Psychosocial Impact of False-Positive Newborn Screening Results: A Scoping Review
Abstract
1. Introduction
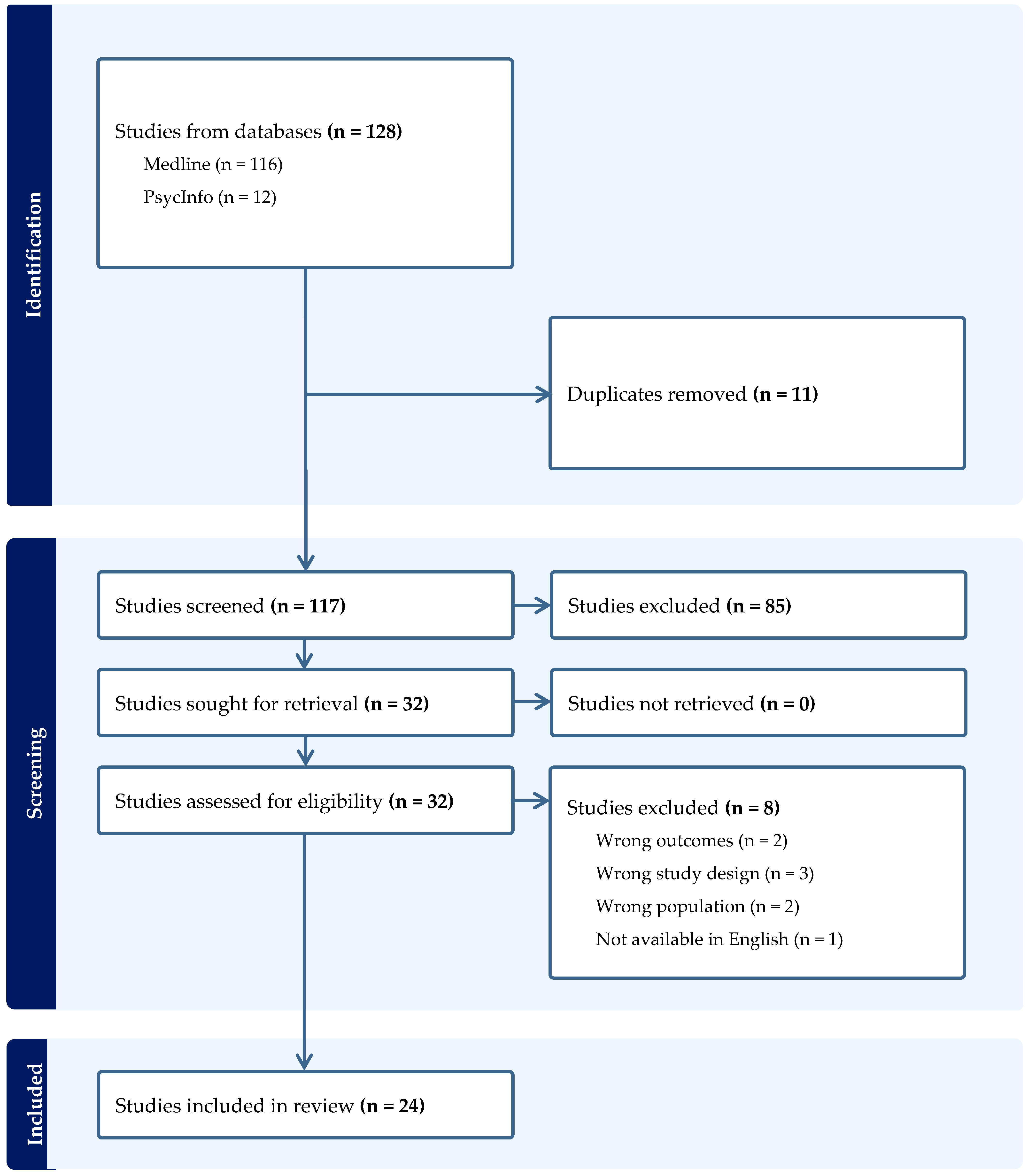
2. Materials and Methods
3. Results
3.1. Reactions to the Initial NBS Result
3.2. Emotional Reactions to False-Positive NBS Results
3.3. Impact of False-Positive NBS Results on Reproductive Decision Making
3.4. Parental Perceptions of Child Vulnerability following False-Positive NBS Results
3.5. Healthcare Utilization following False-Positive NBS Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Public Health England. Newborn Blood Spot Screening: Programme Handbook; Public Health England: London, UK, 2018.
- The Lancet. Genomic newborn screening: Current concerns and challenges. Lancet 2023, 402, 265. [Google Scholar] [CrossRef] [PubMed]
- Malvagia, S.; Forni, G.; Ombrone, D.; la Marca, G. Development of Strategies to Decrease False Positive Results in Newborn Screening. Int. J. Neonatal Screen. 2020, 6, 84. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Watson, M.S.; Lloyd-Puryear, M.A.; Howell, R.R. The Progress and Future of US Newborn Screening. Int. J. Neonatal Screen. 2022, 8, 41. [Google Scholar] [CrossRef]
- Farre, A.; Rapley, T. The New Old (and Old New) Medical Model: Four Decades Navigating the Biomedical and Psychosocial Understandings of Health and Illness. Healthcare 2017, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Grob, R. Is my sick child healthy? Is my healthy child sick?: Changing parental experiences of cystic fibrosis in the age of expanded newborn screening. Soc. Sci. Med. 2008, 67, 1056–1064. [Google Scholar] [CrossRef] [PubMed]
- Chudleigh, J.; Buckingham, S.; Dignan, J.; O’Driscoll, S.; Johnson, K.; Rees, D.; Wyatt, H.; Metcalfe, A. Parents’ Experiences of Receiving the Initial Positive Newborn Screening (NBS) Result for Cystic Fibrosis and Sickle Cell Disease. J. Genet. Couns. 2016, 25, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.G.; Southern, K.W. Considering consent: A structural equation modelling analysis of factors influencing decisional quality when accepting newborn screening. J. Inherit. Metab. Dis. 2014, 37, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Nicholls, S.G.; Southern, K.W. Parental information use in the context of newborn bloodspot screening. An exploratory mixed methods study. J. Community Genet. 2012, 3, 251–257. [Google Scholar] [CrossRef][Green Version]
- Chudleigh, J.; Holder, P.; Fusco, F.; Bonham, J.R.; Bryon, M.; Moody, L.; Morris, S.; Olander, E.K.; Simpson, A.; Chinnery, H.; et al. Co-designed strategies for delivery of positive newborn bloodspot screening results to parents: The ReSPoND mixed-methods study. Health Soc. Care Deliv. Res. 2022, 10, 19. [Google Scholar] [CrossRef]
- Arksey, H.; O’Malley, L. Scoping studies: Towards a methodological framework. Int. J. Soc. Res. Methodol. 2005, 8, 19–32. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Pollock, D.; Peters, M.D.J.; Khalil, H.; McInerney, P.; Alexander, L.; Tricco, A.C.; Evans, C.; de Moraes, É.B.; Godfrey, C.M.; Pieper, D.; et al. Recommendations for the extraction, analysis, and presentation of results in scoping reviews. JBI Evid. Synth. 2023, 21, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Popay, J.; Roberts, H.; Sowden, A.; Petticrew, M.; Arai, L.; Rodgers, M.; Britten, N.; Roen, K.; Duffy, S. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews: A Product from the ESRC Methods Programme; Lancaster University: Lancaster, UK, 2006. [Google Scholar]
- Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. Expanded newborn screening for biochemical disorders: The effect of a false-positive result. Pediatrics 2006, 117, 1915–1921. [Google Scholar] [CrossRef]
- O’Connor, K.; Jukes, T.; Goobie, S.; DiRaimo, J.; Moran, G.; Potter, B.K.; Chakraborty, P.; Rupar, C.A.; Gannavarapu, S.; Prasad, C. Psychosocial impact on mothers receiving expanded newborn screening results. Eur. J. Hum. Genet. 2018, 26, 477–484. [Google Scholar] [CrossRef] [PubMed Central]
- Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Kerr, E.; Tam, K.; Carroll, J.C.; Potter, B.K.; Chakraborty, P.; Davies, C.; et al. Parent Experience with False-Positive Newborn Screening Results for Cystic Fibrosis. Pediatrics 2016, 138, e20161052. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.; Quirk, K.; Duff, A.J.; Brownlee, K.G. Newborn screening for CF in a regional paediatric centre: The psychosocial effects of false-positive IRT results on parents. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2007, 6, 250–254. [Google Scholar] [CrossRef]
- Beucher, J.; Leray, E.; Deneuville, E.; Roblin, M.; Pin, I.; Bremont, F.; Turck, D.; Ginies, J.-L.; Foucaud, P.; Rault, G.; et al. Psychological effects of false-positive results in cystic fibrosis newborn screening: A two-year follow-up. J. Pediatr. 2010, 156, 771–776.e1. [Google Scholar] [CrossRef]
- Tluczek, A.; Orland, K.M.; Cavanagh, L. Psychosocial consequences of false-positive newborn screens for cystic fibrosis. Qual. Health Res. 2011, 21, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. Effect of expanded newborn screening for biochemical genetic disorders on child outcomes and parental stress. JAMA 2003, 290, 2564–2572. [Google Scholar] [CrossRef]
- Morrison, D.R.; Clayton, E.W. False positive newborn screening results are not always benign. Public Health Genom. 2011, 14, 173–177. [Google Scholar] [CrossRef]
- Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. The impact of false-positive newborn screening results on families: A qualitative study. Genet. Med. 2012, 14, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Lipstein, E.A.; Perrin, J.M.; Waisbren, S.E.; Prosser, L.A. Impact of false-positive newborn metabolic screening results on early health care utilization. Genet. Med. 2009, 11, 716–721. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hayeems, R.Z.; Miller, F.A.; Vermeulen, M.; Potter, B.K.; Chakraborty, P.; Davies, C.; Carroll, J.C.; Ratjen, F.; Guttmann, A. False-Positive Newborn Screening for Cystic Fibrosis and Health Care Use. Pediatrics 2017, 140, e20170604. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.-J.; He, J.; Chen, H.; Shi, X.-D.; Li, Y. Psychological effects of false-positive results in expanded newborn screening in China. PLoS ONE 2012, 7, e36235. [Google Scholar] [CrossRef] [PubMed]
- Karaceper, M.D.; Chakraborty, P.; Coyle, D.; Wilson, K.; Kronick, J.B.; Hawken, S.; Davies, C.; Brownell, M.; Dodds, L.; Feigenbaum, A.; et al. The health system impact of false positive newborn screening results for medium-chain acyl-CoA dehydrogenase deficiency: A cohort study. Orphanet J. Rare Dis. 2016, 11, 12. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hewlett, J.; Waisbren, S.E. A review of the psychosocial effects of false-positive results on parents and current communication practices in newborn screening. J. Inherit. Metab. Dis. 2006, 29, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Mischler, E.H.; Bowers, B.; Peterson, N.M.; Morris, M.E.; Farrell, P.M.; Bruns, W.T.; Colby, H.; McCarthy, C.; Fost, N.; et al. Psychological impact of false-positive results when screening for cystic fibrosis. Pediatr. Pulmonol. Suppl. 1991, 7, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Gapp, S.; Garbade, S.F.; Feyh, P.; Brockow, I.; Nennstiel, U.; Hoffmann, G.F.; Sommerburg, O.; Gramer, G. German newborn screening for Cystic fibrosis: Parental perspectives and suggestions for improvements. Pediatr. Pulmonol. 2023, 58, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.W.; McColley, S.A.; Lester, L.A.; Ross, L.F. Parental understanding of newborn screening for cystic fibrosis after a negative sweat-test. Pediatrics 2011, 127, 276–283. [Google Scholar] [CrossRef]
- Green, J.M.; Hewison, J.; Bekker, H.L.; Bryant, L.D.; Cuckle, H.S. Psychosocial aspects of genetic screening of pregnant women and newborns: A systematic review. Health Technol. Assess. 2004, 8, iii-109. [Google Scholar] [CrossRef]
- Peterson, L.; Siemon, A.; Olewiler, L.; McBride, K.L.; Allain, D.C. A qualitative assessment of parental experiences with false-positive newborn screening for Krabbe disease. J. Genet. Couns. 2022, 31, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Mischler, E.H.; Farrell, P.M.; Fost, N.; Peterson, N.M.; Carey, P.; Bruns, W.T.; McCarthy, C. Parents’ knowledge of neonatal screening and response to false-positive cystic fibrosis testing. J. Dev. Behav. Pediatr. 1992, 13, 181–186. [Google Scholar] [CrossRef] [PubMed]
- van den Heuvel, L.M.; van der Pal, S.M.; Verschoof-Puite, R.K.; Klapwijk, J.E.; Elsinghorst, E.; Dekkers, E.; van der Ploeg, C.P.B.; Henneman, L. Psychosocial Impact of a True-Positive, False-Positive, or Inconclusive Newborn Bloodspot Screening Result: A Questionnaire Study among Parents. Int. J. Neonatal Screen. 2024, 10, 18. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vernooij-van Langen, A.M.; van der Pal, S.M.; Reijntjens, A.J.; Loeber, J.G.; Dompeling, E.; Dankert-Roelse, J.E. Parental knowledge reduces long term anxiety induced by false-positive test results after newborn screening for cystic fibrosis. Mol. Genet. Metab. Rep. 2014, 1, 334–344. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sorenson, J.R.; Levy, H.L.; Mangione, T.W.; Sepe, S.J. Parental response to repeat testing of infants with ‘false-positive’ results in a newborn screening program. Pediatrics 1984, 73, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Tarini, B.A.; Clark, S.J.; Pilli, S.; Dombkowski, K.J.; Korzeniewski, S.J.; Gebremariam, A.; Eisenhandler, J.; Grigorescu, V. False-positive newborn screening result and future health care use in a state Medicaid cohort. Pediatrics 2011, 128, 715–722. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Buchbinder, M.; Timmermans, S. Newborn screening for metabolic disorders: Parental perceptions of the initial communication of results. Clin. Pediatr. 2012, 51, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Salm, A.; Yetter, E.; Tluczek, A. Informing parents about positive newborn screening results: Parents’ recommendations. J. Child Health Care 2012, 16, 367–381. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, J.M.; Kearney, M.H.; Norton, S.A.; Arnold, G.L. Parents’ experiences of expanded newborn screening evaluations. Pediatrics 2011, 128, 53–61. [Google Scholar] [CrossRef]
- Barben, J.; Castellani, C.; Munck, A.; Davies, J.C.; de Winter-de Groot, K.M.; Gartner, S.; Kashirskaya, N.; Linnane, B.; Mayell, S.J.; McColley, S.; et al. Updated guidance on the management of children with cystic fibrosis transmembrane conductance regulator-related metabolic syndrome/cystic fibrosis screen positive, inconclusive diagnosis (CRMS/CFSPID). J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2021, 20, 810–819. [Google Scholar] [CrossRef]
- Munck, A.; Mayell, S.J.; Winters, V.; Shawcross, A.; Derichs, N.; Parad, R.; Barben, J.; Southern, K.W. Cystic Fibrosis Screen Positive, Inconclusive Diagnosis (CFSPID): A new designation and management recommendations for infants with an inconclusive diagnosis following newborn screening. J. Cyst. Fibros. Off. J. Eur. Cyst. Fibros. Soc. 2015, 14, 706–713. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Screening Programmes: A Short Guide. Increase Effectiveness, Maximise Benefits and Minimize Harm; World Health Organisation: Copenhagen, Denmark, 2020; pp. 1–70. [Google Scholar]
- Wright, S.J.; Ulph, F.; Dharni, N.; Payne, K. Eliciting Preferences for Information Provision in Newborn Bloodspot Screening Programs. Value Health 2017, 20, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Chudleigh, J.; Chinnery, H.; Bonham, J.R.; Olander, E.; Moody, L.; Simpson, A.; Morris, S.; Ulph, F.; Bryon, M.; Southern, K. Qualitative exploration of health professionals’ experiences of communicating positive newborn bloodspot screening results for nine conditions in England. BMJ Open 2020, 10, e037081. [Google Scholar] [CrossRef] [PubMed]
- Chudleigh, J.; Chinnery, H.; Holder, P.; Carling, R.S.; Southern, K.; Olander, E.; Moody, L.; Morris, S.; Ulph, F.; Bryon, M.; et al. Processing of positive newborn screening results: A qualitative exploration of current practice in England. BMJ Open 2020, 10, e044755. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Ersig, A.L.; Lee, S. Psychosocial Issues Related to Newborn Screening: A Systematic Review and Synthesis. Int. J. Neonatal Screen. 2022, 8, 53. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ulph, F.; Cullinan, T.; Qureshi, N.; Kai, J. Parents’ responses to receiving sickle cell or cystic fibrosis carrier results for their child following newborn screening. Eur. J. Hum. Genet. 2015, 23, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Tluczek, A.; Clark, R.; McKechnie, A.C.; Brown, R.L. Factors affecting parent-child relationships one year after positive newborn screening for cystic fibrosis or congenital hypothyroidism. J. Dev. Behav. Pediatr. 2015, 36, 24–34. [Google Scholar] [CrossRef] [PubMed]
- UK Newborn Screening Programme Centre. Health Professional Handbook: A Guide to Newborn Blood Spot Screening for Healthcare Professionals; UK Newborn Screening Programme Centre: London, UK, 2012; pp. 1–56.
- Collins, J.L.; La Pean, A.; O’Tool, F.; Eskra, K.L.; Roedl, S.J.; Tluczek, A.; Farrell, M.H. Factors that influence parents’ experiences with results disclosure after newborn screening identifies genetic carrier status for cystic fibrosis or sickle cell hemoglobinopathy. Patient Educ. Couns. 2012, 90, 378–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tluczek, A.; Koscik, R.L.; Farrell, P.M.; Rock, M.J. Psychosocial risk associated with newborn screening for cystic fibrosis: Parents’ experience while awaiting the sweat-test appointment. Pediatrics 2005, 115, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Brockow, I.; Nennstiel, U. Parents’ experience with positive newborn screening results for cystic fibrosis. Eur. J. Pediatr. 2019, 178, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Edwards, D.J.; Wicking, K.; Smyth, W.; Shields, L.; Douglas, T. Information needs of parents of infants diagnosed with cystic fibrosis: Results of a pilot study. J. Child Health Care Prof. Work. Child. Hosp. Community 2018, 22, 382–392. [Google Scholar] [CrossRef] [PubMed]
| Author, Year, and Country | Condition(s) | Research Design/Data Collection Methods | Sample | Psychosocial Impact of False-Positive NBS Results |
|---|---|---|---|---|
| [15] Gurian, E.A.; Kinnamon, D.D.; Henry, J.J.; Waisbren, S.E. (2006) US | Biochemical genetic disorders | Mixed methods: structured interview; Parenting Stress Index | Parents of 173 children with false-positive NBS results and 67 children with normal NBS results. | Parents of children with a false-positive NBS result scored higher on the Parenting Stress Index. |
| [16] O’Connor, K.; Jukes, T.; Goobie, S.; DiRaimo, J.; Moran, G.; Potter, B.K.; Chakraborty, P.; Rupar, C.A.; Gannavarapu, S.; Prasad, C. (2018) Canada | Inborn error of metabolism, an endocrine disorder (congenital adrenal hyperplasia and congenital hypothyroidism) or cystic fibrosis (CF) | Cross-sectional study: Parenting Stress Index; Depression, Anxiety and Stress Scale | Mothers who had received a true-negative result (n = 31), true-positive result (n = 8), and a false-positive result (n = 18). | There were no significant differences regardless of NBS outcome in terms of overall anxiety, stress and depression. |
| [17] Hayeems, R.Z.; Miller, F.A.; Barg, C.J.; Bombard, Y.; Kerr, E.; Tam, K.; Carroll, J.C.; Potter, B.K.; Chakraborty, P.; Davies, C.; et al. (2016) Canada | CF | Mixed-methods cohort design: prospective self-report data; qualitative interviews | 134 mothers of infants with false-positive CF NBS results completed questionnaires. 54 mothers of infants with false-positive CF NBS results were interviewed. | Mothers reported psychosocial distress but this was not detected via the psychosocial response measures at the newborn stage or 1 year later. |
| [18] Moran, J.; Quirk, K.; Duff, A.J.; Brownlee, K.G. (2007) UK | CF | Qualitative study: semi-structured interview | 21 parents. | Mothers described experiencing anxiety, stress and upset during the screening process. |
| [19] Beucher, J.; Leray, E.; Deneuville, E.; Roblin, M.; Pin, I.; Bremont, F.; Turck, D.; Ginies, J.L.; Foucaud, P.; Rault, G.; et al. (2009) France | CF | Mixed Methods: interviews; Perceived Stress Scale; Vulnerable Child Scale | 86 children with CF. | Mean Perceived Stress Scale Score did not differ when compared with the French population. Mean Vulnerable Child Scale was high. Results at 1 and 2 years did not differ significantly. |
| [20] Tluczek, A.; Orland, K.M.; Cavanagh, L. (2011) US | CF | Qualitative study: interviews | 87 parents of 44 infants who had received false-positive NBS results for CF. | Repercussions of receiving genetic information following NBS included concern about test accuracy, the child’s health, questioning paternity, wondering if other relatives were carriers, and searching for the genetic source Positive outcomes included gaining new perspectives and strengthening relationships. |
| [21] Waisbren, S.E.; Albers, S.; Amato, S.; Ampola, M.; Brewster, T.G.; Demmer, L.; Eaton, R.B.; Greenstein, R.; Korson, M.; Larson, C.; et al. (2003) US | Biochemical genetic disorders | Mixed methods: children’s health and development; Parental Stress Index; interviews | Families of 50 affected children identified through expanded NBS; 33 affected children identified clinically, 94 children with false-positive results, 81 children with negative NBS results. | Children with a false-positive NBS result were twice as likely as children in the negative NBS result group to experience hospitalization. Mothers of children in the false-positive group obtained higher scores on the Parental Stress Index compared to mothers of children in the negative NBS group. |
| [22] Morrison, D.R.; Clayton, E.W. (2011) US | Metabolic or endocrine conditions included in NBS | Mixed methods: telephone interviews; Parental Stress Index-Short Form | Parents of children with false-positive but otherwise healthy NBS results (n = 28), true positive (n = 20) and false positive with other medical conditions (n = 12). | Parents who had received a false-positive NBS result expressed concern about having more children. Almost 10% of parents who had received a false-positive NBS result reported clinically significant stress as well as concern about their child’s health and future. |
| [23] Schmidt, J.L.; Castellanos-Brown, K.; Childress, S.; Bonhomme, N.; Oktay, J.S.; Terry, S.F.; Kyler, P.; Davidoff, A.; Greene, C. (2012) US | Any condition included in NBS | Qualitative study: semi-structured interviews; focus groups | 27 parents of children who had received a false-negative NBS result. | Most parents did not report long-term negative impacts following a false positive NBS result but some experienced residual worry. Some parents identified positive outcomes after receiving the false positive NSB result. |
| [24] Lipstein, E.A.; Perrin, J.M.; Waisbren, S.E.; Prosser, L.A. (2009) US | Any of 20 biochemical disorders | Mixed methods: telephone survey; Parental Stress Index | 200 children with false-positive NBS results and 137 children with negative NBS results. | There were no significant differences between NBS results and child healthcare utilization. |
| [25] Hayeems, R.Z.; Miller, F.A.; Vermeulen, M.; Potter, B.K.; Chakraborty, P.; Davies, C.; Carroll, J.C.; Ratjen, F.; Guttmann, A. (2017) Canada | CF | Population-based cohort study using linked health administrative data | 1564 false-positive CF results and 6256 screen-negative matched controls. | A greater proportion of infants with false-positive results had more than 2 outpatient visits and more than 2 hospital admissions compared with control. No differences in healthcare use among mothers were detected. |
| [26] Tu, W.J.; He, J.; Chen, H.; Shi, X.D.; Li, Y. (2012) China | Metabolic disorders | Mixed methods: interviews; Parental Stress Index | Parents of 49 children with false-positive NBS results and 42 children with negative NBS results. | More mothers who had received a false-positive NBS result, worried about their child’s future compared to the negative NBS group. There were no differences in terms of worry for fathers in both groups. Children with false-positive NBS results were three times more likely to experience hospitalization. |
| [27] Karaceper, M.D.; Chakraborty, P.; Coyle, D.; Wilson, K.; Kronick, J.B.; Hawken, S.; Davies, C.; Brownell, M.; Dodds, L.; Feigenbaum, A.; et al. (2016) Canada | Medium-chain acyl-CoA dehydrogenase deficiency | Cohort study using healthcare administrative data sets | 43 infants with a false-positive NBS result. | Children who had received a false positive NBS result experienced significantly higher physician visits (IRR 1.42) and hospitalizations (IR 2.32) during the first year compared with children who had received a negative (normal) NBS result. |
| [28] Hewlett, J.; Waisbren, S.E. (2006) US | CF Metabolic disorders Congenital hypothyroidism Newborn hearing screening | Literature review: using Medline (via PubMed) and Journals@OVID | Nine studies were included. | Improved education and communication with parents, specifically at the time of follow up screening, can reduce parental stress and anxiety associated with a false-positive NBS result. |
| [29] Tluczek, A.; Mischler, E.H.; Bowers, B.; Peterson, N.M.; Morris, M.E.; Farrell, P.M.; Bruns, W.T.; Colby, H.; McCarthy, C.; Fost, N.; et al. (1991) US | CF | Qualitative: semi-structured interviews | Parents of infants with a false-positive result (n = 104); control group (n = 11). | Communication via telephone was more likely to lead to misunderstanding of the sweat test result than in-person communcation. Some parents expressed ongoing concerns that their child may still have CF. Most parents did not change their reproductive plans. Parents in both groups experienced strong emotional responses. These included worry but also gratitude in terms of their child being identified early if they had of had a problem. |
| [30] Gapp, S.; Garbade, S.F.; Feyh, P.; Brockow, I.; Nennstiel, U.; Hoffman, G.F. Sommerburg, O.; Gramer G, G.; 2022 Germany | CF | Cross-sectional: prospective questionnaire-based survey | 178 families responded to the emainedaire (33.7% had a confirmed CF diagnosis). | 17% of families with a false-positive NBS result remained anxious following confirmatory diagnostics. |
| [31] Lang, C.W.; McColley S.A.; Lester, L.A.; Friedman Ross, L. 2011 US | CF | Cross-sectional: telephone survey | 90 parents. | 94% of parents understood their child did not have CF after a negative sweat test. 79% of those identified as carriers understood their result. Parents expressed frustration related to the lack of knowledge and sensitivity by those who notified them of the initial positive NSB result. Speaking to a genetic counsellor while waiting for the sweat test decreased anxiety. |
| [32] Green, J.M.; Hewison J.; Bekker, H.l.; Bryant, L.D.; Cuckle, H.S. 2004 UK | All screened conditions | Systematic review | 106 papers were included: 78 about antenatal screening and 28 about NBS. | Anxiety in parents who had received false positive NBS resultmay not return to normal levels. Residual anxiety may continue, possibly over extended periods of time. |
| [33] Peterson, L.; Siemon, A.; Olewiler, L.; McBride, K.L.;Allain, D.D.; 2021 US | Krabbe Disease | Qualitative: cross-sectional semistructured, audio-recorded, phone interviews | 11 families were included in the analysis. | Parents reported experiencing emotional distress and negative emotions, specifically uncertainty during the initial communication of the NBS result, and leading up to and during the follow-up appointment with a genetic counsellor. |
| [34] Tluczek, A.; Mischler E.H.; Farrell P.M.; Fost, N.; Peterson, N.M.; Carey, P.; Bruns, W.T.; McCarthy, C. 1992 US | CF | Cross-sectional: survey | Parents of 104 infants with a false-positive NBS result for CF | Parents expressed shock, disbelief and confusion in response to the initial positive NBS result. Most parents (92%) were relieved following the negative sweat test and (88%) were aware that a negative sweat test meant their child did not have CF. Communication via telephone was more likely to lead to misunderstanding of the sweat test results compared to those told in person. Six parents who had lingering concerns that their child might have CF were informed of the sweat test via the telephone. The children also had lower APGAR scores at birth. 69% did not change their reproductive plans as a result of the false-positive result, 8% definitely changed their plans and 22% were uncertain. |
| [35] van den Heuvel, L.M.; van der Pal, S.M.; Verschoof-Puite, R.K.; Klapwijk, J.E.; Elsinghorst, E.; Dekker, E.; van der Ploeg C.P.B.; Henneman, L. 2024 The Netherlands | All screened conditions | Cross-Sectional: questionnaire; Hospital Anxiety and Depression Scale; Child Vulnerability Scale; TNO-AZL Preschool Children Quality of Life Scale (TAPQoL); NL-TiC-P questionnaire | 112 parents completed the questionnaires, of whom 35 had received a true-positive result, 20 a false-positive, 57 an inconclusive result and 268 controls 5 weeks after NBS. 60 parents completed the questionnaires, of whom 19 had received a true-positive result, 14 a false-positive, 27 an inconclusive result and 116 controls 4 months after NBS. | Negative emotions were more commonly associated with parents who had received true-positive, false-positive and inconclusive results both following the initial NBS result and four months later. No significant differences were observed in parental perceptions of child vulnerability between true-positive, false-positive, inconclusive and control groups after receiving the initial NBS result and four months later. Parents of children born at term who received true-positive and false-positive NBS results reported more frequent visits to a paediatrician compared with controls both after receiving the initial NBS result and four months later. |
| [36] Vernooij-van Langen, A.M.M.; van der Pal, S.M.; Reijntjens, A.J.T.; Loeber, J.G.; Dompeling, E.; Dankert, Roelse, J.E. 2014 The Netherlands | CF | Prospective controlled study using mixed methods: semi-structured interviews; questionnaires; Hospital Anxiety and Depression Scale; TNO-AZL Preschool Children Quality of Life Scale (TAPQoL) | Questionnaires were received from 62 parents who received a false-positive result, and 146 controls. Twenty-four mothers and three fathers took part in 25 interviews. | Parents in both the false-positive and control groups considered their child to be low risk in terms of being affected by CF. Parents in the false-positive group considered CF NBS less reliable. Scores related to anxiety and depression were not significantly different between the false-positive and control groups. Parental concern about their child’s health and number of GP visits did not differ between groups. |
| [37] Sorenson, J.R.; Levy, H.L.; Mangione, T.W.; Sepe, S.J. 1984 US | Metabolic conditions | Mixed methods: telephone interviews; parental psychological status was assessed using the Multipled Affect Adjective Checklist | 60 parents. | Parents who received an abnormal NBS result were no more anxious or depressed while waiting for the repeat test results than other parents. Parents were less anxious and depressed after the repeat test was found to be ‘normal’. However, 36% of parents reported concern about the health of their infant because of the need for the repeat test. |
| [38] Tarini, B.A.; Clark, S.J.; Pilli, S.; Dombkowski, K.J.; Korzeniewski, S.j.; Gebremariam, A.; Eisenhandler, J.; Grigorescu, V. 2011 US | All screened conditions | Cohort study using medicaid data | 818 infants with a false-positive NBS result. | Pre-term but not term infants with false-positive NBS results had more acute outpatient visits than infants who had received normal NBS results. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chudleigh, J.; Holder, P. Psychosocial Impact of False-Positive Newborn Screening Results: A Scoping Review. Children 2024, 11, 507. https://doi.org/10.3390/children11050507
Chudleigh J, Holder P. Psychosocial Impact of False-Positive Newborn Screening Results: A Scoping Review. Children. 2024; 11(5):507. https://doi.org/10.3390/children11050507
Chicago/Turabian StyleChudleigh, Jane, and Pru Holder. 2024. "Psychosocial Impact of False-Positive Newborn Screening Results: A Scoping Review" Children 11, no. 5: 507. https://doi.org/10.3390/children11050507
APA StyleChudleigh, J., & Holder, P. (2024). Psychosocial Impact of False-Positive Newborn Screening Results: A Scoping Review. Children, 11(5), 507. https://doi.org/10.3390/children11050507