Definition and Assessment of Paediatric Breakthrough Pain: A Qualitative Interview Study
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. The Elusive Nature of BTP
3.1.1. Pain in Relation to Background Pain Management
3.1.2. Nature of BTP
3.1.3. Types and Causes of BTP
3.1.4. BTP Management
3.2. BTP Assessment
3.2.1. Questionnaires Used in Clinical Practice
3.2.2. Reliance on Reports, Observations, and Clinical Judgement
3.2.3. Tailoring Assessments
3.3. A Validated Questionnaire Is Needed-Positive Attitudes
3.3.1. A Questionnaire Would Be Valuable
3.3.2. A Questionnaire for Family Caregivers and HCPs
3.4. Reservations towards a Paediatric BTP Assessment Questionnaire
3.4.1. Questionnaire Development Challenges
3.4.2. A New Pain Assessment Questionnaire May Be Unnecessary
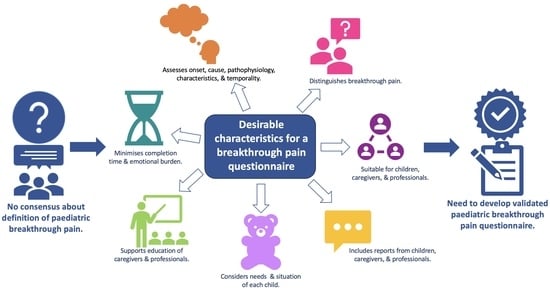
3.5. Features to Include in an Assessment
3.5.1. Identifying and Differentiating Pain Types
3.5.2. Improving Understanding of Assessment and Management
3.5.3. Adaptability to Individuals and Circumstances
3.5.4. Quantity of Information Collected
3.5.5. Questionnaire Design and Format
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fraser, L.; Gibson-Smith, D.; Jarvis, S.; Norman, P.; Parslow, R.C. ‘Make Every Child Count’ Estimating Current and Future Prevalence of Children and Young People with Life-Limiting Conditions in the United Kingdom; University of York: York, UK, 2020. [Google Scholar]
- Greenfield, K.; Schoth, D.E.; Hain, R.; Bailey, S.; Mott, C.; Rajapakse, D.; Harrop, E.; Renton, K.; Anderson, A.-K.; Carter, B.; et al. A rapid systematic review of breakthrough pain definitions and descriptions. Br. J. Pain 2023. [Google Scholar] [CrossRef]
- Davies, A.; Dickman, A.; Reid, C.; Stevens, A.-M.; Zeppetella, G. The management of cancer-related breakthrough pain: Recommendations of a task group of the Science Committee of the Association for Palliative Medicine of Great Britain and Ireland. Eur. J. Pain 2009, 13, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsdorf, S.J.; Postier, A. Management of breakthrough pain in children with cancer. J. Pain Res. 2014, 7, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Oostendorp, L.J.; Rajapakse, D.; Kelly, P.; Crocker, J.; Dinsdale, A.; Fraser, L.; Bluebond-Langner, M. Documentation of breakthrough pain in narrative clinical records of children with life-limiting conditions: Feasibility of a retrospective review. J. Child. Health Care 2018, 23, 564–578. [Google Scholar] [CrossRef] [PubMed]
- Snaman, J.M.; Baker, J.N.; Ehrentraut, J.H.; Anghelescu, D.L. Pediatric oncology: Managing pain at the end of life. Paediatr. Drugs 2016, 18, 161–180. [Google Scholar] [CrossRef] [PubMed]
- Vellucci, R.; Fanelli, G.; Pannuti, R.; Peruselli, C.; Adamo, S.; Alongi, G.; Amato, F.; Consoletti, L.; Lamarca, L.; Liguori, S.; et al. What to do, and what not to do, when diagnosing and treating breakthrough cancer pain (BTcP): Expert opinion. Drugs 2016, 76, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Webber, K.; Davies, A.; Zeppetella, G.; Cowie, M.R. Development and validation of the breakthrough pain assessment tool (BAT) in cancer patients. J. Pain Symptom Manage 2014, 48, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, J.; Orellana, L.; Ullrich, C.; Cook, E.F.; Kang, T.I.; Rosenberg, A.; Geyer, R.; Feudtner, C.; Dussel, V. Symptoms and distress in children with advanced cancer: Prospective patient-reported outcomes from the PediQUEST study. J. Clin. Oncol. 2015, 33, 1928–1935. [Google Scholar] [CrossRef]
- Narayana, A.; Katz, N.; Shillington, A.C.; Stephenson, J.J.; Harshaw, Q.; Frye, C.B.; Portenoy, R.K. National breakthrough pain study: Prevalence, characteristics, and associations with health outcomes. Pain 2015, 156, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Portenoy, R. Breakthrough cancer pain: Twenty-five years of study. Pain 2016, 157, 2657–2663. [Google Scholar] [CrossRef] [PubMed]
- Greco, M.T.; Corli, O.; Montanari, M.; Deandrea, S.; Zagonel, V.; Apolone, G. Epidemiology and pattern of care of breakthrough cancer pain in a longitudinal sample of cancer patients: Results from The Cancer Pain Outcome Research Study Group. Clin. J. Pain 2011, 27, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Davies, A.; Poulain, P.; Cortés-Funes, H.; Panchal, S.; Fanelli, G. Guidelines for the management of breakthrough pain in patients with cancer. J. Natl. Compr. Cancer Netw. 2013, 11, S29–S36. [Google Scholar] [CrossRef] [PubMed]
- Løhre, E.T.; Klepstad, P.; Bennett, M.I.; Brunelli, C.; Caraceni, A.; Fainsinger, R.L.; Knudsen, A.K.; Mercadante, S.; Sjøgren, P.; Kaasa, S. From “Breakthrough” to “Episodic” Cancer Pain? A European Association for Palliative Care Research Network Expert Delphi Survey Toward a Common Terminology and Classification of Transient Cancer Pain Exacerbations. J. Pain Symptom Manag. 2016, 51, 1013–1019. [Google Scholar] [CrossRef]
- Portenoy, R.; Hagen, N.A. Breakthrough pain: Definition and management. Oncology 1989, 3, 25–29. [Google Scholar] [PubMed]
- Portenoy, R.; Hagen, N.A. Breakthrough pain: Definition, prevalence and characteristics. Pain 1990, 41, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Caraceni, A.; Hanks, G.; Kaasa, S.; Bennett, M.I.; Brunelli, C.; Cherny, N.; Dale, O.; De Conno, F.; Fallon, M.; Hanna, M. Use of opioid analgesics in the treatment of cancer pain: Evidence-based recommendations from the EAPC. Lancet Oncolol. 2012, 13, e58–e68. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. WHO Guidelines on the Pharmacological Treatment of Persisting Pain in Children with Medical Illnesses; WHO: Geneva, Switzerland, 2012. [Google Scholar] [PubMed]
- Payne, R. Recognition and diagnosis of breakthrough pain. Pain Med. 2007, 8, S3–S7. [Google Scholar] [CrossRef] [PubMed]
- Daeninck, P.; Gagnon, B.; Gallagher, R.; Henderson, J.D.; Shir, Y.; Zimmermann, C.; Lapointe, B. Canadian recommendations for the management of breakthrough cancer pain. Curr. Oncol. 2016, 23, 96–108. [Google Scholar] [CrossRef]
- Hagen, N.A.; Biondo, P.; Stiles, C. Assessment and management of breakthrough pain in cancer patients: Current approaches and emerging research. Curr. Pain. Headache Rep. 2008, 12, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Mercadante, S.; Lazzari, M.; Reale, C.; Cuomo, A.; Fusco, F.; Marchetti, P.; Mediati, R.D.; Chiurazzi, B.; Ciuffedra, L.; Caraceni, A.; et al. Italian Oncological Pain Survey (IOPS): A multicentre Italian study of breakthrough pain performed in different settings. Clin. J. Pain 2015, 31, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Zeppetella, G.; Andersen, S.; Damkier, A.; Vejlgaard, T.; Nauck, F.; Radbruch, L.; Sjolund, K.-F.; Stenberg, M.; Buchanan, A. Multi-centre European study of breakthrough cancer pain: Pain characteristics and patient perceptions of current and potential management strategies. Eur. J. Pain 2011, 15, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.S.; Chang, V.T.; Kasimis, B. Cancer breakthrough pain characteristics and responses to treatment at a VA medical center. Pain 2003, 101, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Zeppetella, G.; Davies, A. Opioids for the management of breakthrough pain in cancer patients. Cochrane Database Syst. Rev. 2013, CD004311, Update in: Cochrane Database Syst. Rev. 2015, CD004311. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsdorf, S.J.; Postier, A.C. Recent advances in pain treatment for children with serious illness. Pain Manag. 2019, 9, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.B.; Andersen, S.; Arnason, S.; Arnér, S.; Breivik, H.; Heiskanen, T.; Kalso, E.; Kongsgaard, U.E.; Sjogren, P.; Strang, P.; et al. Breakthrough pain in malignant and non-malignant diseases: A review of prevalence, characteristics and mechanisms. Eur. J. Pain 2005, 9, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Davies, A. Cancer-related breakthrough pain. Br. J. Hosp. Med. 2006, 67, 414–416. [Google Scholar] [CrossRef] [PubMed]
- Feudtner, C.; Kang, T.I.; Hexem, K.R.; Friedrichsdorf, S.J.; Osenga, K.; Siden, H.; Friebert, S.E.; Hays, R.M.; Dussel, V.; Wolfe, J. Pediatric palliative care patients: A prospective multicenter cohort study. Pediatrics 2011, 127, 1094–1101. [Google Scholar] [CrossRef] [PubMed]
- Downing, J.; Jassal, S.S.; Mathews, L.; Brits, H.; Friedrichsdorf, S.J. Pediatric pain management in palliative care. Pain Manage 2015, 5, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H. Overcoming barriers in cancer pain management. J. Clin. Oncol. 2014, 32, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Jara, C.; del Barco, S.; Grávalos, C.; Hoyos, S.; Hernández, B.; Muñoz, M.; Quintanar, T.; Meana, J.A.; Rodriguez, C.; de las Peñas, R. SEOM clinical guideline for treatment of cancer pain (2017). Clin. Transl. Oncol. 2018, 20, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Fallon, M.; Giusti, R.; Aielli, F.; Hoskin, P.; Rolke, R.; Sharma, M.; Ripamonti, C.I. Management of cancer pain in adult patients: ESMO Clinical Practice Guidelines. Ann. Oncol. 2018, 29 (Suppl. S4), iv166–iv191. [Google Scholar] [CrossRef] [PubMed]
- Friedrichsdorf, S.J.; Finney, D.; Bergin, M.; Stevens, M.; Collins, J.J. Breakthrough pain in children with cancer. J. Pain. Symptom Manage 2007, 34, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, K.; Holley, S.; Schoth, D.E.; Bayliss, J.; Anderson, A.-K.; Jassal, S.; Rajapakse, D.; Fraser, L.K.; Mott, C.; Johnson, M. A protocol for a systematic review and meta-analysis to identify measures of breakthrough pain and evaluate their psychometric properties. BMJ Open 2020, 10, e035541. [Google Scholar] [CrossRef]
- Liossi, C.; Greenfield, K.; Schoth, D.E.; Mott, C.; Jassal, S.; Fraser, L.K.; Rajapakse, D.; Howard, R.F.; Johnson, M.; Anderson, A.-K. A systematic review of measures of breakthrough pain and their psychometric properties. J. Pain. Symptom Manage 2021, 62, 1041–1064. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, G.; Murphy, M.S.; Vickers, D.; Harrop, E.; Dworzynski, K. End of life care for infants, children and young people with life limiting conditions: Summary of NICE guidance. BMJ 2016, 355, i6385. [Google Scholar] [CrossRef] [PubMed]
- Saunders, C.H.; Sierpe, A.; von Plessen, C.; Kennedy, A.M.; Leviton, L.C.; Bernstein, S.L.; Goldwag, J.; King, J.R.; Marx, C.M.; Pogue, J.A.; et al. Practical thematic analysis: A guide for multidisciplinary health services research teams engaging in qualitative analysis. BMJ 2023, 381, e074256. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Clarke, V. Using thematic analysis in psychology. Qual. Res. Psycho 2006, 3, 77–101. [Google Scholar] [CrossRef]
- O’Brien, B.C.; Harris, I.B.; Beckman, T.J.; Reed, D.A.; Cook, D.A. Standards for reporting qualitative research: A synthesis of recommendations. Acad. Med. 2014, 89, 1245–1251. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.L.; Brook, L.; Mpundu-Kaambwa, C.; Harris, N.; Lapwood, S.; Randall, D. The Spectrum of Children’s Palliative Care Needs: A classification framework for children with life-limiting or life-threatening conditions. BMJ Support. Palliat. Care 2015, 5, 249. [Google Scholar] [CrossRef] [PubMed]
- Guest, G.; Bunce, A.; Johnson, L. How many interviews are enough?: An experiment with data saturation and variability. Field Methods 2006, 18, 59–82. [Google Scholar] [CrossRef]
- Greenfield, K.; Holley, S.; Schoth, D.E.; Harrop, E.; Howard, R.F.; Bayliss, J.; Brook, L.; Jassal, S.S.; Johnson, M.; Wong, I.; et al. A mixed-methods systematic review and meta-analysis of barriers and facilitators to paediatric symptom management at end of life. Palliat. Med. 2020, 34, 689–707. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, K.; Holley, S.; Schoth, D.E.; Harrop, E.; Howard, R.; Bayliss, J.; Brook, L.; Jassal, S.; Johnson, M.; Wong, I. Barriers and facilitators experienced by patients, carers and healthcare professionals when managing symptoms in infants, children and young people at end-of-life: A mixed methods systematic review protocol. BMJ Open 2019, 9, e030566. [Google Scholar] [CrossRef] [PubMed]
- Greenfield, K.; Carter, B.; Harrop, E.; Jassal, S.; Bayliss, M.J.; Renton, K.; Holley, S.; Howard, R.F.; Johnson, M.M.; Liossi, C. Healthcare professionals’ experiences of the barriers and facilitators to pediatric pain management in the community at end-of-life: A qualitative interview study. J. Pain Symptom Manage 2022, 63, 98–105. [Google Scholar] [CrossRef] [PubMed]
- QSR International Pty Ltd. NVivo Qualitative Data Analysis Software, version 12; QSR International Pty Lt: Victoria, Australia, 2018.
- Lincoln, Y.S.; Guba, E.G. Naturalistic Inquiry; Sage: Newbury Park, CA, USA, 1985. [Google Scholar]
- Bieri, D.; Reeve, R.A.; Champion, G.D.; Addicoat, L.; Ziegler, J.B. The Faces Pain Scale for the self-assessment of the severity of pain experienced by children: Development, initial validation, and preliminary investigation for ratio scale properties. Pain 1990, 41, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Merkel, S.I.; Voepel-Lewis, T.; Shayevitz, J.R.; Malviya, S. The FLACC: A behavioral scale for scoring postoperative pain in young children. Pediatr. Nurs. 1997, 23, 293–297. [Google Scholar] [PubMed]
- Mokkink, L.B.; Terwee, C.B.; Patrick, D.L.; Alonso, J.; Stratford, P.W.; Knol, D.L.; Bouter, L.M.; De Vet, H.C. The COSMIN checklist for assessing the methodological quality of studies on measurement properties of health status measurement instruments: An international Delphi study. Qual. Life Res. 2010, 19, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Kim, D.Y.; Lee, J.; Choi, Y.S.; Hwang, I.G.; Baek, S.K.; Seo, M.S.; Shim, J.Y. Practice patterns in distinguishing between background pain and breakthrough pain during patient education: A Korean physician survey. J. Cancer Educ. 2018, 33, 284–292. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Cancer Pain Relief and Palliative Care in Children; WHO: Geneva, Switzerland, 1998. [Google Scholar]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A. The revised IASP definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976. [Google Scholar] [CrossRef] [PubMed]
- Hechler, T.; Blankenburg, M.; Friedrichsdorf, S.J.; Garske, D.; Hübner, B.; Menke, A.; Wamsler, C.; Wolfe, J.; Zernikow, B. Parents’ perspective on symptoms, quality of life, characteristics of death and end-of-life decisions for children dying from cancer. Klin. Padiatr. 2008, 220, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rustøen, T.; Geerling, J.I.; Pappa, T.; Rundström, C.; Weisse, I.; Williams, S.C.; Zavratnik, B.; Wengström, Y. How nurses assess breakthrough cancer pain, and the impact of this pain on patients’ daily lives -results of a European survey. Eur. J. Oncol. Nurs. 2013, 17, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Haugen, D.F.; Hjermstad, M.J.; Hagen, N.A.; Caraceni, A.; Kaasa, S. Assessment and classification of cancer breakthrough pain: A systematic literature review. Pain 2010, 149, 476–482. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence. End of Life Care for Infants, Children and Young People with Life-Limiting Conditions: Planning and Management (Nice Guideline: NG61); National Institute for Health and Care Excellence: London, UK, 2016; pp. 1–45. [Google Scholar]
- Lyon, A.R.; Dorsey, S.; Pullmann, M.; Silbaugh-Cowdin, J.; Berliner, L. Clinician use of standardized assessments following a common elements psychotherapy training and consultation program. Adm. Policy Ment. Health 2015, 42, 47–60. [Google Scholar] [CrossRef] [PubMed]
- O’Hagan, P.; Mercadante, S. Breakthrough cancer pain: The importance of the right treatment at the right time. Eur. J. Pain 2018, 22, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Ammentorp, J.; Mainz, J.; Sabroe, S. Parents’ priorities and satisfaction with acute pediatric care. Arch. Pediatr. Adolesc. Med. 2005, 159, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Breau, L.M. Non-communicating children’s pain checklist: Better pain assessment for severely disabled children. Expert. Rev. Pharmacoecon Outcomes Res. 2003, 3, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Palermo, T.M.; Valenzuela, D.; Stork, P.P. A randomized trial of electronic versus paper pain diaries in children: Impact on compliance, accuracy, and acceptability. Pain 2004, 107, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; West, N.; Ansermino, J.M.; Montgomery, C.J.; Myers, D.; Dunsmuir, D.; Lauder, G.R.; von Baeyer, C.L. A smartphone version of the Faces Pain Scale-Revised and the Color Analog Scale for postoperative pain assessment in children. Paediatr. Anaesth. 2015, 25, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yao, N.A.; Liu, Y.; Geng, Z.; Wang, Y.; Shen, N.; Zhang, X.; Shen, M.; Yuan, C. Development of a smartphone application to monitor pediatric patient-reported outcomes. Comput. Inform. Nurs. 2017, 35, 590–598. [Google Scholar] [CrossRef] [PubMed]
- Camps Herrero, C.; Batista, N.; Díaz Fernández, N.; Escobar Álvarez, Y.; Gonzalo Gómez, A.; Isla Casado, D.; Salud, A.; Terrasa Pons, J.; Guillem Porta, V. Breakthrough cancer pain: Review and calls to action to improve its management. Clin. Transl. Oncol. 2020, 22, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Bunn, R.J.; Griffiths, M.; Bunn, R. Understanding and managing breakthrough pain: Ray Bunn & Professor Matt Griffiths discuss the management of breakthrough cancer pain with a focus on the community setting. J. Community Nurs. 2011, 25, 25–29. [Google Scholar]
- Hjermstad, M.J.; Kaasa, S.; Caraceni, A.; Loge, J.H.; Pedersen, T.; Haugen, D.F.; Aass, N. Characteristics of breakthrough cancer pain and its influence on quality of life in an international cohort of patients with cancer. BMJ Support. Palliat. Care 2016, 6, 344–352. [Google Scholar] [CrossRef]


| Definition |
|---|
| Portenoy and Hagen, 1989 [15]: ‘a transitory increase in pain to greater than moderate intensity which occurs on a baseline of pain of moderate intensity or less’ (p. 25) |
| Portenoy and Hagen, 1990 [16]: ‘a transitory exacerbation of pain that occurs on a background of otherwise stable pain in a patient receiving chronic opioid therapy’ (p. 273) |
| Davies, Dickman et al., 2009 [3]: ‘a transient exacerbation of pain that occurs either spontaneously, or in relation to a specific predictable or unpredictable trigger, despite relatively stable and adequately controlled background pain’ (p. 332) |
| European Association of Palliative Care, 2012 [17]: ‘transitory exacerbations of pain that occur on a background of stable pain otherwise adequately controlled by around-the-clock opioid therapy’ (p. e62) |
| World Health Organisation, 2012 [18]: ‘a temporary increase in the severity of pain over and above the pre-existing baseline pain level’ (p. 8) |
|
|
|
|
|
|
|
| Demographics | |
|---|---|
| Age (average ± SD) | 44.6 ± 8.1 years |
| Age range | 26–61 years |
| Female | n = 25 (86.2%) |
| Role | |
| Nurse | n = 12 (41.4%) |
| GP | n = 5 (17.2%) |
| Consultants and registrar doctors | n = 5 (17.2%) |
| Pharmacist | n = 2 (6.9%) |
| Psychological, social & physical support therapists | n = 5 (17.2%) |
| Work setting | |
| Community | n = 9 (31.0%) |
| Hospice | n = 10 (34.5%) |
| Hospital | n = 10 (34.5%) |
| Years in paediatric palliative care (average ± SD) | 11.1 ± 8.1 years |
| Years in paediatric palliative care (range) | 2 months–25 years |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawson, E.; Greenfield, K.; Carter, B.; Bailey, S.; Anderson, A.-K.; Rajapakse, D.; Renton, K.; Mott, C.; Hain, R.; Harrop, E.; et al. Definition and Assessment of Paediatric Breakthrough Pain: A Qualitative Interview Study. Children 2024, 11, 485. https://doi.org/10.3390/children11040485
Dawson E, Greenfield K, Carter B, Bailey S, Anderson A-K, Rajapakse D, Renton K, Mott C, Hain R, Harrop E, et al. Definition and Assessment of Paediatric Breakthrough Pain: A Qualitative Interview Study. Children. 2024; 11(4):485. https://doi.org/10.3390/children11040485
Chicago/Turabian StyleDawson, Eleanor, Katie Greenfield, Bernie Carter, Simon Bailey, Anna-Karenia Anderson, Dilini Rajapakse, Kate Renton, Christine Mott, Richard Hain, Emily Harrop, and et al. 2024. "Definition and Assessment of Paediatric Breakthrough Pain: A Qualitative Interview Study" Children 11, no. 4: 485. https://doi.org/10.3390/children11040485