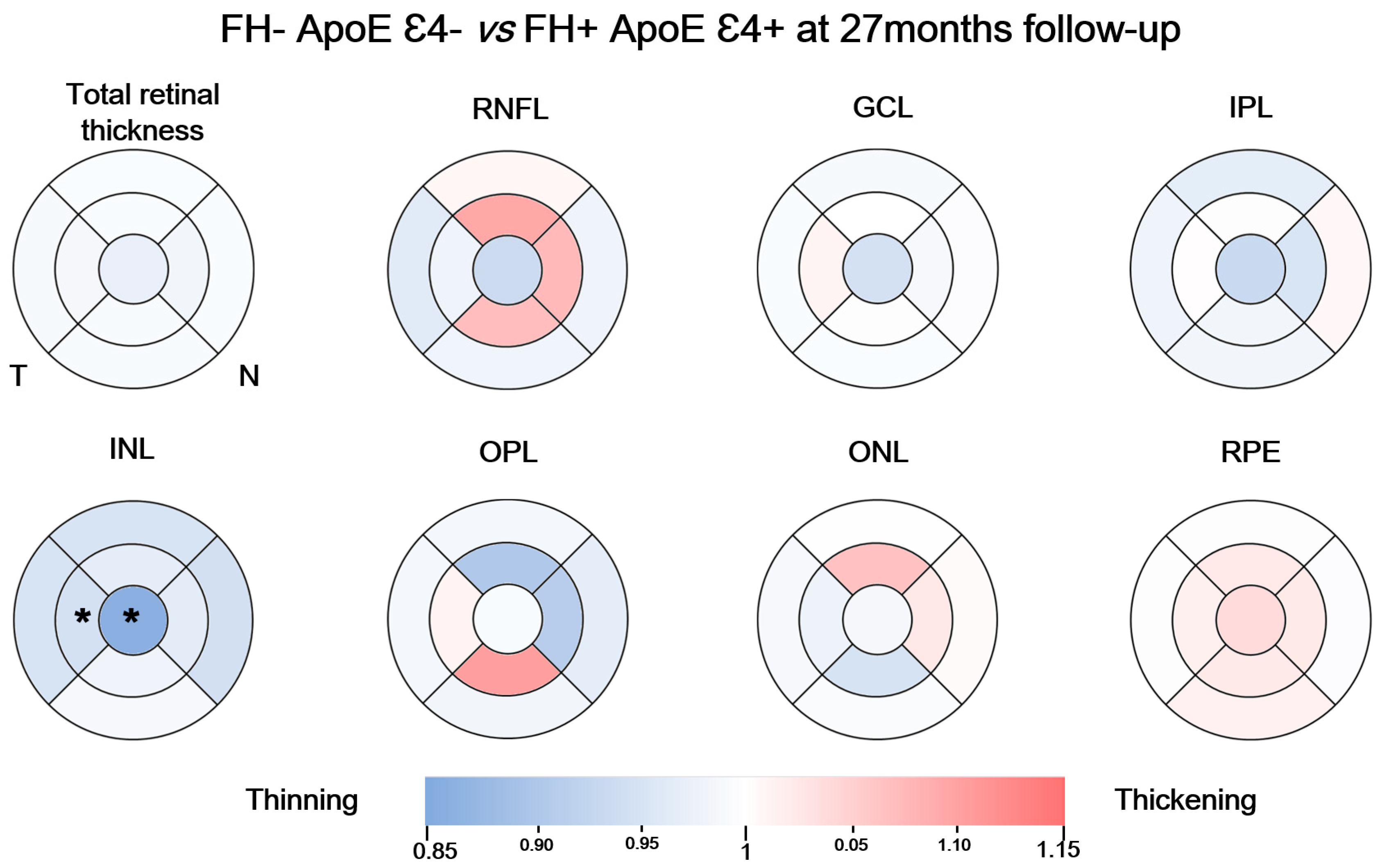
3.4. Analysis between the Study Groups at 27-Month Follow-Up
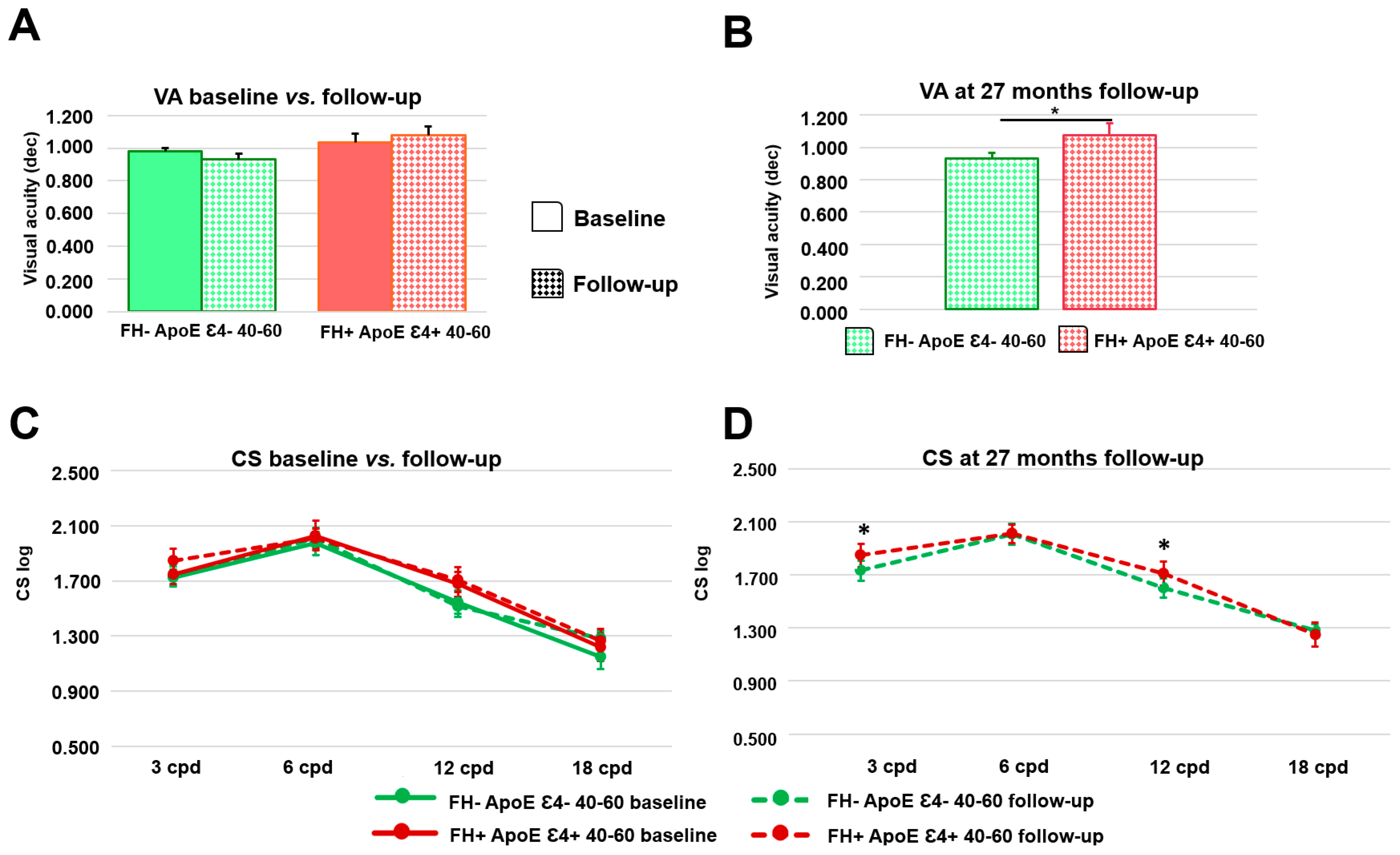
3.5. Longitudinal Psychophysical Test
3.5.1. Longitudinal Study in the FH− ApoE ε4− 40–60 yrs and FH− ApoE ε4− > 60 yrs Groups
3.5.2. Longitudinal Study in the FH− ApoE Ɛ4− 40–60 yrs and FH− ApoE Ɛ4− > 60 yrs Groups
3.5.3. Analysis of Psychophysical Test between the Study Groups at 27-Month Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Dementia. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 16 May 2023).
- Vieira, R.T.; Caixeta, L.; Machado, S.; Silva, A.C.; Nardi, A.E.; Arias-Carrión, O.; Carta, M.G. Epidemiology of early-onset dementia: A review of the literature. Clin. Pract. Epidemiol. Ment. Health CP EMH 2013, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R., Jr.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzheimer’s association. 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.P.; Robbins, C.B.; Lee, J.M.; Soundararajan, S.; Stinnett, S.S.; Agrawal, R.; Plassman, B.L.; Lad, E.M.; Whitson, H.; Grewal, D.S.; et al. Longitudinal Analysis of the Retina and Choroid in Cognitively Normal Individuals at Higher Genetic Risk of Alzheimer Disease. Ophthalmol. Retin. 2022, 6, 607–619. [Google Scholar] [CrossRef]
- Ramos, A.A.; Galiano-Castillo, N.; Machado, L. Cognitive Functioning of Unaffected First-degree Relatives of Individuals With Late-onset Alzheimer’s Disease: A Systematic Literature Review and Meta-analysis. Neuropsychol. Rev. 2022, 1–16. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; de Hoz, R.; Salobrar-García, E.; Elvira-Hurtado, L.; Rojas, P.; Fernández-Albarral, J.A.; Barabash, A.; Salazar, J.J.; Ramírez, A.I.; Ramírez, J.M. Macular Thickness Decrease in Asymptomatic Subjects at High Genetic Risk of Developing Alzheimer’s Disease: An OCT Study. J. Clin. Med. 2020, 9, 1728. [Google Scholar] [CrossRef]
- Aschenbrenner, A.J.; Balota, D.A.; Gordon, B.A.; Ratcliff, R.; Morris, J.C. A diffusion model analysis of episodic recognition in preclinical individuals with a family history for Alzheimer’s disease: The adult children study. Neuropsychology 2016, 30, 225–238. [Google Scholar] [CrossRef]
- Yang, A.; Kantor, B.; Chiba-Falek, O. APOE: The New Frontier in the Development of a Therapeutic Target towards Precision Medicine in Late-Onset Alzheimer’s. Int. J. Mol. Sci. 2021, 22, 1244. [Google Scholar] [CrossRef]
- Farrer, L.A.; Cupples, L.A.; Haines, J.L.; Hyman, B.; Kukull, W.A.; Mayeux, R.; Myers, R.H.; Pericak-Vance, M.A.; Risch, N.; Van Duijn, C.M. Effects of Age, Sex, and Ethnicity on the Association Between Apolipoprotein E Genotype and Alzheimer Disease: A Meta-analysis. JAMA 1997, 278, 1349–1356. [Google Scholar] [CrossRef]
- Scarabino, D.; Gambina, G.; Broggio, E.; Pelliccia, F.; Corbo, R.M. Influence of family history of dementia in the development and progression of late-onset Alzheimer’s disease. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2015, 171, 250–256. [Google Scholar] [CrossRef]
- Donix, M.; Burggren, A.C.; Suthana, N.A.; Siddarth, P.; Ekstrom, A.D.; Krupa, A.K.; Jones, M.; Martin-Harris, L.; Ercoli, L.M.; Miller, K.J.; et al. Family History of Alzheimer’s Disease and Hippocampal Structure in Healthy People. Am. J. Psychiatry 2010, 167, 1399–1406. [Google Scholar] [CrossRef] [PubMed]
- Mosconi, L.; Murray, J.; Tsui, W.H.; Li, Y.; Spector, N.; Goldowsky, A.; Williams, S.; Osorio, R.; McHugh, P.; Glodzik, L.; et al. Brain imaging of cognitively normal individuals with 2 parents affected by late-onset AD. Neurology 2014, 82, 752–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Cuenca, I.; de Hoz, R.; Alcántara-Rey, C.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Barabash, A.; Ramírez-Toraño, F.; de Frutos-Lucas, J.; Salazar, J.J.; et al. Foveal Avascular Zone and Choroidal Thickness Are Decreased in Subjects with Hard Drusen and without High Genetic Risk of Developing Alzheimer’s Disease. Biomedicines 2021, 9, 638. [Google Scholar] [CrossRef]
- López-Cuenca, I.; Salobrar-García, E.; Sánchez-Puebla, L.; Espejel, E.; del Arco, L.G.; Rojas, P.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Ramírez-Toraño, F.; Barabash, A.; et al. Retinal Vascular Study Using OCTA in Subjects at High Genetic Risk of Developing Alzheimer’s Disease and Cardiovascular Risk Factors. J. Clin. Med. 2022, 11, 3248. [Google Scholar] [CrossRef] [PubMed]
- López-Cuenca, I.; Marcos-Dolado, A.; Yus-Fuertes, M.; Salobrar-García, E.; Elvira-Hurtado, L.; Fernández-Albarral, J.A.; Salazar, J.J.; Ramírez, A.I.; Sánchez-Puebla, L.; Fuentes-Ferrer, M.E.; et al. The relationship between retinal layers and brain areas in asymptomatic first-degree relatives of sporadic forms of Alzheimer’s disease: An exploratory analysis. Alzheimer’s Res. Ther. 2022, 14, 79. [Google Scholar] [CrossRef]
- Tzekov, R.; Mullan, M. Vision function abnormalities in Alzheimer disease. Surv. Ophthalmol. 2014, 59, 414–433. [Google Scholar] [CrossRef]
- López-Cuenca, I.; Nebreda, A.; García-Colomo, A.; Salobrar-García, E.; de Frutos-Lucas, J.; Bruña, R.; Ramírez, A.I.; Ramirez-Toraño, F.; Salazar, J.J.; Barabash, A.; et al. Early visual alterations in individuals at-risk of Alzheimer’s disease: A multidisciplinary approach. Alzheimer’s Res. Ther. 2023, 15, 19. [Google Scholar] [CrossRef]
- Golzan, S.; Goozee, K.; Georgevsky, D.; Avolio, A.; Chatterjee, P.; Shen, K.; Gupta, V.; Chung, R.; Savage, G.; Orr, C.F.; et al. Retinal vascular and structural changes are associated with amyloid burden in the elderly: Ophthalmic biomarkers of preclinical Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Van De Kreeke, J.A.; Nguyen, H.; Haan, J.D.; Konijnenberg, E.; Tomassen, J.; Braber, A.D.; Kate, M.T.; Collij, L.; Yaqub, M.; Van Berckel, B.; et al. Retinal layer thickness in preclinical Alzheimer’s disease. Acta Ophthalmol. 2019, 97, 798–804. [Google Scholar] [CrossRef]
- Santos, C.Y.; Johnson, L.N.; Sinoff, S.E.; Festa, E.; Heindel, W.C.; Snyder, P.J. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimer’s Dementia: Diagn. Assess. Dis. Monit. 2018, 10, 196–209. [Google Scholar] [CrossRef]
- Salobrar-Garcia, E.; Hoyas, I.; Leal, M.; de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; et al. Analysis of Retinal Peripapillary Segmentation in Early Alzheimer’s Disease Patients. BioMed. Res. Int. 2015, 2015, 636548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth, A. Test-28 hue de Roth selon Farnsworth-Munsell (Manual); Luneau: Paris, France, 1966. [Google Scholar]
- Salobrar-García, E.; de Hoz, R.; Ramírez, A.I.; López-Cuenca, I.; Rojas, P.; Vazirani, R.; Amarante, C.; Yubero, R.; Gil, P.; Pinazo-Durán, M.D.; et al. Changes in visual function and retinal structure in the progression of Alzheimer’s disease. PLoS ONE 2019, 14, e0220535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rami, L.; Serradell, M.; Bosch, B.; Villar, A.; Molinuevo, J.L. Perception Digital Test (PDT) for the assessment of incipient visual disorder in initial Alzheimer’s disease. Neurologia 2007, 22, 342–347. [Google Scholar]
- Lozupone, M.; Panza, F. Impact of apolipoprotein E isoforms on sporadic Alzheimer’s disease: Beyond the role of amyloid beta. Neural Regen. Res. 2024, 19, 80–83. [Google Scholar] [CrossRef]
- Salvadó, G.; Grothe, M.J.; Groot, C.; Moscoso, A.; Schöll, M.; Gispert, J.D.; Ossenkoppele, R.; Initiative, F.T.A.D.N. Differential associations of APOE-ε2 and APOE-ε4 alleles with PET-measured amyloid-β and tau deposition in older individuals without dementia. Eur. J. Nucl. Med. 2021, 48, 2212–2224. [Google Scholar] [CrossRef]
- Papassotiropoulos, A.; Fountoulakis, M.; Dunckley, T.; Stephan, D.A.; Reiman, E.M. Genetics, transcriptomics and proteomics of Alzheimer’s disease. J. Clin. Psychiatry 2006, 67, 652. [Google Scholar] [CrossRef] [PubMed]
- Jáñez-Escalada, L.; Jáñez-García, L.; Salobrar-García, E.; Santos-Mayo, A.; De Hoz, R.; Yubero, R.; Gil, P.; Ramírez, J.M. Spatial analysis of thickness changes in ten retinal layers of Alzheimer’s disease patients based on optical coherence tomography. Sci. Rep. 2019, 9, 13000. [Google Scholar] [CrossRef] [Green Version]
- Alamouti, B.; Funk, J. Retinal thickness decreases with age: An OCT study. Br. J. Ophthalmol. 2003, 87, 899–901. [Google Scholar] [CrossRef]
- Ramírez, A.I.; Fernández-Albarral, J.A.; de Hoz, R.; López-Cuenca, I.; Salobrar-García, E.; Rojas, P.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; et al. Microglial changes in the early aging stage in a healthy retina and an experimental glaucoma model. Prog. Brain Res. 2020, 256, 125–149. [Google Scholar]
- Trapp, B.D.; Wujek, J.R.; Criste, G.A.; Jalabi, W.; Yin, X.; Kidd, G.J.; Stohlman, S.; Ransohoff, R. Evidence for synaptic stripping by cortical microglia. Glia 2007, 55, 360–368. [Google Scholar] [CrossRef]
- De Hoz, R.; Gallego, B.I.; Ramírez, A.I.; Rojas, B.; Salazar, J.J.; Valiente-Soriano, F.J.; Avilés-Trigueros, M.; Villegas-Perez, M.P.; Vidal-Sanz, M.; Triviño, A.; et al. Rod-Like Microglia Are Restricted to Eyes with Laser-Induced Ocular Hypertension but Absent from the Microglial Changes in the Contralateral Untreated Eye. PLoS ONE 2013, 8, e83733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van de Kreeke, J.A.; Nguyen, H.T.; Konijnenberg, E.; Tomassen, J.; Braber, A.D.; Kate, M.T.; Yaqub, M.; van Berckel, B.; Lammertsma, A.A.; Boomsma, D.I.; et al. Longitudinal retinal layer changes in preclinical Alzheimer’s disease. Acta Ophthalmol. 2020, 99, 538–544. [Google Scholar] [CrossRef]
- Jansen, W.J.; Ossenkoppele, R.; Knol, D.L.; Tijms, B.M.; Scheltens, P.; Verhey, F.R.; Visser, P.J.; Aalten, P.; Aarsland, D.; Alcolea, D.; et al. Faculty Opinions recommendation of Prevalence of cerebral amyloid pathology in persons without dementia: A meta-analysis. JAMA 2017, 313, 1924–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marquié, M.; on behalf of the FACEHBI Study Group; Valero, S.; Castilla-Marti, M.; Martínez, J.; Rodríguez-Gómez, O.; Sanabria, Á.; Tartari, J.P.; Monté-Rubio, G.C.; Sotolongo-Grau, O.; et al. Association between retinal thickness and β-amyloid brain accumulation in individuals with subjective cognitive decline: Fundació ACE Healthy Brain Initiative. Alzheimer’s Res. Ther. 2020, 12, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, D.L.; Thompson, L.; Snyder, P.J. A Potential Association Between Retinal Changes, Subjective Memory Impairment, and Anxiety in Older Adults at Risk for Alzheimer’s Disease: A 27-Month Pilot Study. Front. Aging Neurosci. 2019, 11, 288. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Salinas-Navarro, M.; Jiménez-López, M.; Sobrado-Calvo, P.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Displaced retinal ganglion cells in albino and pigmented rats. Front. Neuroanat. 2014, 8, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannibal, J.; Christiansen, A.T.; Heegaard, S.; Fahrenkrug, J.; Kiilgaard, J.F. Melanopsin expressing human retinal ganglion cells: Subtypes, distribution, and intraretinal connectivity. J. Comp. Neurol. 2017, 525, 1934–1961. [Google Scholar] [CrossRef]
- Sjöstrand, J.; Popovic, Z.; Conradi, N.; Marshall, J. Morphometric study of the displacement of retinal ganglion cells subserving cones within the human fovea. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 1014–1023. [Google Scholar] [CrossRef]
- Shin, J.Y.; Choi, E.Y.; Kim, M.; Lee, H.K.; Byeon, S.H. Changes in retinal microvasculature and retinal layer thickness in association with apolipoprotein E genotype in Alzheimer’s disease. Sci. Rep. 2021, 11, 1847. [Google Scholar] [CrossRef]
- Armstrong, G.W.; Kim, L.A.; Vingopoulos, F.; Park, J.Y.; Garg, I.; Kasetty, M.; Silverman, R.F.; Zeng, R.; Douglas, V.P.; Lopera, F.; et al. Retinal Imaging Findings in Carriers With PSEN1-Associated Early-Onset Familial Alzheimer Disease Before Onset of Cognitive Symptoms. JAMA Ophthalmol. 2021, 139, 49–56. [Google Scholar] [CrossRef]
- Gupta, V.B.; Chitranshi, N.; den Haan, J.; Mirzaei, M.; You, Y.; Lim, J.K.; Basavarajappa, D.; Godinez, A.; Di Angelantonio, S.; Sachdev, P.; et al. Retinal changes in Alzheimer’s disease—Integrated prospects of imaging, functional and molecular advances. Prog. Retin. Eye Res. 2021, 82, 100899. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martin, E.S.; Rojas, B.; Ramirez, A.I.; de Hoz, R.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; Ramirez, J.M. Macular Thickness as a Potential Biomarker of Mild Alzheimer’s Disease. Ophthalmology 2014, 121, 1149–1151.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salobrar-Garcia, E.; de Hoz, R.; Rojas, B.; Ramirez, A.I.; Salazar, J.J.; Yubero, R.; Gil, P.; Triviño, A.; Ramirez, J.M. Ophthalmologic Psychophysical Tests Support OCT Findings in Mild Alzheimer’s Disease. J. Ophthalmol. 2015, 2015, 736949. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Zheng, H.; Hu, J.; Jiang, L.; Cao, X.; Chen, Y.; Mei, X.; Li, C.; Shen, Y. Retinal Nerve Fiber Layer Thinning Is Associated With Brain Atrophy: A Longitudinal Study in Nondemented Older Adults. Front. Aging Neurosci. 2019, 11, 69. [Google Scholar] [CrossRef] [Green Version]
- Mishra, S.; Blazey, T.M.; Holtzman, D.M.; Cruchaga, C.; Su, Y.; Morris, J.C.; Benzinger, T.L.S.; Gordon, B.A. Longitudinal brain imaging in preclinical Alzheimer disease: Impact of APOE ε4 genotype. Brain 2018, 141, 1828–1839. [Google Scholar] [CrossRef]
- Pegueroles, J.; Vilaplana, E.; Montal, V.; Sampedro, F.; Alcolea, D.; Carmona-Iragui, M.; Clarimon, J.; Blesa, R.; Lleó, A.; Fortea, J.; et al. Longitudinal brain structural changes in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2016, 13, 499–509. [Google Scholar] [CrossRef]
- Fjell, A.M.; McEvoy, L.; Holland, D.; Dale, A.M.; Walhovd, K.B. What is normal in normal aging? Effects of aging, amyloid and Alzheimer’s disease on the cerebral cortex and the hippocampus. Prog. Neurobiol. 2014, 117, 20–40. [Google Scholar] [CrossRef] [Green Version]
- Bakkour, A.; Morris, J.C.; Wolk, D.A.; Dickerson, B.C. The effects of aging and Alzheimer’s disease on cerebral cortical anatomy: Specificity and differential relationships with cognition. Neuroimage 2013, 76, 332–344. [Google Scholar] [CrossRef] [Green Version]
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef]
- Ren, S.-Q.; Yao, W.; Yan, J.-Z.; Jin, C.; Yin, J.-J.; Yuan, J.; Yu, S.; Cheng, Z. Amyloid β causes excitation/inhibition imbalance through dopamine receptor 1-dependent disruption of fast-spiking GABAergic input in anterior cingulate cortex. Sci. Rep. 2018, 8, 302. [Google Scholar] [CrossRef] [Green Version]
- Ortuño-Lizarán, I.; Sánchez-Sáez, X.; Lax, P.; Serrano, G.E.; Beach, T.G.; Adler, C.H.; Cuenca, N. Dopaminergic Retinal Cell Loss and Visual Dysfunction in Parkinson Disease. Ann. Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Margeta, M.A.; Yin, Z.; Madore, C.; Pitts, K.M.; Letcher, S.M.; Tang, J.; Jiang, S.; Gauthier, C.D.; Silveira, S.R.; Schroeder, C.M.; et al. Apolipoprotein E4 impairs the response of neurodegenerative retinal microglia and prevents neuronal loss in glaucoma. Immunity 2022, 55, 1627–1644.e7. [Google Scholar] [CrossRef] [PubMed]
- Baird, P.N.; Richardson, A.J.; Robman, L.D.; Dimitrov, P.N.; Tikellis, G.; McCarty, C.A.; Guymer, R.H. Apolipoprotein (APOE) gene is associated with progression of age-related macular degeneration (AMD). Hum. Mutat. 2006, 27, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Hoffmann, T.J.; Melles, R.B.; Sakoda, L.C.; Kvale, M.N.; Banda, Y.; Schaefer, C.; Risch, N.; Jorgenson, E. Differences in the Genetic Susceptibility to Age-Related Macular Degeneration Clinical Subtypes. Investig. Opthalmol. Vis. Sci. 2015, 56, 4290–4299. [Google Scholar] [CrossRef] [Green Version]


| FH− ApoE ɛ4− 40–60 yrs | FH+ ApoE ɛ4+ 40–60 yrs | p-Value at 27 Months FH− ApoE ɛ4− 40–60 yrs vs. FH+ ApoE ɛ4− 40–60 yrs | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | |||
| Visual acuity | 0.981 ± 0.040 | 0.933 ± 0.070 | 0.102 | 1.036 ± 0.095 | 1.077 ± 0.148 | 0.285 | 0.014 * | |
| Contrast sensitivity | 3 cpd | 1.724 ± 0.121 | 1.731 ± 0.148 | 0.131 | 1.749 ± 0.148 | 1.845 ± 0.176 | 0.100 | 0.047 * |
| 6 cpd | 1.972 ± 0.171 | 2.007 ± 0.158 | 0.943 | 2.028 ± 0.213 | 2.008 ± 0.139 | 0.823 | 0.932 | |
| 12 cpd | 1.544 ± 0.170 | 1.511 ± 0.142 | 0.311 | 1.675 ± 0.181 | 1.708 ± 0.181 | 0.259 | 0.008 ** | |
| 18 cpd | 1.144 ± 0.164 | 1.283 ± 0.100 | 0.119 | 1.219 ± 0.197 | 1.261 ± 0.176 | 0.059 | 0.777 | |
| Color perception | Total errors | 1.500 (0.000–6.000) | 2.000 (0.000–6.000) | 0.127 | 3.000 (0.000–3.000) | 2.000 (0.000–4.500) | 0.304 | 0.710 |
| Tritan errors | 1.000 (0.000–2.750) | 0.000 (0.000–3.000) | 0.303 | 0.000 (0.000–3.000) | 0.000 (0.000–1.500) | 0.263 | 0.620 | |
| Deutan errors | 1.000 (0.000–2.750) | 0.000 (0.000–3.000) | 0.347 | 0.000 (0.000–3.000) | 0.000 (0.000–1.500) | 0.205 | 0.853 | |
| PDT | 0.000 (0.000–1.000) | 0.000 (0.000–0.000) | 0.157 | 0.000 (0.000–0.000) | 0.000 (0.000–0.500) | 0.157 | 0.454 | |
| Visual Field | Fixation losses | 0.500 (0.000–1.000) | 1.000 (0.000–1.000) | 0.408 | 0.000 (0.000–1.000) | 0.000 (0.000–1.000) | 0.890 | 0.311 |
| False positives % | 1.000 (0.000–5.000) | 2.000 (0.000–2.500) | 0.414 | 3.000 (0.000–5.750) | 2.000 (0.000–6.500) | 0.552 | 0.460 | |
| %False negatives | 0.000 (0.000–1.000) | 0.000 (0.000–3.500) | 0.273 | 0.000 (0.000–4.000) | 1.000 (0.000–3.000) | 0.959 | 0.954 | |
| VFI (%) | 99.500 (99.000–100.000) | 99.000 (98.500–100.000) | 0.083 | 100.000 (99.000–100.000) | 99.000 (97.000–100.000) | 0.305 | 0.735 | |
| Mean deviation (MD) | −0.885 (−1.868–2.950) | −0.420 (−1.695–0.115) | 0.484 | −0.650 (−1.675–0.013) | −0.560 (−2.155–0.165) | 0.999 | 0.936 | |
| Pattern standard deviation (PSD) | 1.635 (1.410–1.833) | 1.640 (1.445–2.085) | 0.575 | 1.535 (1.318–1.848) | 1.660 (1.4650–2.420) | 0.064 | 0.500 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Cuenca, I.; Sánchez-Puebla, L.; Salobrar-García, E.; Álvarez-Gutierrez, M.; Elvira-Hurtado, L.; Barabash, A.; Ramírez-Toraño, F.; Fernández-Albarral, J.A.; Matamoros, J.A.; Nebreda, A.; et al. Exploratory Longitudinal Study of Ocular Structural and Visual Functional Changes in Subjects at High Genetic Risk of Developing Alzheimer’s Disease. Biomedicines 2023, 11, 2024. https://doi.org/10.3390/biomedicines11072024
López-Cuenca I, Sánchez-Puebla L, Salobrar-García E, Álvarez-Gutierrez M, Elvira-Hurtado L, Barabash A, Ramírez-Toraño F, Fernández-Albarral JA, Matamoros JA, Nebreda A, et al. Exploratory Longitudinal Study of Ocular Structural and Visual Functional Changes in Subjects at High Genetic Risk of Developing Alzheimer’s Disease. Biomedicines. 2023; 11(7):2024. https://doi.org/10.3390/biomedicines11072024
Chicago/Turabian StyleLópez-Cuenca, Inés, Lidia Sánchez-Puebla, Elena Salobrar-García, María Álvarez-Gutierrez, Lorena Elvira-Hurtado, Ana Barabash, Federico Ramírez-Toraño, José A. Fernández-Albarral, José A. Matamoros, Alberto Nebreda, and et al. 2023. "Exploratory Longitudinal Study of Ocular Structural and Visual Functional Changes in Subjects at High Genetic Risk of Developing Alzheimer’s Disease" Biomedicines 11, no. 7: 2024. https://doi.org/10.3390/biomedicines11072024