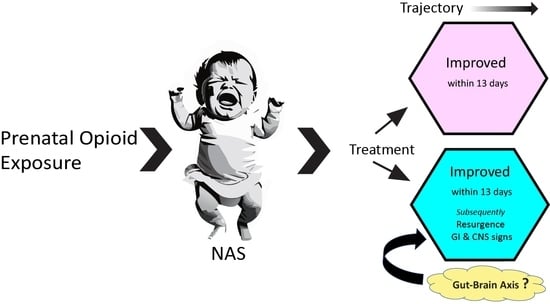
Neonatal Abstinence Signs during Treatment: Trajectory, Resurgence and Heterogeneity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Methods
2.2. Statistical Analyses
3. Results
3.1. Sample of Infants Studied
3.2. Mean FS and Prediction of the LOT
3.3. FNAST Item Prevalence and LOT
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ko, J.Y.; Yoon, J.; Tong, V.T.; Haight, S.C.; Patel, R.; Rockhill, K.M.; Luck, J.; Shapiro-Mendoza, C. Maternal opioid exposure, neonatal abstinence syndrome, and infant healthcare utilization: A retrospective cohort analysis. Drug Alcohol Depend. 2021, 223, 108704. [Google Scholar] [CrossRef]
- Ramphul, K.; Mejias, S.G.; Joynauth, J. Increase in Incidence of Neonatal Abstinence Syndrome among In-Hospital Birth in the United States. JAMA Pediatr. 2021, 175, 99–100. [Google Scholar] [CrossRef]
- Hirai, A.H.; Ko, J.Y.; Owens, P.L.; Stocks, C.; Patrick, S.W. Neonatal Abstinence Syndrome and Maternal Opioid-Related Diagnoses in the US, 2010–2017. JAMA 2021, 325, 146–155. [Google Scholar] [CrossRef]
- Reddy, U.M.; Davis, J.M.; Ren, Z.; Greene, M.F.; Workshop Invited Speakers. Opioid Use in Pregnancy, Neonatal Abstinence Syndrome, and Childhood Outcomes: Executive Summary of a Joint Workshop by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, American College of Obstetricians and Gynecologists, American Academy of Pediatrics, Society for Maternal-Fetal Medicine, Centers for Disease Control and Prevention, and the March of Dimes Foundation. Obstet. Gynecol. 2017, 130, 10–28. [Google Scholar] [CrossRef]
- Oei, J.L.; Blythe, S.; Dicair, L.; Didden, D.; Preisz, A.; Lantos, J. What’s in a name? The ethical implications and opportunities in diagnosing an infant with neonatal abstinence syndrome (NAS). Addiction 2022, 118, 4–6. [Google Scholar] [CrossRef]
- Patrick, S.W.; Barfield, W.D.; Poindexter, B.B.; Committee on Fetus and Newborn; Committee on Substance Use and Prevention. Neonatal Opioid Withdrawal Syndrome. Pediatrics 2020, 146, e2020029074. [Google Scholar] [CrossRef] [PubMed]
- Patrick, S.W.; Schumacher, R.E.; Benneyworth, B.D.; Krans, E.E.; McAllister, J.M.; Davis, M.M. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000–2009. JAMA 2012, 307, 1934–1940. [Google Scholar] [CrossRef] [PubMed]
- Desmond, M.M.; Wilson, G.S. Neonatal abstinence syndrome: Recognition and diagnosis. Addict. Dis. 1975, 2, 113–121. [Google Scholar] [PubMed]
- Kolb, L.; Himmelsbach, C.K. Clinical studies of drug addiction, III: A critical review of the withdrawal treatments with method of evaluating abstinence syndromes. Am. J. Psychiatr. 1938, 94, 759–799. [Google Scholar] [CrossRef]
- Finnegan, L.P.; Kron, R.E.; Connaughton, J.F.; Emich, J.P. Assessment and treatment of abstinence in the infant of the drug-dependent mother. Int. J. Clin. Pharmacol. Biopharm. 1975, 12, 19–32. [Google Scholar] [PubMed]
- Finnegan, L.P.; MacNew, B.A. Care of the addicted infant. Am. J. Nurs. 1974, 74, 685–693. [Google Scholar]
- Maguire, D.; Cline, G.J.; Parnell, L.; Tai, C.Y. Validation of the Finnegan neonatal abstinence syndrome tool-short form. Adv. Neonatal. Care 2013, 13, 430–437. [Google Scholar] [CrossRef]
- Gomez Pomar, E.; Finnegan, L.P.; Devlin, L.; Bada, H.; Concina, V.A.; Ibonia, K.T.; Westgate, P.M. Simplification of the Finnegan Neonatal Abstinence Scoring System: Retrospective study of two institutions in the USA. BMJ Open 2017, 7, e016176. [Google Scholar] [CrossRef] [PubMed]
- Devlin, L.A.; Breeze, J.L.; Terrin, N.; Gomez Pomar, E.; Bada, H.; Finnegan, L.P.; O’Grady, K.E.; Jones, H.E.; Lester, B.; Davis, J.M. Association of a Simplified Finnegan Neonatal Abstinence Scoring Tool with the Need for Pharmacologic Treatment for Neonatal Abstinence Syndrome. JAMA Netw. Open 2020, 3, e202275. [Google Scholar] [CrossRef] [PubMed]
- Chervoneva, I.; Adeniyi-Jones, S.C.; Blanco, F.; Kraft, W.K. Development of an abbreviated symptom score for the neonatal abstinence syndrome. J. Perinatol. 2020, 40, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Davis, J.M. Escaping the Finnegan—Is it time? Semin. Fetal Neonatal Med. 2021, 26, 101218. [Google Scholar] [CrossRef] [PubMed]
- Schiff, D.M.; Grossman, M.R. Beyond the Finnegan scoring system: Novel assessment and diagnostic techniques for the opioid-exposed infant. Semin. Fetal Neonatal Med. 2019, 24, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.R.; Berkwitt, A.K.; Osborn, R.R.; Xu, Y.; Esserman, D.A.; Shapiro, E.D.; Bizzarro, M.J. An Initiative to Improve the Quality of Care of Infants With Neonatal Abstinence Syndrome. Pediatrics 2017, 139, e20163360. [Google Scholar] [CrossRef] [PubMed]
- Schubach, N.E.; Mehler, K.; Roth, B.; Korsch, E.; Laux, R.; Singer, D.; von der Wense, A.; Treszl, A.; Hunseler, C. Skin conductance in neonates suffering from abstinence syndrome and unexposed newborns. Eur. J. Pediatr. 2016, 175, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Heil, S.H.; Gaalema, D.E.; Johnston, A.M.; Sigmon, S.C.; Badger, G.J.; Higgins, S.T. Infant pupillary response to methadone administration during treatment for neonatal abstinence syndrome: A feasibility study. Drug Alcohol Depend. 2012, 126, 268–271. [Google Scholar] [CrossRef]
- Manigault, A.W.; Sheinkopf, S.J.; Silverman, H.F.; Lester, B.M. Newborn Cry Acoustics in the Assessment of Neonatal Opioid Withdrawal Syndrome Using Machine Learning. JAMA Netw. Open 2022, 5, e2238783. [Google Scholar] [CrossRef]
- Miller, J.S.; Bada, H.S.; Leggas, M.; Westgate, P.M. Assessment of the relative clinical utility of shortened Finnegan neonatal abstinence scoring tools. J. Perinatol. 2022, 42, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Tolia, V.N.; Patrick, S.W.; Bennett, M.M.; Murthy, K.; Sousa, J.; Smith, P.B.; Clark, R.H.; Spitzer, A.R. Increasing incidence of the neonatal abstinence syndrome in U.S. neonatal ICUs. N. Engl. J. Med. 2015, 372, 2118–2126. [Google Scholar] [CrossRef] [PubMed]
- Oei, J.L.; Wouldes, T. Will Simplifying the Finnegan Neonatal Abstinence Scoring Tool Improve Outcomes for Infants with Opioid Exposure? JAMA Netw. Open 2020, 3, e202271. [Google Scholar] [CrossRef] [PubMed]
- MacMillan, K.D.L.; Rendon, C.P.; Verma, K.; Riblet, N.; Washer, D.B.; Volpe Holmes, A. Association of Rooming-in With Outcomes for Neonatal Abstinence Syndrome: A Systematic Review and Meta-analysis. JAMA Pediatr. 2018, 172, 345–351. [Google Scholar] [CrossRef]
- Welle-Strand, G.K.; Skurtveit, S.; Jansson, L.M.; Bakstad, B.; Bjarko, L.; Ravndal, E. Breastfeeding reduces the need for withdrawal treatment in opioid-exposed infants. Acta Paediatr. 2013, 102, 1060–1066. [Google Scholar] [CrossRef] [PubMed]
- Velez, M.; Jansson, L.M. The Opioid dependent mother and newborn dyad: Non-pharmacologic care. J. Addict. Med. 2008, 2, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, G.M.; Laird, N.M.; Ware, J.H. Applied Longitudinal Analysis, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Liang, K.Y.; Zeger, S.L. Longitudinal data analysis using generalized liner models. Biometrika 1986, 73, 13–22. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing. Available online: https://www.R-project.org/ (accessed on 28 December 2023).
- Murrell, P. R Graphics; Chapman & Hall/CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- GraphPad Prism Version 9.3.1 for Windows, GraphPad Software LLC, Boston, Massachusetts USA. Available online: www.graphpad.com (accessed on 28 December 2023).
- SAS Institute. SAS/ACCESS 9.4 Interface to ADABAS: Reference; SAS Institute, Inc.: Cary, NC, USA, 2013. [Google Scholar]
- Grossman, M.R.; Lipshaw, M.J.; Osborn, R.R.; Berkwitt, A.K. A Novel Approach to Assessing Infants With Neonatal Abstinence Syndrome. Hosp. Pediatr. 2018, 8, 1–6. [Google Scholar] [CrossRef]
- Wachman, E.M.; Grossman, M.; Schiff, D.M.; Philipp, B.L.; Minear, S.; Hutton, E.; Saia, K.; Nikita, F.; Khattab, A.; Nolin, A.; et al. Quality improvement initiative to improve inpatient outcomes for Neonatal Abstinence Syndrome. J. Perinatol. 2018, 38, 1114–1122. [Google Scholar] [CrossRef]
- Young, L.W.; Ounpraseuth, S.T.; Merhar, S.L.; Hu, Z.; Simon, A.E.; Bremer, A.A.; Lee, J.Y.; Das, A.; Crawford, M.M.; Greenberg, R.G.; et al. Eat, Sleep, Console Approach or Usual Care for Neonatal Opioid Withdrawal. N. Engl. J. Med. 2023, 388, 2326–2337. [Google Scholar] [CrossRef]
- Gomez Pomar, E. A mini review of what matters in the management of NAS, is ESC the best care? Front. Pediatr. 2023, 11, 1239107. [Google Scholar] [CrossRef]
- Jansson, L.M.; Velez, M.L. Optimal Care for NAS: Are We Moving in the Wrong Direction? Hosp. Pediatr. 2019, 9, 655–658. [Google Scholar] [CrossRef]
- Surran, B.; Visintainer, P.; Chamberlain, S.; Kopcza, K.; Shah, B.; Singh, R. Efficacy of clonidine versus phenobarbital in reducing neonatal morphine sulfate therapy days for neonatal abstinence syndrome. A prospective randomized clinical trial. J. Perinatol. 2013, 33, 954–959. [Google Scholar] [CrossRef] [PubMed]
- Agthe, A.G.; Kim, G.R.; Mathias, K.B.; Hendrix, C.W.; Chavez-Valdez, R.; Jansson, L.; Lewis, T.R.; Yaster, M.; Gauda, E.B. Clonidine as an adjunct therapy to opioids for neonatal abstinence syndrome: A randomized, controlled trial. Pediatrics 2009, 123, e849–e856. [Google Scholar] [CrossRef]
- Hayhurst, C.J.; Durieux, M.E. Differential Opioid Tolerance and Opioid-induced Hyperalgesia: A Clinical Reality. Anesthesiology 2016, 124, 483–488. [Google Scholar] [CrossRef]
- Tse, D.; Chow, M.K.; Fung, W.; De Lima, J. Unexpectied opioid response ininfants: A retrospective case series. J. Paedatr. Neonatal. Dis. 2021, 1, 104–110. [Google Scholar]
- Lee, M.; Silverman, S.M.; Hansen, H.; Patel, V.B.; Manchikanti, L. A comprehensive review of opioid-induced hyperalgesia. Pain Physician 2011, 14, 145–161. [Google Scholar] [CrossRef]
- Pokela, M.L.; Olkkola, K.T.; Seppala, T.; Koivisto, M. Age-related morphine kinetics in infants. Dev. Pharmacol. Ther. 1993, 20, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, N.J.; Anderson, B.J.; Tibboel, D.; Holford, N.H. Developmental pharmacokinetics of morphine and its metabolites in neonates, infants and young children. Br. J. Anaesth. 2004, 92, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Matic, M.; Simons, S.H.; van Lingen, R.A.; van Rosmalen, J.; Elens, L.; de Wildt, S.N.; Tibboel, D.; van Schaik, R.H. Rescue morphine in mechanically ventilated newborns associated with combined OPRM1 and COMT genotype. Pharmacogenomics 2014, 15, 1287–1295. [Google Scholar] [CrossRef]
- Odekon, L.; Landau, R.; Blouin, J.L.; Brodow, D.; Wang, S.; Smiley, R.M. The Effect of beta2-Adrenoceptor Genotype on Phenylephrine Dose Administered During Spinal Anesthesia for Cesarean Delivery. Anesth. Analg. 2015, 120, 1309–1316. [Google Scholar] [CrossRef]
- Hahn, D.; Emoto, C.; Euteneuer, J.C.; Mizuno, T.; Vinks, A.A.; Fukuda, T. Influence of OCT1 Ontogeny and Genetic Variation on Morphine Disposition in Critically Ill Neonates: Lessons from PBPK Modeling and Clinical Study. Clin. Pharmacol. Ther. 2019, 105, 761–768. [Google Scholar] [CrossRef]
- Hahn, D.; Fukuda, T.; Euteneuer, J.C.; Mizuno, T.; Vinks, A.A.; Sadhasivam, S.; Emoto, C. Influence of MRP3 Genetics and Hepatic Expression Ontogeny for Morphine Disposition in Neonatal and Pediatric Patients. J. Clin. Pharmacol. 2020, 60, 992–998. [Google Scholar] [CrossRef]
- Baldo, B.A. Neonatal opioid toxicity: Opioid withdrawal (abstinence) syndrome with emphasis on pharmacogenomics and respiratory depression. Arch. Toxicol. 2023, 97, 2575–2585. [Google Scholar] [CrossRef]
- Holzer, P. Opioid receptors in the gastrointestinal tract. Regul. Pept. 2009, 155, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A. Toxicities of opioid analgesics: Respiratory depression, histamine release, hemodynamic changes, hypersensitivity, serotonin toxicity. Arch. Toxicol. 2021, 95, 2627–2642. [Google Scholar] [CrossRef]
- Smith, H.S.; Laufer, A. Opioid induced nausea and vomiting. Eur. J. Pharmacol. 2014, 722, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Maguire, D.; Groer, M. Neonatal abstinence syndrome and the gastrointestinal tract. Med. Hypotheses 2016, 97, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Sealschott, S.D.; Pickler, R.H.; Fortney, C.A.; Bailey, M.T. Integrative Review of Gut Microbiota and Expression of Symptoms Associated with Neonatal Abstinence Syndrome. Nurs. Res. 2020, 69, S66–S78. [Google Scholar] [CrossRef] [PubMed]
- Rhoads, J.M.; Collins, J.; Fatheree, N.Y.; Hashmi, S.S.; Taylor, C.M.; Luo, M.; Hoang, T.K.; Gleason, W.A.; Van Arsdall, M.R.; Navarro, F.; et al. Infant Colic Represents Gut Inflammation and Dysbiosis. J. Pediatr. 2018, 203, 55–61.e53. [Google Scholar] [CrossRef]
- Simpson, S.; McLellan, R.; Wellmeyer, E.; Matalon, F.; George, O. Drugs and Bugs: The Gut-Brain Axis and Substance Use Disorders. J. Neuroimmune Pharmacol. 2022, 17, 33–61. [Google Scholar] [CrossRef]
- Oei, J.L. After NAS. Semin. Fetal Neonatal Med. 2019, 24, 161–165. [Google Scholar] [CrossRef]
- Miller, J.S.; Bada, H.; Dunworth, C.; Charnigo, R. Recent and lifetime maternal substance use: Rurality and economic distress. Res. Nurs. Health 2023, 46, 502–514. [Google Scholar] [CrossRef] [PubMed]
- Heil, S.H.; Sigmon, S.C.; Jones, H.E.; Wagner, M. Comparison of characteristics of opioid-using pregnant women in rural and urban settings. Am. J. Drug Alcohol Abus. 2008, 34, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Gaalema, D.E.; Scott, T.L.; Heil, S.H.; Coyle, M.G.; Kaltenbach, K.; Badger, G.J.; Arria, A.M.; Stine, S.M.; Martin, P.R.; Jones, H.E. Differences in the profile of neonatal abstinence syndrome signs in methadone- versus buprenorphine-exposed neonates. Addiction 2012, 107 (Suppl. S1), 53–62. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Greenough, A.; Gerada, C. Maternal drug use and length of neonatal unit stay. Addiction 2003, 98, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Sanlorenzo, L.A.; Cooper, W.O.; Dudley, J.A.; Stratton, S.; Maalouf, F.I.; Patrick, S.W. Increased Severity of Neonatal Abstinence Syndrome Associated with Concomitant Antenatal Opioid and Benzodiazepine Exposure. Hosp. Pediatr. 2019, 9, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Kozhimannil, K.B.; Chantarat, T.; Ecklund, A.M.; Henning-Smith, C.; Jones, C. Maternal Opioid Use Disorder and Neonatal Abstinence Syndrome among Rural US Residents, 2007–2014. J. Rural Health 2019, 35, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Wachman, E.M.; Warden, A.H.; Thomas, Z.; Thomas-Lewis, J.A.; Shrestha, H.; Nikita, F.N.U.; Shaw, D.; Saia, K.; Schiff, D.M. Impact of psychiatric medication co-exposure on Neonatal Abstinence Syndrome severity. Drug Alcohol Depend. 2018, 192, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Wachman, E.M.; Hayes, M.J.; Brown, M.S.; Paul, J.; Harvey-Wilkes, K.; Terrin, N.; Huggins, G.S.; Aranda, J.V.; Davis, J.M. Association of OPRM1 and COMT single-nucleotide polymorphisms with hospital length of stay and treatment of neonatal abstinence syndrome. JAMA 2013, 309, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Yen, E.; Gaddis, N.; Jantzie, L.; Davis, J.M. A review of the genomics of neonatal abstinence syndrome. Front. Genet. 2023, 14, 1140400. [Google Scholar] [CrossRef] [PubMed]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miller, J.S.; Bada, H.S.; Westgate, P.M.; Sithisarn, T.; Leggas, M. Neonatal Abstinence Signs during Treatment: Trajectory, Resurgence and Heterogeneity. Children 2024, 11, 203. https://doi.org/10.3390/children11020203
Miller JS, Bada HS, Westgate PM, Sithisarn T, Leggas M. Neonatal Abstinence Signs during Treatment: Trajectory, Resurgence and Heterogeneity. Children. 2024; 11(2):203. https://doi.org/10.3390/children11020203
Chicago/Turabian StyleMiller, Jennifer S., Henrietta S. Bada, Philip M. Westgate, Thitinart Sithisarn, and Markos Leggas. 2024. "Neonatal Abstinence Signs during Treatment: Trajectory, Resurgence and Heterogeneity" Children 11, no. 2: 203. https://doi.org/10.3390/children11020203