Motor Adaptation Deficits in Children with Developmental Coordination Disorder and/or Reading Disorder
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedure
2.3. Material
2.4. Data Analysis
2.4.1. Data Processing
2.4.2. Statistical Analysis
3. Results
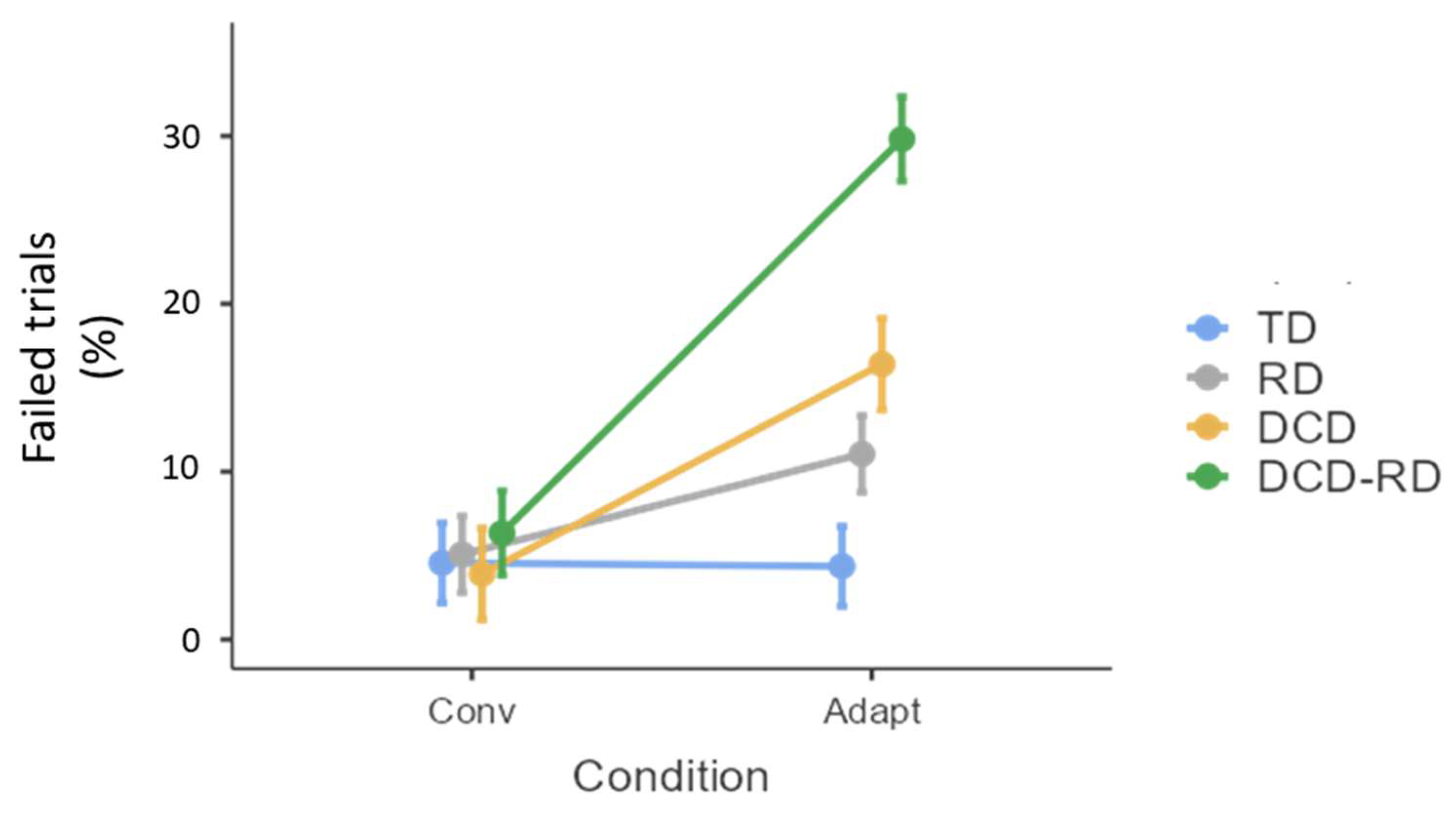
3.1. Trials Failed
3.2. Product Analysis
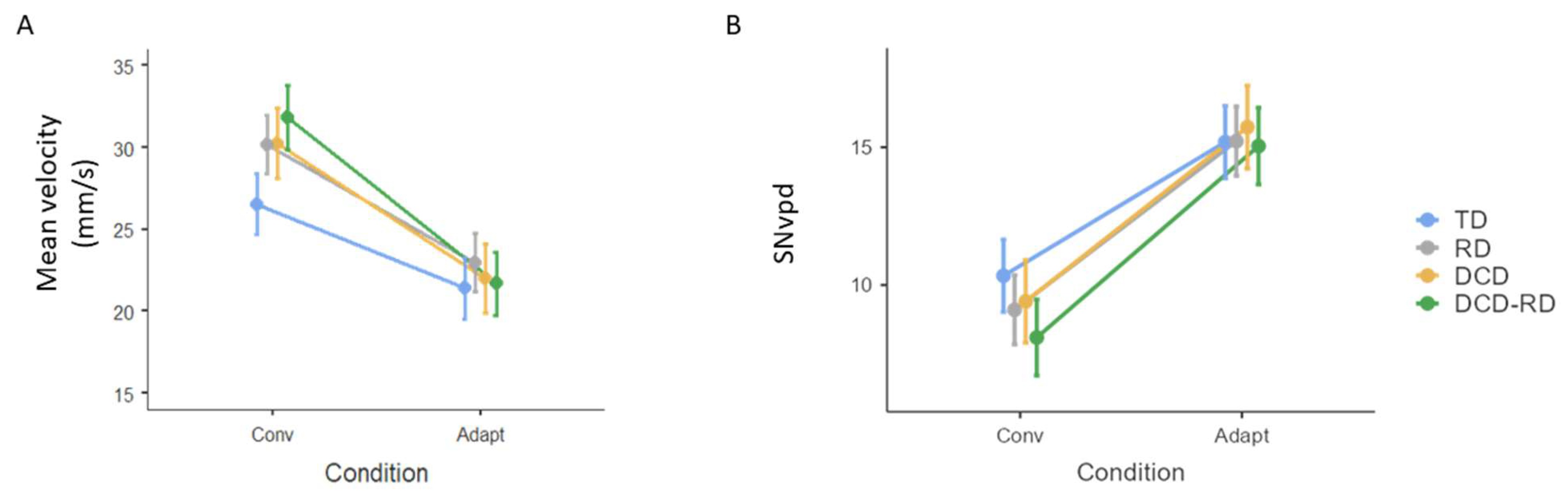
3.3. Process Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doyon, J.; Ungerleider, L.G. Functional anatomy of motor skill learning. In Neuropsychology of Memory; Squire, L.R., Schacter, D.L., Eds.; The Guilford Press: New York, NY, USA, 2002; pp. 225–238. [Google Scholar]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Procedural learning difficulties: Reuniting the developmental disorders? Trends Neurosci. 2007, 30, 135–141. [Google Scholar] [CrossRef]
- Nicolson, R.I.; Fawcett, A.J. Dyslexia, dysgraphia, procedural learning and the cerebellum. Cortex 2011, 47, 117–127. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Publishing: Washington, DC, USA, 2013. [Google Scholar]
- Bo, J.; Lee, C.M. Motor skill learning in children with developmental coordination disorder. Res. Dev. Disabil. 2013, 34, 2047–2055. [Google Scholar] [CrossRef]
- Biotteau, M.; Chaix, Y.; Albaret, J.M. What do we really know about motor learning in children with developmental coordination disorder? Curr. Dev. Disord. Rep. 2016, 3, 152–160. [Google Scholar] [CrossRef]
- Clark, G.M.; Lum, J.A.G. Procedural learning in Parkinson’s disease, specific language impairment, dyslexia, schizophrenia, developmental coordination disorder, and autism spectrum disorders: A second-order meta-analysis. Brain Cogn. 2017, 117, 41–48. [Google Scholar] [CrossRef]
- Dionne, E.; Bolduc, M.È.; Majnemer, A.; Beauchamp, M.H.; Brossard-Racine, M. Academic Challenges in Developmental Coordination Disorder: A Systematic Review and Meta-Analysis. Phys. Occup. Ther. Pediatr. 2023, 43, 34–57. [Google Scholar] [CrossRef]
- Lum, J.A.; Ullman, M.T.; Conti-Ramsden, G. Procedural learning is impaired in dyslexia: Evidence from a meta-analysis of serial reaction time studies. Res. Dev. Disabil. 2013, 34, 3460–3476. [Google Scholar] [CrossRef]
- Lejeune, C.; Catale, C.; Willems, S.; Meulemans, T. Intact procedural motor sequence learning in developmental coordination disorder. Res. Dev. Disabil. 2013, 34, 1974–1981. [Google Scholar] [CrossRef]
- West, G.; Clayton, F.J.; Shanks, D.R.; Hulme, C. Procedural and declarative learning in dyslexia. Dyslexia 2019, 25, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Blais, M.; Amarantini, D.; Albaret, J.M.; Chaix, Y.; Tallet, J. Atypical inter-hemispheric communication correlates with altered motor inhibition during learning of a new bimanual coordination pattern in developmental coordination disorder. Dev. Sci. 2018, 21, e12563. [Google Scholar] [CrossRef]
- Blais, M.; Baly, C.; Biotteau, M.; Albaret, J.M.; Chaix, Y.; Tallet, J. Lack of motor inhibition as a marker of learning difficulties of bimanual coordination in teenagers with developmental coordination disorder. Dev. Neuropsychol. 2017, 42, 207–219. [Google Scholar] [CrossRef]
- Lê, M.; Blais, M.; Jucla, M.; Chauveau, N.; Maziero, S.; Biotteau, M.; Albaret, J.-M.; Péran, P.; Chaix, Y.; Tallet, J. Procedural learning and retention of audio-verbal temporal sequence is altered in children with developmental coordination disorder but cortical thickness matters. Dev. Sci. 2021, 24, e13009. [Google Scholar] [CrossRef]
- Huau, A.; Velay, J.-L.; Jover, M. Graphomotor skills in children with developmental coordination disorder (DCD): Handwriting and learning a new letter. Hum. Mov. Sci. 2015, 42, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Smits-Engelsman, B.; Jelsma, L.D.; Ferguson, G.D.; Geuze, R.H. Motor learning: An analysis of 100 trials of a ski slalom game in children with and without developmental coordination disorder. PLoS ONE 2015, 10, e0140470. [Google Scholar] [CrossRef] [PubMed]
- Smits-Engelsman, B.; Bonney, E.; Ferguson, G. Motor skill learning in children with and without Developmental Coordination Disorder. Hum. Mov. Sci. 2020, 74, 102687. [Google Scholar] [CrossRef]
- Adi-Japha, E.; Brestel, G. Motor skill learning with impaired transfer by children with developmental coordination disorder. Res. Dev. Disabil. 2020, 103, 103671. [Google Scholar] [CrossRef] [PubMed]
- Lejeune, C.; Wansard, M.; Geurten, M.; Meulemans, T. Procedural learning, consolidation, and transfer of a new skill in Developmental Coordination Disorder. Child Neuropsychol. 2016, 22, 143–154. [Google Scholar] [CrossRef]
- Kagerer, F.A.; Bo, J.; Contreras-Vidal, J.L.; Clark, J.E. Visuomotor adaptation in children with developmental coordination disorder. Motor Control 2004, 8, 450–460. [Google Scholar] [CrossRef]
- Cantin, N.; Polatajko, H.J.; Thach, W.T.; Jaglal, S. Developmental coordination disorder: Exploration of a cerebellar hypothesis. Hum. Mov. Sci. 2007, 26, 491–509. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Moya, R.; Diaz, R.; Vaca-Palomares, I.; Fernandez-Ruiz, J. Procedural and strategic visuomotor learning deficits in children with developmental coordination disorder. Res. Q. Exerc. Sport 2020, 91, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Vicari, S.; Finzi, A.; Menghini, D.; Marotta, L.; Baldi, S.; Petrosini, L. Do children with developmental dyslexia have an implicit learning deficit? J. Neurol. Neurosurg. Psychiatry 2005, 76, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- Dewey, D. What is comorbidity and why does it matter in neurodevelopmental disorders? Curr. Dev. Disord. Rep. 2018, 5, 235–242. [Google Scholar] [CrossRef]
- Kaplan, B.J.; Dewey, D.M.; Crawford, S.G.; Wilson, B.N. The term comorbidity is of questionable value in reference to developmental disorders: Data and theory. J. Learn. Disabil. 2001, 34, 555–565. [Google Scholar] [CrossRef]
- Pennington, B.F. How neuropsychology informs our understanding of developmental disorders. J. Child Psychol. Psychiatry 2009, 50, 72–78. [Google Scholar] [CrossRef]
- Smits-Engelsman, B.; Jover, M.; Green, D.; Ferguson, G.; Wilson, P. DCD and comorbidity in neurodevelopmental disorder: How to deal with complexity? Hum. Mov. Sci. 2017, 53, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Biotteau, M.; Chaix, Y.; Albaret, J.M. Procedural learning and automatization process in children with developmental coordination disorder and/or developmental dyslexia. Hum. Mov. Sci. 2015, 43, 78–89. [Google Scholar] [CrossRef]
- Bellocchi, S.; Muneaux, M.; Huau, A.; Lévêque, Y.; Jover, M.; Ducrot, S. Exploring the Link between Visual Perception, Visual–Motor Integration, and Reading in Normal Developing and Impaired Children using DTVP-2. Dyslexia 2017, 23, 296–315. [Google Scholar] [CrossRef]
- Ho, C.S.-H.; Chan, D.W.-O.; Leung, P.W.L.; Lee, S.-H.; Tsang, S.-M. Reading-related cognitive deficits in developmental dyslexia, attention-deficit/hyperactivity disorder, and developmental coordination disorder among Chinese children. Read. Res. Quart. 2005, 40, 318–337. [Google Scholar] [CrossRef]
- Biotteau, M.; Péran, P.; Vayssière, N.; Tallet, J.; Albaret, J.M.; Chaix, Y. Neural changes associated to procedural learning and automatization process in developmental coordination disorder and/or developmental dyslexia. Eur. J. Paediatr. Neurol. 2017, 21, 286–299. [Google Scholar] [CrossRef]
- Downing, C.; Caravolas, M. Prevalence and cognitive profiles of children with comorbid literacy and motor disorders. Front. Psychol. 2020, 11, 1097. [Google Scholar] [CrossRef]
- Maziero, S.; Tallet, J.; Bellocchi, S.; Jover, M.; Chaix, Y.; Jucla, M. Influence of comorbidity on working memory profile in dyslexia and developmental coordination disorder. J. Clin. Exp. Neuropsyc. 2020, 42, 660–674. [Google Scholar] [CrossRef]
- Bellocchi, S.; Ducrot, S.; Tallet, J.; Jucla, M.; Jover, M. Effect of comorbid developmental dyslexia on oculomotor behavior in children with developmental coordination disorder: A study with the Developmental Eye Movement test. Hum. Mov. Sci. 2021, 76, 102764. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Jover, M.; Danna, J. Dysgraphia differs between children with developmental coordination disorder and/or reading disorder. J. Learn. Disabil. 2024. [Google Scholar] [CrossRef]
- Jongmans, M.J.; Smits-Engelsman, B.; Schoemaker, M.M. Consequences of comorbidity of developmental coordination disorders and learning disabilities for severity and pattern of perceptual—Motor dysfunction. J. Learn. Disabil. 2003, 36, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Cignetti, F.; Vaugoyeau, M.; Fontan, A.; Jover, M.; Livet, M.O.; Hugonenq, C.; Audic, F.; Chabrol, B.; Assaiante, C. Feedforward motor control in developmental dyslexia and developmental coordination disorder: Does comorbidity matter? Res. Dev. Disabil. 2018, 76, 25–34. [Google Scholar] [CrossRef]
- Blais, M.; Jucla, M.; Maziero, S.; Albaret, J.M.; Chaix, Y.; Tallet, J. Specific cues can improve procedural learning and retention in developmental coordination disorder and/or developmental dyslexia. Front. Hum. Neurosci. 2021, 15, 744562. [Google Scholar] [CrossRef]
- Brookes, R.L.; Nicolson, R.I.; Fawcett, A.J. Prisms throw light on developmental disorders. Neuropsychologia 2007, 45, 1921–1930. [Google Scholar] [CrossRef] [PubMed]
- Afonso, O.; Connelly, V.; Barnett, A. Struggling with writing: An examination of writing difficulties in specific language impairment, developmental dyslexia and developmental coordination disorder. In Spelling and Writing Words: Theoretical and Methodological Advances; Studies in Writing Book Series; Peret, C., Olive, T., Eds.; Brill Publishing: Leiden, The Netherlands, 2019; pp. 112–130. [Google Scholar]
- Wechsler, D. WISC-IV—Echelle d’intelligence de Wechsler pour Enfants et Adolescents—4ème Edition [Wechsler Intelligence Scale for Children and Adolescents, 4th ed.; Éditions du Centre de Psychologie Appliquée: Paris, France, 2005. [Google Scholar]
- Flessas, J.; Lussier, F. EVAC–Epreuve Verbale d’Aptitudes Cognitives [EVAC–Verbal Cognitive Aptitude Test]; Éditions du Centre de Psychologie Appliquée: Paris, France, 2003. [Google Scholar]
- Lecocq, P. E.CO.S.SE: Epreuve de Compréhension Syntaxico-Sémantique [E.CO.S.SE: Syntactic-Semantic Comprehension Test]; Presses Universitaires du Septentrion: Lille, France, 1996. [Google Scholar]
- Lefavrais, P. Test de l’Alouette, Version Révisée [Alouette Test, Revised Version]; Éditions du Centre de Psychologie Appliquée: Paris, France, 2005. [Google Scholar]
- Jacquier-Roux, M.; Valdois, S.; Zorman, M.; Lequette, C.; Pouget, G. Odédys-2: Outils de Dépistage des Dyslexies [Odedys-2: Dyslexia Screening Tools]; Laboratoire Cogni-Sciences, IUFM de Grenoble: Grenoble, France, 2005. [Google Scholar]
- Soppelsa, R.; Albaret, J.M. Manuel de la Batterie d’Evaluation du Mouvement chez l’Enfant (M-ABC) [Manual of the Movement Assessment Battery for Children]; Éditions du Centre de Psychologie Appliquée: Paris, France, 2004. [Google Scholar]
- Grégoire, J. L’examen Clinique de l’intelligence de l’enfant: Fondements et Pratique du WISC IV [The Clinical Examination of Children’s Intelligence: Foundations and Practice of the WISC IV]; Mardaga: Sprimont, Belgium, 2006. [Google Scholar]
- Keith, T.Z.; Fine, J.G.; Taub, G.E.; Reynolds, M.R.; Kranzler, J.H. Higher order, multisample, confirmatory factor analysis of the Wechsler Intelligence Scale for Children—Fourth Edition: What does it measure. School Psychol. Rev. 2006, 35, 108–127. [Google Scholar] [CrossRef]
- Kleiner, M.; Brainard, D.; Pelli, D.; Ingling, A.; Murray, R.; Broussard, C. What’s new in psychtoolbox-3. Perception 2007, 36, 1–16. [Google Scholar]
- Danna, J.; Paz-Villagrán, V.; Velay, J.-L. Signal-to-Noise velocity peak difference: A new method for evaluating the handwriting movement fluency in children with dysgraphia. Res. Dev. Disabil. 2013, 34, 4375–4384. [Google Scholar] [CrossRef]
- The Jamovi Project. Jamovi (Version 1.2) [Computer Software]. The Jamovi Project. 2020. Available online: https://www.jamovi.org (accessed on 1 May 2023).
- He, J.L.; Fuelscher, I.; Coxon, J.; Barhoun, P.; Parmar, D.; Enticott, P.G.; Hyde, C. Impaired motor inhibition in developmental coordination disorder. Brain Cogn. 2018, 127, 23–33. [Google Scholar] [CrossRef]
- Wilson, P.H.; Ruddock, S.; Smits-Engelsman, B.; Polatajko, H.; Blank, R. Understanding performance deficits in developmental coordination disorder: A meta-analysis of recent research. Dev. Med. Child Neurol. 2013, 55, 217–228. [Google Scholar] [CrossRef]
- Peng, P.; Zhang, Z.; Wang, W.; Lee, K.; Wang, T.; Wang, C.; Luo, J.; Lin, J. A meta-analytic review of cognition and reading difficulties: Individual differences, moderation, and language mediation mechanisms. Psychol. Bull. 2022, 148, 227–272. [Google Scholar] [CrossRef]
- Longcamp, M.; Anton, J.-L.; Roth, M.; Velay, J.-L. Visual presentation of single letters activates a premotor area involved in writing. Neuroimage 2003, 19, 1492–1500. [Google Scholar] [CrossRef] [PubMed]
- Roux, F.-E.; Dufor, O.; Giussani, C.; Wamain, Y.; Draper, L.; Longcamp, M.; Démonet, J.-F. The graphemic/motor frontal area Exner’s area revisited. Ann. Neurol. 2009, 66, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Wolpert, D.M.; Ghahramani, Z. Computational principles of movement neuroscience. Nat. Neurosci. 2000, 3, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Körding, K.P.; Wolpert, D.M. Bayesian decision theory in sensorimotor control. Trends Cogn. Sci. 2006, 10, 319–326. [Google Scholar] [CrossRef]
- Palmis, S.; Danna, J.; Velay, J.-L.; Longcamp, M. Motor control of handwriting in the developing brain: A review. Cognitive Neuropsych. 2017, 34, 187–204. [Google Scholar] [CrossRef]
- Biotteau, M.; Chaix, Y.; Blais, M.; Tallet, J.; Péran, P.; Albaret, J.M. Neural signature of DCD: A critical review of MRI neuroimaging studies. Front. Neurol. 2016, 7, 227. [Google Scholar] [CrossRef]
- Debrabant, J.; Gheysen, F.; Caeyenberghs, K.; Van Waelvelde, H.; Vingerhoets, G. Neural underpinnings of impaired predictive motor timing in children with Developmental Coordination Disorder. Res. Dev. Disabil. 2013, 34, 1478–1487. [Google Scholar] [CrossRef]
- Gill, K.K.; Lang, D.; Zwicker, J.G. Cerebellar and brainstem differences in children with developmental coordination disorder: A voxel-based morphometry study. Front. Hum. Neurosci. 2022, 16, 921505. [Google Scholar] [CrossRef]
- Nemmi, F.; Cignetti, F.; Vaugoyeau, M.; Assaiante, C.; Chaix, Y.; Péran, P. Developmental dyslexia, developmental coordination disorder and comorbidity discrimination using multimodal structural and functional neuroimaging. Cortex 2023, 160, 43–54. [Google Scholar] [CrossRef]
- Zwicker, J.G.; Missiuna, C.; Harris, S.R.; Boyd, L.A. Brain activation of children with developmental coordination disorder is different than peers. Pediatrics 2010, 126, e678–e686. [Google Scholar] [CrossRef] [PubMed]
- Zwicker, J.G.; Missiuna, C.; Harris, S.R.; Boyd, L.A. Brain activation associated with motor skill practice in children with developmental coordination disorder: An fMRI study. Int. J. Dev. Neurosci. 2011, 29, 145–152. [Google Scholar] [CrossRef]
- Schoemaker, M.M.; Lingam, R.; Jongmans, M.J.; van Heuvelen, M.J.; Emond, A. Is severity of motor coordination difficulties related to co-morbidity in children at risk for developmental coordination disorder? Res. Dev. Disabil. 2013, 34, 3084–3091. [Google Scholar] [CrossRef] [PubMed]
- Jover, M.; Huau, A. A dimensional approach to comorbidity between developmental coordination disorder and dyslexia: A study of graphomotor abilities. Approch. Neuropsychol. Des Apprentiss. Chez L’enfant 2021, 175, 673–688. [Google Scholar]
- Soltani, A.; Koechlin, E. Computational models of adaptive behavior and prefrontal cortex. Neuropsychopharmacology 2022, 47, 58–71. [Google Scholar] [CrossRef]
- Martin, E.; Scotté-Barranoff, C.; Tallet, J. What neurological diseases tell us about procedural perceptual-motor learning? A systematic review of the literature. Neurol. Sci. 2023, 44, 2645–2665. [Google Scholar] [CrossRef]
- Guilbert, J.; Guilbert, J.; Alamargot, D.; Morin, M.-F. Handwriting on a tablet screen: Role of visual and proprioceptive feedback in the control of movement by children and adults. Hum. Mov. Sci. 2019, 65, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Cappelli, G. The Impact of Dyslexia on Lexico-Semantic Abilities: An Overview. In A Linguistic Approach to the Study of Dyslexia; Cappelli, G., Noccetti, S., Eds.; Multilingual Matters: Bristol, UK, 2022; pp. 211–239. [Google Scholar]
- Nicolson, R.J.; Fawcett, P.D. Developmental dyslexia: The cerebellar deficit hypothesis. Trends Neurosci. 2001, 24, 508–511. [Google Scholar] [CrossRef] [PubMed]
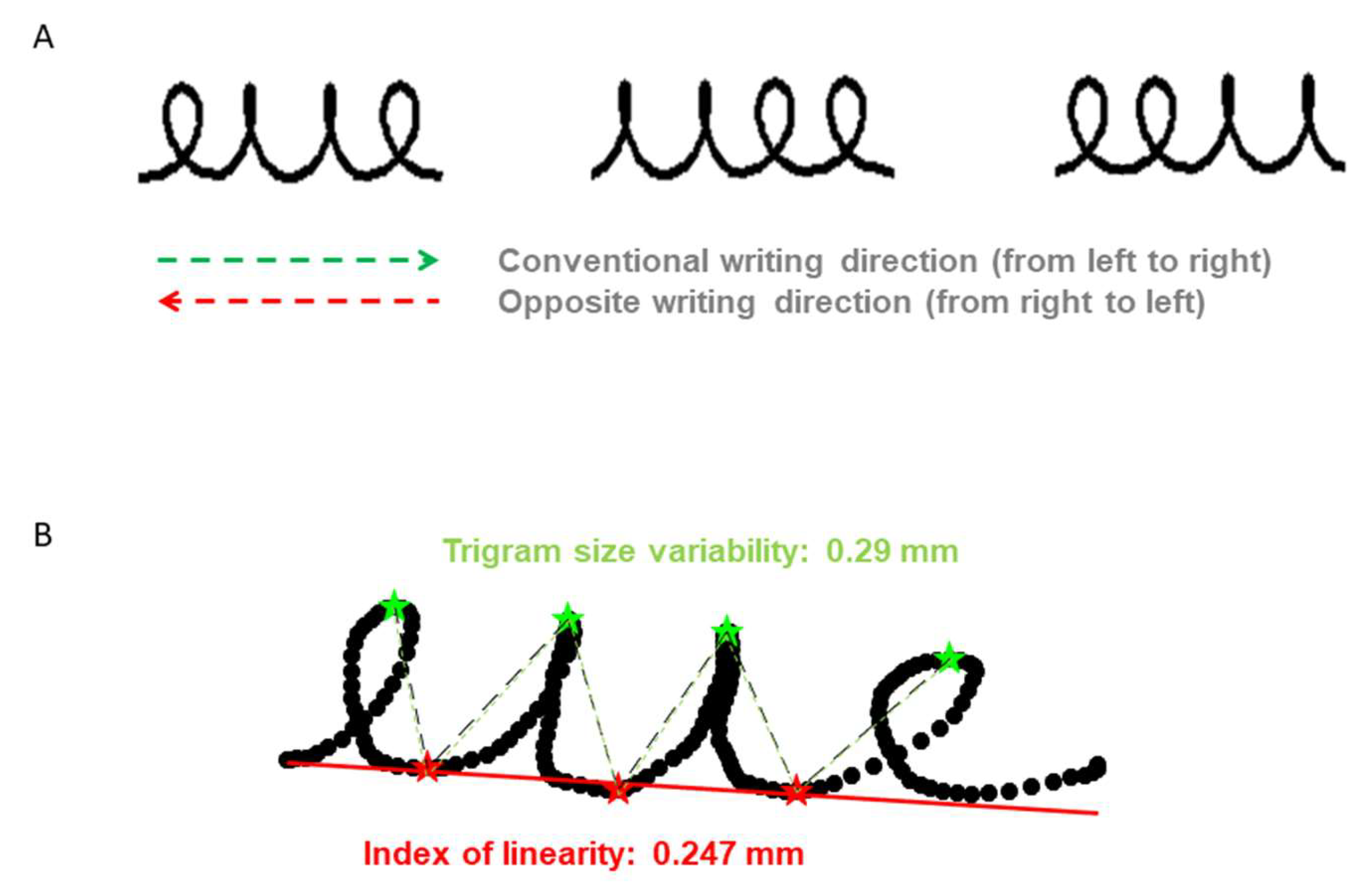

| Group | TD | DCD | RD | DCD-RD | p | ||
|---|---|---|---|---|---|---|---|
| Number, Gender and Age | N (female) | 21 (10) | 16 (6) | 23 (9) | 19 (7) | ns | |
| Age—Mean (SD) | 10 (0.9) | 10 (1.3) | 10.1 (1.2) | 9.8 (1.3) | ns | ||
| Intelligence (WISC) | Similarities (scaled score)—Mean (SD) | 16.3 (2.33) | 14.1 (3.87) | 13.2 (2.15) | 11.7 (3.36) | ns | |
| Picture Concepts (scaled score)—Mean (SD) | 10.76 (1.95) | 10.13 (2.55) | 11.65 (2.39) | 10.21 (2.27) | <0.001 | DCD-RD; RD < TD | |
| Motor (MABC-1) | Total Mean perc (SD) | 46.7 (25) | 4.1 (3.3) | 34.8 (19.2) | 3.5 (2.9) | <0.001 | DCD-RD; DCD < TD; RD |
| Dexterity (% chidren < 15th perc) | 9.5 | 75 | 30.4 | 68.4 | <0.005 | ||
| Ball skills (% children < 15th perc) | 14.3 | 50 | 8.7 | 47.4 | <0.001 | ||
| Balance (% children < 15th perc) | 0 | 100 | 0 | 100 | <0.001 | ||
| Reading | Alouette z score—Mean (SD) | 1.20 (0.82) | 0.40 (0.73) | −1.50 (0.72) | −1.55 0.50) | <0.001 | DCD-RD; RD < TD; DCD |
| ODEDYS non-words—Mean (SD) | 0.72 (0.59) | 0.16 (0.64) | −1.67 (1.32) | −1.47 (1.18) | <0.001 | DCD-RD; RD < TD; DCD | |
| ODEDYS irregular words—Mean (SD) | 0.97 (0.59) | 0.66 (0.82) | −1.50 (0.97) | −1.69 (1.07) | <0.001 | DCD-RD; RD < TD; DCD |
| Dependant Variable | Condition | TD (Mean, SD) | RD (Mean, SD) | DCD (Mean, SD) | DCD-RD (Mean, SD) |
|---|---|---|---|---|---|
| Trials failed | Conv | 4.56 (20.9) | 5.07 (22) | 3.91 (19.4) | 6.37 (24.5) |
| Adapt | 4.37 (20.5) | 11.1 (31.4) | 16.4 (37.1) | 29.8 (45.8) | |
| Spatial variability (mm) | Conv | 1.45 (0.86) | 1.62 (0.84) | 1.89 (1.02) | 1.75 (1.02) |
| Adapt | 1.44 (0.66) | 1.73 (0.81) | 1.95 (0.99) | 1.87 (0.79) | |
| Trigram linearity (mm) | Conv | 0.25 (0.2) | 0.27 (0.22) | 0.32 (0.26) | 0.31 (0.25) |
| Adapt | 0.28 (0.23) | 0.36 (0.29) | 0.41 (0.36) | 0.39 (0.33) | |
| Mean velocity (mm/s) | Conv | 26.5 (10.2) | 30 (13.1) | 30.2 (14.3) | 31.7 (15.4) |
| Adapt | 21.4 (7.65) | 22.9 (8.71) | 21.9 (8.34) | 22.9 (10.2) | |
| SNvpd | Conv | 10.3 (7.14) | 9.05 (8.2) | 9.43 (7.72) | 7.84 (6.43) |
| Adapt | 15.1 (7.89) | 15.3 (9.89) | 15.9 (7.97) | 14.5 (8.9) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danna, J.; Lê, M.; Tallet, J.; Albaret, J.-M.; Chaix, Y.; Ducrot, S.; Jover, M. Motor Adaptation Deficits in Children with Developmental Coordination Disorder and/or Reading Disorder. Children 2024, 11, 491. https://doi.org/10.3390/children11040491
Danna J, Lê M, Tallet J, Albaret J-M, Chaix Y, Ducrot S, Jover M. Motor Adaptation Deficits in Children with Developmental Coordination Disorder and/or Reading Disorder. Children. 2024; 11(4):491. https://doi.org/10.3390/children11040491
Chicago/Turabian StyleDanna, Jérémy, Margaux Lê, Jessica Tallet, Jean-Michel Albaret, Yves Chaix, Stéphanie Ducrot, and Marianne Jover. 2024. "Motor Adaptation Deficits in Children with Developmental Coordination Disorder and/or Reading Disorder" Children 11, no. 4: 491. https://doi.org/10.3390/children11040491





