Enhancing Executive Functions in Pediatric Epilepsy: Feasibility and Efficacy of a Computerized Cognitive Training Program
Abstract
:1. Introduction
1.1. The Importance of Executive Functions
1.2. Cognitive Intervention for Epilepsy
1.3. Our Proposal
2. Methods
2.1. Participants
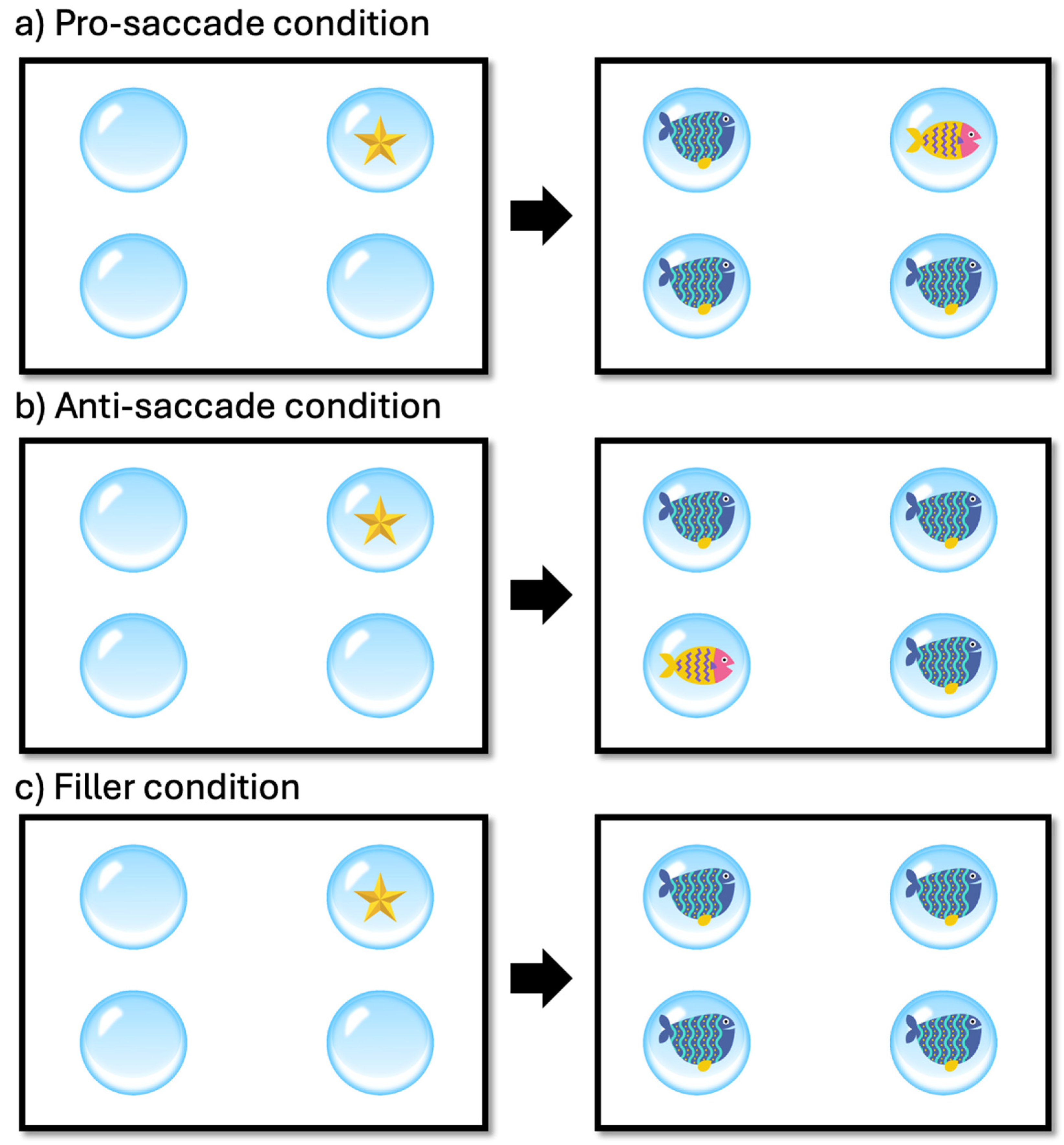
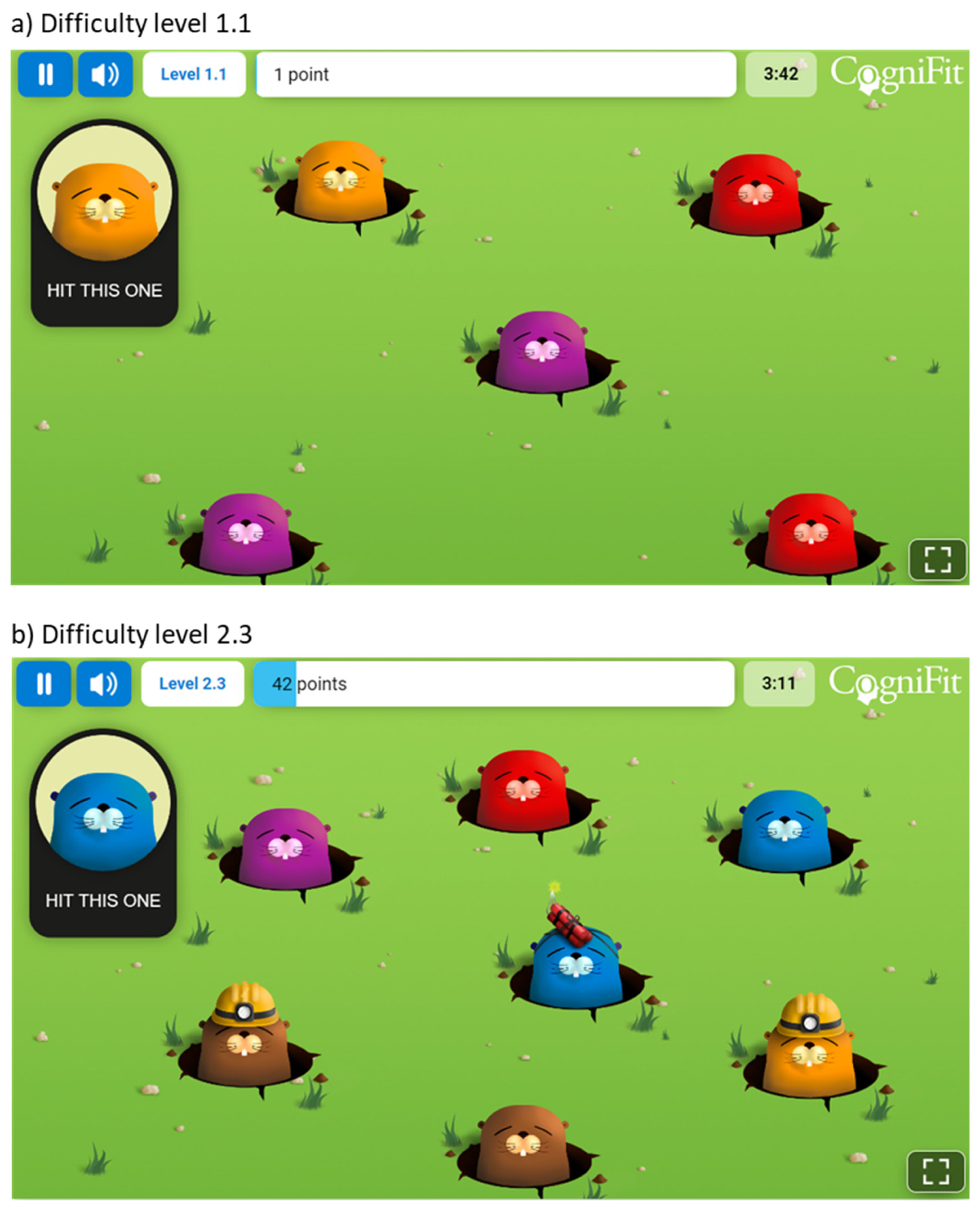
2.2. Materials and Procedure
3. Results
3.1. Reaction Time Analysis
3.2. Error Rate Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Murray, C.J.L.; Vos, T.; Lozano, R.; Naghavi, M.; Flaxman, A.D.; Michaud, C.; Ezzati, M.; Shibuya, K.; Salomon, J.A.; Abdalla, S.; et al. Disability-Adjusted Life Years (DALYs) for 291 Diseases and Injuries in 21 Regions, 1990–2010: A Systematic Analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380, 2197–2223. [Google Scholar] [CrossRef] [PubMed]
- Fiest, K.M.; Sauro, K.M.; Wiebe, S.; Patten, S.B.; Kwon, C.-S.; Dykeman, J.; Pringsheim, T.; Lorenzetti, D.L.; Jetté, N. Prevalence and Incidence of Epilepsy. Neurology 2017, 88, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E. The Epidemiology of Epilepsy. Neuroepidemiology 2020, 54, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Biset, G.; Abebaw, N.; Gebeyehu, N.A.; Estifanos, N.; Birrie, E.; Tegegne, K.D. Prevalence, Incidence, and Trends of Epilepsy among Children and Adolescents in Africa: A Systematic Review and Meta-Analysis. BMC Public Health 2024, 24, 771. [Google Scholar] [CrossRef] [PubMed]
- Strzelczyk, A.; Aledo-Serrano, A.; Coppola, A.; Didelot, A.; Bates, E.; Sainz-Fuertes, R.; Lawthom, C. The Impact of Epilepsy on Quality of Life: Findings from a European Survey. Epilepsy Behav. 2023, 142, 109179. [Google Scholar] [CrossRef] [PubMed]
- Operto, F.F.; Pastorino, G.M.G.; Viggiano, A.; Dell’Isola, G.B.; Dini, G.; Verrotti, A.; Coppola, G. Epilepsy and Cognitive Impairment in Childhood and Adolescence: A Mini-Review. Curr. Neuropharmacol. 2023, 21, 1646–1665. [Google Scholar] [CrossRef]
- Cairós-González, M.; Verche, E.; Hernández, S.; Alonso, M.Á. Cognitive Flexibility Impairment in Temporal Lobe Epilepsy: The Impact of Epileptic Foci Lateralization on Executive Functions. Epilepsy Behav. 2024, 151, 109587. [Google Scholar] [CrossRef]
- Koganti, H.; Paneyala, S.; Sundaramurthy, H.; Sc, N.; Kashyap, R.S.; Joshi, S.; Colaco, V. The Impact of Idiopathic Generalized Epilepsy on Executive Functions. Ann. Neurosci. 2020, 27, 131–135. [Google Scholar] [CrossRef]
- Diamond, A. Executive Functions. Annu. Rev. Psychol. 2013, 64, 135–168. [Google Scholar] [CrossRef] [PubMed]
- Sung, D.; Park, B.; Kim, B.; Kim, H.; Jung, K.-I.; Lee, S.-Y.; Kim, B.-N.; Park, S.; Park, M.-H. Gray Matter Volume in the Developing Frontal Lobe and Its Relationship with Executive Function in Late Childhood and Adolescence: A Community-Based Study. Front. Psychiatry 2021, 12, 686174. [Google Scholar] [CrossRef]
- Sánchez-Vincitore, L.V.; Cubilla-Bonnetier, D.; Marte-Santana, H.; Duñabeitia, J.A. Cognitive Decline Monitoring through a Web-Based Application. Front. Aging Neurosci. 2023, 15, 1212496. [Google Scholar] [CrossRef] [PubMed]
- Privitera, A.J.; Zhou, Y.; Xie, X. Inhibitory Control as a Significant Predictor of Academic Performance in Chinese High Schoolers. Child Neuropsychol. 2023, 29, 457–473. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wu, D.; Chen, Y.; Wang, Z.; Xiao, W. The Effect of Response Inhibition Training on Risky Decision-Making Task Performance. Front. Psychol. 2020, 11, 1806. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, Q.; Tian, M.; Nan, W.; Yang, G.; Liang, J.; Liu, X. Deficits in Voluntary Pursuit and Inhibition of Risk Taking in Sensation Seeking. Hum. Brain Mapp. 2017, 38, 6019–6028. [Google Scholar] [CrossRef] [PubMed]
- Berkman, E.T. The Neuroscience of Goals and Behavior Change. Consult. Psychol. J. Pract. Res. 2018, 70, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Coe, B.C.; Munoz, D.P. Mechanisms of Saccade Suppression Revealed in the Anti-Saccade Task. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2017, 372, 20160192. [Google Scholar] [CrossRef] [PubMed]
- Triplett, R.L.; Velanova, K.; Luna, B.; Padmanabhan, A.; Gaillard, W.D.; Asato, M.R. Investigating Inhibitory Control in Children with Epilepsy: An fMRI Study. Epilepsia 2014, 55, 1667. [Google Scholar] [CrossRef] [PubMed]
- Borai, A.; Aly, H.Y.; Ibrahim, H.K. Executive Functions Assessment in Adult Patients with Idiopathic Epilepsy. J. Behav. Brain Sci. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Veloso, A.; Vicente, S.G.; Filipe, M.G. Effectiveness of Cognitive Training for School-Aged Children and Adolescents with Attention Deficit/Hyperactivity Disorder: A Systematic Review. Front. Psychol. 2020, 10, 2983. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, L.; Wu, Z.; Cao, X.; Xue, G.; Wang, Y.; Yang, B. Computer-Based Multiple Component Cognitive Training in Children with ADHD: A Pilot Study. Child Adolesc. Psychiatry Ment. Health 2023, 17, 9. [Google Scholar] [CrossRef]
- Pasqualotto, A.; Mazzoni, N.; Bentenuto, A.; Mulè, A.; Benso, F.; Venuti, P. Effects of Cognitive Training Programs on Executive Function in Children and Adolescents with Autism Spectrum Disorder: A Systematic Review. Brain Sci. 2021, 11, 1280. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.L.; Puertas, F.J.; Duñabeitia, J.A. Study Protocol for a Randomized Controlled Trial Assessing the Effectiveness of Personalized Computerized Cognitive Training for Individuals with Insomnia. Front. Behav. Neurosci. 2022, 16, 779990. [Google Scholar] [CrossRef]
- Tapia, J.L.; Taberner-Bonastre, M.T.; Collado-Martínez, D.; Pouptsis, A.; Núñez-Abad, M.; Duñabeitia, J.A. Effectiveness of a Computerized Home-Based Cognitive Stimulation Program for Treating Cancer-Related Cognitive Impairment. Int. J. Environ. Res. Public Health 2023, 20, 4953. [Google Scholar] [CrossRef]
- Duñabeitia, J.A.; Mera, F.; Baro, Ó.; Jadad-Garcia, T.; Jadad, A.R. Personalized Computerized Training for Cognitive Dysfunction after COVID-19: A Before-and-After Feasibility Pilot Study. Int. J. Environ. Res. Public Health 2023, 20, 3100. [Google Scholar] [CrossRef] [PubMed]
- Fitapelli, B.; Lindenmayer, J.-P. Advances in Cognitive Remediation Training in Schizophrenia: A Review. Brain Sci. 2022, 12, 129. [Google Scholar] [CrossRef]
- Khaleghi, A.; Naderi, F.; Joharifard, R.; Javadzadeh, M. Comparing the Effectiveness of Computer-Based and Task-Oriented Cognitive Rehabilitation Programs on Epileptic Children’s Attention in Tehran. J. Compr. Ped 2024, 15, e137309. [Google Scholar] [CrossRef]
- Barnes, S.P.; Bailey, R.; Jones, S.M. Evaluating the Impact of a Targeted Approach Designed to Build Executive Function Skills: A Randomized Trial of Brain Games. Front. Psychol. 2021, 12, 655246. [Google Scholar] [CrossRef] [PubMed]
- Engelberts, N.H.J.; Klein, M.; Adèr, H.J.; Heimans, J.J.; Trenité, D.G.A.K.-N.; Van der Ploeg, H.M. The Effectiveness of Cognitive Rehabilitation for Attention Deficits in Focal Seizures: A Randomized Controlled Study. Epilepsia 2002, 43, 587–595. [Google Scholar] [CrossRef]
- Helmstaedter, C.; Loer, B.; Wohlfahrt, R.; Hammen, A.; Saar, J.; Steinhoff, B.J.; Quiske, A.; Schulze-Bonhage, A. The Effects of Cognitive Rehabilitation on Memory Outcome after Temporal Lobe Epilepsy Surgery. Epilepsy Behav. 2008, 12, 402–409. [Google Scholar] [CrossRef]
- Koorenhof, L.; Baxendale, S.; Smith, N.; Thompson, P. Memory Rehabilitation and Brain Training for Surgical Temporal Lobe Epilepsy Patients: A Preliminary Report. Seizure 2012, 21, 178–182. [Google Scholar] [CrossRef]
- Wan, H.; Liu, Q.; Chen, C.; Dong, W.; Wang, S.; Shi, W.; Li, C.; Ren, J.; Wang, Z.; Cui, T.; et al. An Integrative Nomogram for Identifying Cognitive Impairment Using Seizure Type and Cerebral Small Vessel Disease Neuroimaging Markers in Patients with Late-Onset Epilepsy of Unknown Origin. Neurol. Ther. 2024, 13, 107–125. [Google Scholar] [CrossRef] [PubMed]
- de Vries, S.T.; Mol, P.G.M.; de Zeeuw, D.; Haaijer-Ruskamp, F.M.; Denig, P. Development and Initial Validation of a Patient-Reported Adverse Drug Event Questionnaire. Drug Saf. 2013, 36, 765–777. [Google Scholar] [CrossRef] [PubMed]
- The Jamovi Project (2024). Jamovi (Version 2.5) [Computer Software]. Available online: https://www.jamovi.org (accessed on 16 April 2024).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Gallucci, M. GAMLj: General Analyses for Linear Models. 2019. Available online: https://gamlj.github.io/ (accessed on 16 April 2024).
- Luna, B.; Marek, S.; Larsen, B.; Tervo-Clemmens, B.; Chahal, R. An Integrative Model of the Maturation of Cognitive Control. Annu. Rev. Neurosci. 2015, 38, 151–170. [Google Scholar] [CrossRef] [PubMed]
- Dhir, S.; Teo, W.-P.; Chamberlain, S.R.; Tyler, K.; Yücel, M.; Segrave, R.A. The Effects of Combined Physical and Cognitive Training on Inhibitory Control: A Systematic Review and Meta-Analysis. Neurosci. Biobehav. Rev. 2021, 128, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Klingberg, T.; Fernell, E.; Olesen, P.J.; Johnson, M.; Gustafsson, P.; Dahlström, K.; Gillberg, C.G.; Forssberg, H.; Westerberg, H. Computerized Training of Working Memory in Children with ADHD-A Randomized, Controlled Trial. J. Am. Acad. Child Adolesc. Psychiatry 2005, 44, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhu, X.; Ziegler, A.; Shi, J. The Effects of Inhibitory Control Training for Preschoolers on Reasoning Ability and Neural Activity. Sci. Rep. 2015, 5, 14200. [Google Scholar] [CrossRef] [PubMed]
- Rhoades, B.L.; Greenberg, M.T.; Domitrovich, C.E. The Contribution of Inhibitory Control to Preschoolers’ Social–Emotional Competence. J. Appl. Dev. Psychol. 2009, 30, 310–320. [Google Scholar] [CrossRef]
- Garcia-Ramos, C.; Jackson, D.C.; Lin, J.J.; Dabbs, K.; Jones, J.E.; Hsu, D.A.; Stafstrom, C.E.; Zawadzki, L.; Seidenberg, M.; Prabhakaran, V.; et al. Cognition and Brain Development in Children with Benign Epilepsy with Centrotemporal Spikes. Epilepsia 2015, 56, 1615–1622. [Google Scholar] [CrossRef]
- O’Muircheartaigh, J.; Richardson, M.P. Epilepsy and the Frontal Lobes. Cortex 2012, 48, 144–155. [Google Scholar] [CrossRef]
- Wesnes, K.A.; Edgar, C.; Dean, A.D.P.; Wroe, S.J. The Cognitive and Psychomotor Effects of Remacemide and Carbamazepine in Newly Diagnosed Epilepsy. Epilepsy Behav. 2009, 14, 522–528. [Google Scholar] [CrossRef]
- Witt, J.-A.; Elger, C.E.; Helmstaedter, C. Adverse Cognitive Effects of Antiepileptic Pharmacotherapy: Each Additional Drug Matters. Eur. Neuropsychopharmacol. 2015, 25, 1954–1959. [Google Scholar] [CrossRef] [PubMed]
- Fastenau, P.S.; Johnson, C.S.; Perkins, S.M.; Byars, A.W.; deGrauw, T.J.; Austin, J.K.; Dunn, D.W. Neuropsychological Status at Seizure Onset in Children. Neurology 2009, 73, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Witt, J.-A.; Helmstaedter, C. Cognition in the Early Stages of Adult Epilepsy. Seizure 2015, 26, 65–68. [Google Scholar] [CrossRef] [PubMed]
- Oostrom, K.J.; Smeets-Schouten, A.; Kruitwagen, C.L.J.J.; Peters, A.C.B.; Jennekens-Schinkel, A.; Dutch Study Group of Epilepsy in Childhood (DuSECh). Not Only a Matter of Epilepsy: Early Problems of Cognition and Behavior in Children with “Epilepsy Only”—A Prospective, Longitudinal, Controlled Study Starting at Diagnosis. Pediatrics 2003, 112, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.; Kolamunnage-Dona, R.; Marson, A.G.; Smith, P.E.M.; Aldenkamp, A.P.; Baker, G.A.; SANAD Study Group. Patients with Epilepsy: Cognitively Compromised before the Start of Antiepileptic Drug Treatment? Epilepsia 2010, 51, 48–56. [Google Scholar] [CrossRef]
- Operto, F.F.; Pastorino, G.M.G.; Mazza, R.; Carotenuto, M.; Roccella, M.; Marotta, R.; di Bonaventura, C.; Verrotti, A. Effects on Executive Functions of Antiepileptic Monotherapy in Pediatric Age. Epilepsy Behav. 2020, 102, 106648. [Google Scholar] [CrossRef]
- Albsoul-Younes, A.M.; Masri, A.T.; Gharaibeh, L.F.; Murtaja, A.A.; Al-Qudah, A.A. Frequency of Antiepileptic Drugs and Response Change in Pediatric Patients Receiving 2 or More Antiepileptic Drugs. Neurosci. J. 2020, 25, 269–275. [Google Scholar] [CrossRef]
| ID | Gender | Age | Condition | Associated Condition(s) |
|---|---|---|---|---|
| 1 | Male | 9 | Refractory focal epilepsy | ADHD Combined |
| 2 | Male | 11 | Epilepsy | ADHD |
| 3 | Female | 8 | Epilepsy | - |
| 4 | Female | 16 | Dravet Syndrome | - |
| 5 | Female | 9 | Epilepsy due to PCDH19 gene mutation | - |
| 6 | Male | 10 | Focal epilepsy | Dyslexia and dysgraphia |
| 7 | Male | 13 | Refractory epilepsy | - |
| 8 | Male | 16 | Dravet Syndrome | - |
| 9 | Male | 13 | Dravet Syndrome | - |
| 10 | Male | 12 | Idiopathic epilepsy | Non-verbal learning disorder |
| 11 | Female | 14 | Epilepsy | ASD features, Genetic Syndrome |
| 12 | Female | 16 | Refractory epilepsy | ASD features |
| 13 | Male | 7 | Epilepsy | CSWS, Polymicrogyria, ADHD |
| 14 | Male | 15 | Refractory epilepsy | Double Hit, Craniotomy |
| 15 | Male | 14 | Rolandic epilepsy | - |
| 16 | Female | 11 | Generalized epilepsy | - |
| 17 | Male | 13 | Epilepsy | Attention deficit |
| 18 | Male | 15 | Epilepsy | - |
| 19 | Female | 9 | Focal epilepsy | - |
| 20 | Male | 9 | Refractory focal epilepsy | Cerebral palsy without cognitive impairment and with autonomous gait, ADHD of inattentive type, visual hallucinations (occipital focus) |
| 21 | Male | 10 | Atypical Rolandic epilepsy | - |
| 22 | Female | 17 | Epilepsy | - |
| 23 | Female | 13 | Epilepsy | Ring chromosome 20 mutation, OCD, attention deficit |
| 24 | Female | 16 | Refractory epilepsy | Genetic alteration of the SCN2A gene, ASD |
| 25 | Female | 9 | Refractory epilepsy | ADHD, dyslexia, dyscalculia |
| 26 | Female | 10 | Refractory epilepsy | Congenital CMV infection, bilateral hearing loss with cochlear implants, right hemiparesis |
| 95% Confidence Interval | |||||
|---|---|---|---|---|---|
| Experimental Condition | Time of Measurement | Mean | SD | Lower | Upper |
| Anti-saccade | Post-test | 1763 (17.5%) | 664 (38.1) | 1691 (13.9) | 1835 (21.2) |
| Pro-saccade | Post-test | 1708 (10.3%) | 583 (30.5) | 1648 (7.4) | 1769 (13.3) |
| Filler | Post-test | 1756 (12.1%) | 633 (32.7) | 1709 (9.9) | 1803 (14.4) |
| Anti-saccade | Pre-test | 1929 (16.1%) | 782 (36.8) | 1845 (12.6) | 2013 (19.7) |
| Pro-saccade | Pre-test | 1798 (8.4%) | 703 (27.8) | 1726 (5.7) | 1871 (11.1) |
| Filler | Pre-test | 1899 (13.3%) | 746 (34.0) | 1843 (11.0) | 1954 (15.7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tapia, J.L.; Aras, L.M.; Duñabeitia, J.A. Enhancing Executive Functions in Pediatric Epilepsy: Feasibility and Efficacy of a Computerized Cognitive Training Program. Children 2024, 11, 484. https://doi.org/10.3390/children11040484
Tapia JL, Aras LM, Duñabeitia JA. Enhancing Executive Functions in Pediatric Epilepsy: Feasibility and Efficacy of a Computerized Cognitive Training Program. Children. 2024; 11(4):484. https://doi.org/10.3390/children11040484
Chicago/Turabian StyleTapia, José Luis, Luis Miguel Aras, and Jon Andoni Duñabeitia. 2024. "Enhancing Executive Functions in Pediatric Epilepsy: Feasibility and Efficacy of a Computerized Cognitive Training Program" Children 11, no. 4: 484. https://doi.org/10.3390/children11040484





