The Clinical Pulmonary Infection Score Combined with Procalcitonin and Lung Ultrasound (CPIS-PLUS), a Good Tool for Ventilator Associated Pneumonia Early Diagnosis in Pediatrics
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Definitions
2.3. Variables
2.4. Image Diagnostic Test
- -
- The presence of B-lines and their characteristics (long, spared or confluent), and their position (peri-lesional, unilateral/bilateral).
- -
- The main lesions (consolidation): size (divided into <15 mm, 15–20 mm, >20 mm), if they were single or multiple, location (unilateral or bilateral) [49]
- -
- -
- Presence and type of bronchogram and its dynamicity during breath (fix or dynamic).
- -
- Presence of pleural effusion.
- -
- Presence of pneumothorax (lung point, absence of lung sliding (M-mode).
2.5. PCT Values
2.6. Microbiological Study
2.7. Data Management
2.8. Outcomes
2.9. Statistical Analysis
3. Results
- (1)
- Lobar consolidations were found in 47 (69.1%) vs. 21 (30.9%) patients; and subpleural consolidations in one (12.5%) vs. in seven (87.5%) cases, p < 0.001.
- (2)
- Unilateral consolidations were found in 41 (73.2%) vs. 15 (26.8%) patients; and bilateral consolidations in seven (35%) vs. thirteen (65%) cases, p < 0.001.
- (3)
- Consolidations > 20 mm were found in 39 (76.5%) vs. 12 (23.5%) patients, and those between 15–20 mm in eight (47.1%) vs. nine (52.9%) cases, p < 0.001.
- (4)
- Regarding the presence and type of bronchogram: dynamic bronchogram (DB) was seen in thirty-eight (95%) vs. two (5%) patients; and static bronchogram (SB) in 10 (34.5%) vs. 19 (65.5%) cases (p < 0.001).
- (5)
- PCT resulted with a median of 0.47 (IQR 0.17–1.32) in VAP patients vs. 0.21 (IQR 0.10–0.79) in those without VAP, p = 0.300.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rello, J.; Riera, J.; Serrano, R. What’s new in ventilator-associated pneumonia? Intensive Care Med. 2015, 41, 1954–1956. [Google Scholar] [CrossRef] [PubMed]
- Torres, A.; Niederman, M.S.; Chastre, J.; Ewig, S.; Fernandez-Vandellos, P.; Hanberger, H.; Kollef, M.; Bassi, G.L.; Luna, C.M.; Martin-Loeches, I.; et al. International ERS/ESICM/ESCMID/ALAT guidelines for the management of hospital-acquired pneumonia and ventilator-associated pneumonia. Eur. Respir. J. 2017, 50, 1700582. [Google Scholar] [CrossRef] [PubMed]
- Esteban, E.; Ferrer, R.; Urrea, M.; Suarez, D.; Rozas, L.; Balaguer, M.; Palomeque, A.; Jordan, I. The Impact of a Quality Improvement Intervention to Reduce Nosocomial Infections in a PICU. Pediatr. Crit. Care Med. 2013, 14, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Klompas, M. Complications of mechanical ventilation—The CDC’s new surveillance paradigm. N. Engl. J. Med. 2013, 368, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Lisboa, T.; Koulenti, D. Respiratory infections in patients undergoing mechanical ventilation. Lancet Respir. Med. 2014, 2, 764–774. [Google Scholar] [CrossRef] [PubMed]
- De Carlos, J.C. Resultados y evolución del registro multicentrico ENVIN. Rev. Espec. Peditarica 2016, 72 (Suppl. S1), 26–29. [Google Scholar]
- Palomar, M.; Álvarez Lerma, F.; Olaechea, P.; Gimeno Costa, R.; García Arnillas, M.P.; Seijas Betolaza, I.; Nuvials, X.; Catalán, M. Envin e Helics h. Envin-Helics. 2016. Available online: http://hws.vhebron.net/envin-helics/ (accessed on 5 May 2017).
- Chastre, J.; Fagon, J.Y. State of the Art Ventilator-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2002, 165, 867–903. [Google Scholar] [CrossRef] [PubMed]
- Elward, A.M.; Warren, D.K.; Fraser, V.J. Ventilator-Associated Pneumonia in Pediatric Intensive Care Unit Patients: Risk Factors and Outcomes. Pediatrics 2002, 109, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Rudan, I.; Tomaskovic, L.; Boschi-Pinto, C.; Campbell, H.; WHO Child Health Epidemiology Reference Group. Global estimate of the incidence of clinical pneumonia among children under five years of age. Bull. World Health Organ. 2004, 82, 895–903. [Google Scholar] [PubMed]
- Buonsenso, D.; Musolino, A.; Ferro, V.; De Rose, C.; Morello, R.; Ventola, C.; Liotti, F.M.; De Sanctis, R.; Chiaretti, A.; Biasucci, D.G.; et al. Role of lung ultrasound for the etiological diagnosis of acute lower respiratory tract infection (ALRTI) in children: A prospective study. J. Ultrasound 2021, 25, 185–197. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.M.; Groenwold, R.; Bergmans, D.C.; Camus, C.; Bauer, T.T.; Hanisch, E.; Klarin, B.; Koeman, M.; Krueger, W.A.; et al. Attributable mortality of ventilator-associated pneumonia: A meta-analysis of individual patient data from randomised prevention studies. Lancet Infect. Dis. 2013, 13, 665–671. [Google Scholar] [CrossRef]
- Melsen, W.G.; Rovers, M.; Koeman, M.; Bonten, M.J.M. Estimating the attributable mortality of ventilator-associated pneumonia from randomized prevention studies. Crit. Care Med. 2011, 39, 2736–2742. [Google Scholar] [CrossRef] [PubMed]
- Bouhemad, B.; Brisson, H.; Le-Guen, M.; Arbelot, C.; Lu, Q.; Rouby, J.-J. Bedside Ultrasound Assessment of Positive End-Expiratory Pressure–induced Lung Recruitment. Am. J. Respir. Crit. Care Med. 2011, 183, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Mongodi, S.; Via, G.; Girard, M.; Rouquette, I.; Misset, B.; Braschi, A.; Mojoli, F.; Bouhemad, B. Lung Ultrasound for Early Diagnosis of Ventilator-Associated Pneumonia. Chest 2016, 149, 969–980. [Google Scholar] [CrossRef]
- Mongodi, S.; Bouhemad, B.; Mojoli, F. Specific Ultrasound Signs May Improve Bedside Early Diagnosis of Ventilator-Associated Pneumonia. Respir. Care 2019, 64, 1175–1176. [Google Scholar] [CrossRef] [PubMed]
- Soldati, G.; Testa, A.; Silva, F.R.; Carbone, L.; Portale, G.; Silveri, N.G. Chest Ultrasonography in Lung Contusion. Chest 2006, 130, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Copetti, R.; Soldati, G.; Copetti, P. Chest sonography: A useful tool to differentiate acute cardiogenic pulmonary edema from acute respiratory distress syndrome. Cardiovasc. Ultrasound 2008, 6, 16. [Google Scholar] [CrossRef]
- Nazerian, P.; Vanni, S.; Volpicelli, G.; Gigli, C.; Zanobetti, M.; Bartolucci, M.; Ciavattone, A.; Lamorte, A.; Veltri, A.; Fabbri, A.; et al. Accuracy of Point-of-Care Multiorgan Ultrasonography for the Diagnosis of Pulmonary Embolism. Chest 2014, 145, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Iosifidis, E.; Pitsava, G.; Roilides, E. Ventilator-associated pneumonia in neonates and children: A systematic analysis of diagnostic methods and prevention. Future Microbiol. 2018, 13, 1431–1446. [Google Scholar] [CrossRef]
- Fernando, S.M.; Tran, A.; Cheng, W.; Klompas, M.; Kyeremanteng, K.; Mehta, S.; English, S.W.; Muscedere, J.; Cook, D.J.; Torres, A.; et al. Diagnosis of ventilator-associated pneumonia in critically ill adult patients—A systematic review and meta-analysis. Intensive Care Med. 2020, 46, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Rea-Neto, A.; Youssef, N.C.; Tuche, F.; Brunkhorst, F.; Ranieri, V.M.; Reinhart, K.; Sakr, Y. Diagnosis of ventilator-associated pneumonia: A systematic review of the literature. Crit. Care 2008, 12, R56. [Google Scholar] [CrossRef] [PubMed]
- E Graat, M.; Choi, G.; Wolthuis, E.K.; Korevaar, J.C.; E Spronk, P.; Stoker, J.; Vroom, M.B.; Schultz, M.J. The clinical value of daily routine chest radiographs in a mixed medical–surgical intensive care unit is low. Crit. Care 2005, 10, R11. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Ji, X.; Xu, Y.; Xiang, X. Lung ultrasound: A promising tool to monitor ventilator-associated pneumonia in critically ill patients. Crit. Care 2016, 20, 320. [Google Scholar] [CrossRef] [PubMed]
- Reissig, A.; Copetti, R.; Mathis, G.; Mempel, C.; Schuler, A.; Zechner, P.; Aliberti, S.; Neumann, R.; Kroegel, C.; Hoyer, H. Lung Ultrasound in the Diagnosis and Follow-up of Community-Acquired Pneumonia: A prospective, multicenter, diagnostic accuracy study. Chest 2012, 142, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Guitart, C.; Rodríguez-Fanjul, J.; Bobillo-Perez, S.; Carrasco, J.L.; Clemente, E.J.I.; Cambra, F.J.; Balaguer, M.; Jordan, I. An algorithm combining procalcitonin and lung ultrasound improves the diagnosis of bacterial pneumonia in critically ill children: The PROLUSP study, a randomized clinical trial. Pediatr. Pulmonol. 2022, 57, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Bobillo-Perez, S.; Girona-Alarcon, M.; Rodriguez-Fanjul, J.; Jordan, I.; Gargallo, M.B. Lung ultrasound in children: What does it give us? Paediatr. Respir. Rev. 2020, 36, 136–141. [Google Scholar] [CrossRef] [PubMed]
- American Thoracic Society; Infectious Diseases Society of America. Guidelines for the Management of Adults with Hospital-acquired, Ventilator-associated, and Healthcare-associated Pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, E.H.; Sherman, G.; Ward, S.; Fraser, V.J.; Kollef, M.H. The Influence of Inadequate Antimicrobial Treatment of Bloodstream Infections on Patient Outcomes in the ICU Setting. Chest 2000, 118, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Rello, J.; Ausina, V.; Ricart, M.; Puzo, C.; Quintana, E.; Net, A.; Prats, G. Risk factors for infection by Pseudomonas aeruginosa in patients with ventilator-associated pneumonia. Intensive Care Med. 1994, 20, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Trouillet, J.L.; Chastre, J.; Vuagnat, A.; Joly-Guillou, M.L.; Combaux, D.; Dombret, M.C.; Gibert, C. Ventilator-associated pneumonia caused by potentially drug-resistant bacteria. Am. J. Respir. Crit. Care Med. 1998, 157, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Yu, V.L.; Singh, N. Excessive antimicrobial usage causes measurable harm to patients with suspected ventilator-associated pneumonia. Intensive Care Med. 2004, 30, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Milcent, K.; Faesch, S.; Guen, C.G.-L.; Dubos, F.; Poulalhon, C.; Badier, I.; Marc, E.; Laguille, C.; de Pontual, L.; Mosca, A.; et al. Use of Procalcitonin Assays to Predict Serious Bacterial Infection in Young Febrile Infants. JAMA Pediatr. 2016, 170, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Gauvin, F.; Amre, D.K.; Saint-Louis, P.; Lacroix, J. Serum Procalcitonin and C-Reactive Protein Levels as Markers of Bacterial Infection: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2004, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Bobillo-Perez, S.; Rodríguez-Fanjul, J.; Garcia, I.J. Is Procalcitonin Useful in Pediatric Critical Care Patients? Biomark. Insights 2018, 13, 1177271918792244. [Google Scholar] [CrossRef] [PubMed]
- Pepper, D.J.; Sun, J.; Rhee, C.; Welsh, J.; Powers, J.H.; Danner, R.L.; Kadri, S.S. Procalcitonin-Guided Antibiotic Discontinuation and Mortality in Critically Ill Adults: A Systematic Review and Meta-analysis. Chest 2019, 155, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- van der Does, Y.; Rood, P.P.; Haagsma, J.A.; Patka, P.; van Gorp, E.C.; Limper, M. Procalcitonin-guided therapy for the initiation of antibiotics in the ED: A systematic review. Am. J. Emerg. Med. 2016, 34, 1286–1293. [Google Scholar] [CrossRef]
- Korppi, M.; Remes, S.; Heiskanen-Kosma, T. Serum procalcitonin concentrations in bacterial pneumonia in children: A negative result in primary healthcare settings. Pediatr. Pulmonol. 2003, 35, 56–61. [Google Scholar] [CrossRef]
- Pugin, J.; Auckenthaler, R.; Mili, N.; Janssens, J.-P.; Lew, P.D.; Suter, P.M. Diagnosis of Ventilator-associated Pneumonia by Bacteriologic Analysis of Bronchoscopic and Nonbronchoscopic “Blind” Bronchoalveolar Lavage Fluid. Am. Rev. Respir. Dis. 1991, 143, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Luna, C.M.; Blanzaco, D.; Niederman, M.S.; Matarucco, W.; Baredes, N.C.; Desmery, P.; Palizas, F.; Menga, G.; Rios, F.; Apezteguia, C. Resolution of ventilator-associated pneumonia: Prospective evaluation of the clinical pulmonary infection score as an early clinical predictor of outcome. Crit. Care Med. 2003, 31, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, A.; Chugh, K.; Sethi, M.; Gupta, D.; Wattal, C.; Menon, G. Clinical pulmonary infection score to diagnose ventilator-associated pneumonia in children. Indian Pediatr. 2011, 48, 949–954. [Google Scholar] [CrossRef] [PubMed]
- da Silva, P.S.L.; de Aguiar, V.E.; de Carvalho, W.B.; Fonseca, M.C.M. Value of clinical pulmonary infection score in critically ill children as a surrogate for diagnosis of ventilator-associated pneumonia. J. Crit. Care 2014, 29, 545–550. [Google Scholar] [CrossRef] [PubMed]
- Zilberberg, M.D.; Shorr, A.F. Ventilator-Associated Pneumonia: The Clinical Pulmonary Infection Score as a Surrogate for Diagnostics and Outcome. Clin. Infect. Dis. 2010, 51, S131–S135. [Google Scholar] [CrossRef] [PubMed]
- Zagli, G.; Cozzolino, M.; Terreni, A.; Biagioli, T.; Caldini, A.L.; Peris, A. Diagnosis of Ventilator-Associated Pneumonia: A Pilot, Exploratory Analysis of a New Score Based on Procalcitonin and Chest Echography. Chest 2014, 146, 1578–1585. [Google Scholar] [CrossRef] [PubMed]
- Poddar, B.; Neyaz, Z.; Patnaik, R.; Baronia, A.K.; Singh, R.K. Incorporating Lung Ultrasound in Clinical Pulmonary Infection Score as an Added Tool for Diagnosing Ventilator-associated Pneumonia: A Prospective Observational Study from a Tertiary Care Center. Indian J. Crit. Care Med. 2021, 25, 284–291. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention; National Center for Emerging and Zoonotic Infectious Diseases (NCEZID); Division of Healthcare Quality Promotion (DHQP). Pneumonia (Ventilator-Associated [VAP] and Non-Ventilator-Associated Pneumonia [PNEU]) Event; CDC: Atlanta, GA, USA, 2023. [Google Scholar]
- World Health Organization; Pneumonia Vaccine Trial Investigators’ Group. Standardization of Interpretation of Chest Radiographs for the Diagnosis of Pneumonia in Children/World Health Organization Pneumonia Vaccine Trial Investigators’ Group; World Health Organization: Geneva, Switzerland, 2001. [Google Scholar]
- Volpicelli, G.; Elbarbary, M.; Blaivas, M.; Lichtenstein, D.A.; Mathis, G.; Kirkpatrick, A.W.; Melniker, L.; Gargani, L.; Noble, V.E.; Via, G.; et al. International evidence-based recommendations for point-of-care lung ultrasound. Intensive Care Med. 2012, 38, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Berce, V.; Tomazin, M.; Gorenjak, M.; Berce, T.; Lovrenčič, B. The Usefulness of Lung Ultrasound for the Aetiological Diagnosis of Community-Acquired Pneumonia in Children. Sci. Rep. 2019, 9, 17957. [Google Scholar] [CrossRef] [PubMed]
- Cherian, T.; Mulholland, E.K.; Carlin, J.B.; Ostensen, H.; Amin, R.; Campo, M.D.; Greenberg, D.; Lagos, R.; Lucero, M.; Madhi, S.A.; et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005, 83, 353–359. [Google Scholar] [PubMed]
- Shah, V.P.; Tunik, M.G.; Tsung, J.W. Prospective Evaluation of Point-of-Care Ultrasonography for the Diagnosis of Pneumonia in Children and Young Adults. JAMA Pediatr. 2013, 167, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: A prospective study. Pediatr. Pulmonol. 2019, 54, 1479–1486. [Google Scholar] [CrossRef]
- Varshney, T.; Mok, E.; Shapiro, A.J.; Li, P.; Dubrovsky, A.S. Point-of-care lung ultrasound in young children with respiratory tract infections and wheeze. Emerg. Med. J. 2016, 33, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Bouadma, L.; Luyt, C.E.; Tubach, F.; Cracco, C.; Alvarez, A.; Schwebel, C.; Schortgen, F.; Lasocki, S.; Veber, B.; Dehoux, M.; et al. Use of procalcitonin to reduce patients’ exposure to antibiotics in intensive care units (PRORATA trial): A multicentre randomised controlled trial. Lancet 2010, 375, 463–474. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Barat, L.; Motos, A.; Ranzani, O.T.; Bassi, G.L.; Xiol, E.A.; Senussi, T.; Travierso, C.; Chiurazzi, C.; Idone, F.; Muñoz, L.; et al. Diagnostic Value of Endotracheal Aspirates Sonication on Ventilator-Associated Pneumonia Microbiologic Diagnosis. Microorganisms 2017, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Arango, M.V.; Martí, A.T.; Ordeñana, J.I.; Lerma, F.; Joaquinet, N.C.; Casado, M.H.; León, J.T. Valor diagnóstico del cultivo cuantitativo del aspirado endotraqueal en la neumonía adquirida durante la ventilación mecánica. Estud. Multicéntrico 2003, 39, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Kollef, M.H.; Burnham, C.-A.D. Ventilator-Associated Pneumonia: The Role of Emerging Diagnostic Technologies. Semin. Respir. Crit. Care Med. 2017, 38, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef] [PubMed]
- Subirana, I.; Héctor, C.; Cresib, S.; Vila, J. Building Bivariate Tables: The CompareGroups Package for R. J. Stat. Softw. 2014, 57, 1–16. [Google Scholar] [CrossRef]
- Perez-Jaume, S.; Skaltsa, K.; Pallarès, N.; Carrasco, J.L. ThresholdROC: Optimum Threshold Estimation Tools for Continuous Diagnostic Tests in R. J. Stat. Softw. 2017, 82, 1–21. [Google Scholar] [CrossRef]
- Robin, X.; Turck, N.; Hainard, A.; Tiberti, N.; Lisacek, F.; Sanchez, J.-C.; Müller, M. pROC: An open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinform. 2011, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Staub, L.J.; Biscaro, R.R.M.; Maurici, R. Accuracy and Applications of Lung Ultrasound to Diagnose Ventilator-Associated Pneumonia: A Systematic Review. J. Intensive Care Med. 2018, 33, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, M.B.; Rabiner, J.E. Pediatric Point-of-Care Lung Ultrasonography: A Narrative Review. West. J. Emerg. Med. 2022, 23, 497–504. [Google Scholar] [CrossRef]
- See, K.C.; Ong, V.; Wong, S.H.; Leanda, R.; Santos, J.; Taculod, J.; Phua, J.; Teoh, C.M. Lung ultrasound training: Curriculum implementation and learning trajectory among respiratory therapists. Intensive Care Med. 2016, 42, 63–71. [Google Scholar] [CrossRef]
- Bhalla, D.; Naranje, P.; Jana, M.; Bhalla, A.S. Pediatric lung ultrasonography: Current perspectives. Pediatr. Radiol. 2022, 52, 2038–2050. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Wei, J. The Application of Pulmonary Ultrasound in Neonatal Ventilator-Associated Pneumonia. Front. Pediatr. 2022, 10, 882056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Song, J.; Gong, S.; Hu, W.; Wang, M.; Xiao, A.; Zhang, C.; Dong, Z. Lung Ultrasound Combined With Procalcitonin for a Diagnosis of Ventilator-Associated Pneumonia. Respir. Care 2019, 64, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Gibot, S.; Cravoisy, A.; Levy, B.; Bene, M.-C.; Faure, G.; Bollaert, P.-E. Soluble Triggering Receptor Expressed on Myeloid Cells and the Diagnosis of Pneumonia|Enhanced Reader. N. Engl. J. Med. 2004, 350, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Jung, B.; Embriaco, N.; Roux, F.; Forel, J.M.; Demory, D.; Allardet-Servent, J.; Jaber, S.; La Scola, B.; Papazian, L. Microbiogical data, but not procalcitonin improve the accuracy of the clinical pulmonary infection score. Intensive Care Med. 2010, 36, 790–798. [Google Scholar] [CrossRef] [PubMed]
- Gadappa, S.M.; Behera, M.K. Ventilator associated pneumonia: Incidence, profile and outcome in pediatric intensive care unit of tertiary care centre. Int. J. Contemp. Pediatr. 2018, 5, 2098–2102. [Google Scholar] [CrossRef]
- Patria, M.F.; Chidini, G.; Ughi, L.; Montani, C.; Prandi, E.; Galeone, C.; Calderini, E.; Esposito, S. Ventilator-associated pneumonia in an Italian pediatric intensive care unit: A prospective study. World J. Pediatr. 2013, 9, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Asselin, J.; Gildengorin, G.; Wiener-Kronish, J.; Flori, H. A Prospective Study of Ventilator-Associated Pneumonia in Children. Pediatrics 2009, 123, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Ego, A.; Preiser, J.-C.; Vincent, J.-L. Impact of Diagnostic Criteria on the Incidence of Ventilator-Associated Pneumonia. Chest 2015, 147, 347–355. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Points | ||
|---|---|---|---|
| 0 | 1 | 2 | |
| Temperature, °C | ≥36.5 and ≤38.4 | ≥38.5 and ≤38.9 | ≤36 or ≥39 |
| Blood leukocytes, WBC/mm3 | ≥4.000 and ≤11.000 | <4.000 or >11.000 | <4.000 or >11.000 and bands forms ≥500 |
| Oxygenation: PaO2/FiO2 | >240 or presence of ARDS | ---- | ≤240 and absence of ARDS |
| Tracheal secretion | Absent or minimal (<14 aspirations/day) | Non purulent (≥14 aspirations/day) | Purulent |
| Pulmonary radiography | No infiltrate | Patchy o diffuse infiltrate | Localized infiltrate |
| Culture/microscopy of tracheal aspirate | Negative culture | Positive culture | Positive culture and microscopy 1 (same pathogenic bacteria seen on Gram stain) |
| Total n = 108 | VAP Group n = 51 | No-VAP Group n = 57 | p Value | |
|---|---|---|---|---|
| Gender (male), n (%) | 56 (51.9) | 23 (45) | 33 (57.8) | 0.184 |
| Age (months) median (IQR) | 8.4 (0.76–57) | 15 (1.58–63.25) | 6.31 (3.09–54.7) | 0.827 |
| Weight (kg), median (IQR) | 8 (4.6–19.25) | 9 (4.5–20) | 7.2 (4.65–17) | 0.622 |
| Comorbidities, n (%) | 0.365 | |||
| None | 37 (34.3) | 20 (39) | 17 (29.8) | |
| Respiratory | 26 (24.1) | 10 (19.6) | 16 (28) | |
| Cardiovascular | 21 (19.4) | 9 (17.6) | 12 (21) | |
| Infectious diseases | 1 (0.9) | 1 (1.96) | 0 (0) | |
| Neurological | 10 (9.3) | 3 (5.88) | 7 (12.3) | |
| Hematology-oncology | 5 (4.6) | 2 (3.92) | 3 (5.26) | |
| Other | 8 (7.4) | 6 (11.8) | 2 (3.5) | |
| Reason for admission, n (%) | 0.065 | |||
| Infection 1 | 32 (26.9) | 21 (41.2) | 11 (19.3) | |
| Trauma | 8 (7.4) | 5 (9.8) | 3 (5.26) | |
| CV surgery/heart failure | 18 (16.7) | 7 (13.7) | 11 (19.3) | |
| Oncology | 4 (3.7) | 0 (0) | 4 (7) | |
| Surgery (Abd, trauma, NS) | 21 (19.4) | 8 (15.7) | 13 (22.8) | |
| Other | 25 (23.1) | 10 (19.6) | 15 (26.3) | |
| Severity upon admission, | ||||
| PRISM III, median (IQR) | 3 (0–9.75) | 4 (0–8) | 3 (0–10) | 0.249 |
| Length of stay | ||||
| PICU (days), median (IQR) | 23 (16–60.5) | 23 (16–35) | 27 (12.5–84) | 0.571 |
| Hospitalization (days), median (IQR) | 43 (25–108) | 35 (24–66) | 69 (25.5–127) | 0.128 |
| Clinical symptoms/signs n (%) | ||||
| Fever | 74 (68.5) | 38 (74.5) | 36 (63.2) | 0.205 |
| Tracheal Secretions Absent Non-purulent Purulent | 44 (40.7) 47 (43.5) 17 (15.7) | 12 (23.5) 24 (47.1) 15 (29.4) | 32 (56.1) 23 (40.4) 2 (3.5) | 0.001 |
| Blood test | ||||
| Leukocytes, median (IQR) | 12.400 (8.500–16.200) | 12.400 (8.500–16.200) | 12.600 (8.500–16.200) | 0.568 |
| CRP mg/L, median (IQR) | 58.8 (20.85–98.5) | 56.35 (24–105) | 60 (12–93) | 0.666 |
| PCT ng/mL, median (IQR) | 0.36 (0.16–1.42) | 0.44 (0.17–1.31) | 0.25 (0.11–1.77) | 0.549 |
| Antibiotic therapy, n (%) | 89 (82.4) | 51 (100) | 38 (66.7) | p < 0.01 |
| Days of antibiotic, median (IQR) | 7 (5–7) | 7 (7–9) | 5 (0–7) | p < 0.01 |
| CMV days, median (IQR) | 9 (3–15) | 12 (8–16) | 6 (0–11) | p < 0.01 |
| Inotropic support, n (%) | 37 (34.3) | 21 (41.2) | 16 (28) | 0.152 |
| Exitus, n (%) | 5 (4.6) | 2 (3.9) | 3 (5.3) | 1 |
| CPIS (mean ± SD) | 5.81 ± 2.09 | 6.63 ± 1.74 | 5.09 ± 2.12 | <0.001 |
| LUS Findings | Total n = 84 | VAP n = 49 | No-VAP n = 35 | OR [IQR] | p-Value OR | p-Value Overall |
|---|---|---|---|---|---|---|
| Type of consolidation n (%) | <0.001 | |||||
| No consolidation | 8 (9.52%) | 1 (12.5%) | 7 (87.5%) | |||
| Supleural | 8 (9.52%) | 1 (12.5%) | 7 (87.5%) | 1.00 [0.05–19.4] | 1 | |
| Lobar | 68 (81%) | 47 (69.1%) | 21 (30.9%) | 15.7 [1.81–136] | 0.003 | |
| Laterality n (%) | <0.001 | |||||
| No consolidation | 8 (9.52%) | 1 (12.5%) | 7 (87.5%) | |||
| Bilateral | 20 (23.8%) | 7 (35%) | 13 (65%) | 3.77 [0.38–37.1] | 0.281 | |
| Unilateral | 56 (66.7%) | 41 (73.2%) | 15 (26.8%) | 19.1 [2.17–169] | 0.002 | |
| Size n (%) | <0.001 | |||||
| No consolidation | 8 (9.52%) | 1 (12.5%) | 7 (87.5%) | |||
| <15 mm | 8 (9.52%) | 1 (12.5%) | 7 (87.5%) | 1.00 [0.05–19.4] | 1 | |
| ≥15 mm–20 mm | 17 (20.2%) | 8 (47.1%) | 9 (52.9%) | 6.22 [0.62–62.2] | 0.119 | |
| >20 mm | 51 (60.7%) | 39 (76.5%) | 12 (23.5%) | 22.8 [2.54–204] | 0.001 | |
| Bronchogram n (%) | <0.001 | |||||
| No bronchogram | 15 (17.9%) | 1 (6.67%) | 14 (93.3%) | |||
| Static bronchogram | 29 (34.5%) | 10 (34.5%) | 19 (65.5%) | 7.37 [0.84–64.4] | 0.048 | |
| Dynamic bronchogram | 40 (47.6%) | 38 (95%) | 2 (5%) | 266 [22.3–3168] | <0.001 | |
| PCT ng/mL (median [IQR]) | 0.37 [0.16–1.31] | 0.47 [0.17–1.32] | 0.21 [0.10–0.79] | 0.95 [1.01–0.89] | 0.125 | 0.3 |
| CPIS-PLUS | 0 | 1 | 2 | 3 |
|---|---|---|---|---|
| Temperature, °C | ≥36.5 and <38.4 | ≥38.5 and <38.9 | <36 or ≥39 | - |
| Leukocytes/mm3 | ≥4.000 and ≤11.000 | <4.000 or >11.000 | <4.000 or >11.000 and bands forms ≥500 | - |
| PaO2/FiO2 | >240 or ARDS | ---- | ≤240 and no evidence of ARDS | - |
| Tracheal secretion | Absent or minimal (<14 aspirations/day) | Non purulent (≥14 aspirations/day) | Purulent | - |
| Culture or gram of tracheal aspirate | Negative | Positive | Positive culture and microscopy (same pathogenic bacteria seen on Gram stain) | - |
| Procalcitonin, ng/mL | <0.5 | ≥0.5 and <1 | ≥1 | - |
| LUS findings | ||||
| Type of consolidation | No consolidation | Subpleural | Lobar | - |
| Size of consolidation | <15 mm | 15–20 mm | >20 mm | - |
| Laterality | - | Bilateral | Unilateral | - |
| Bronchogram | - | SB | - | DB |
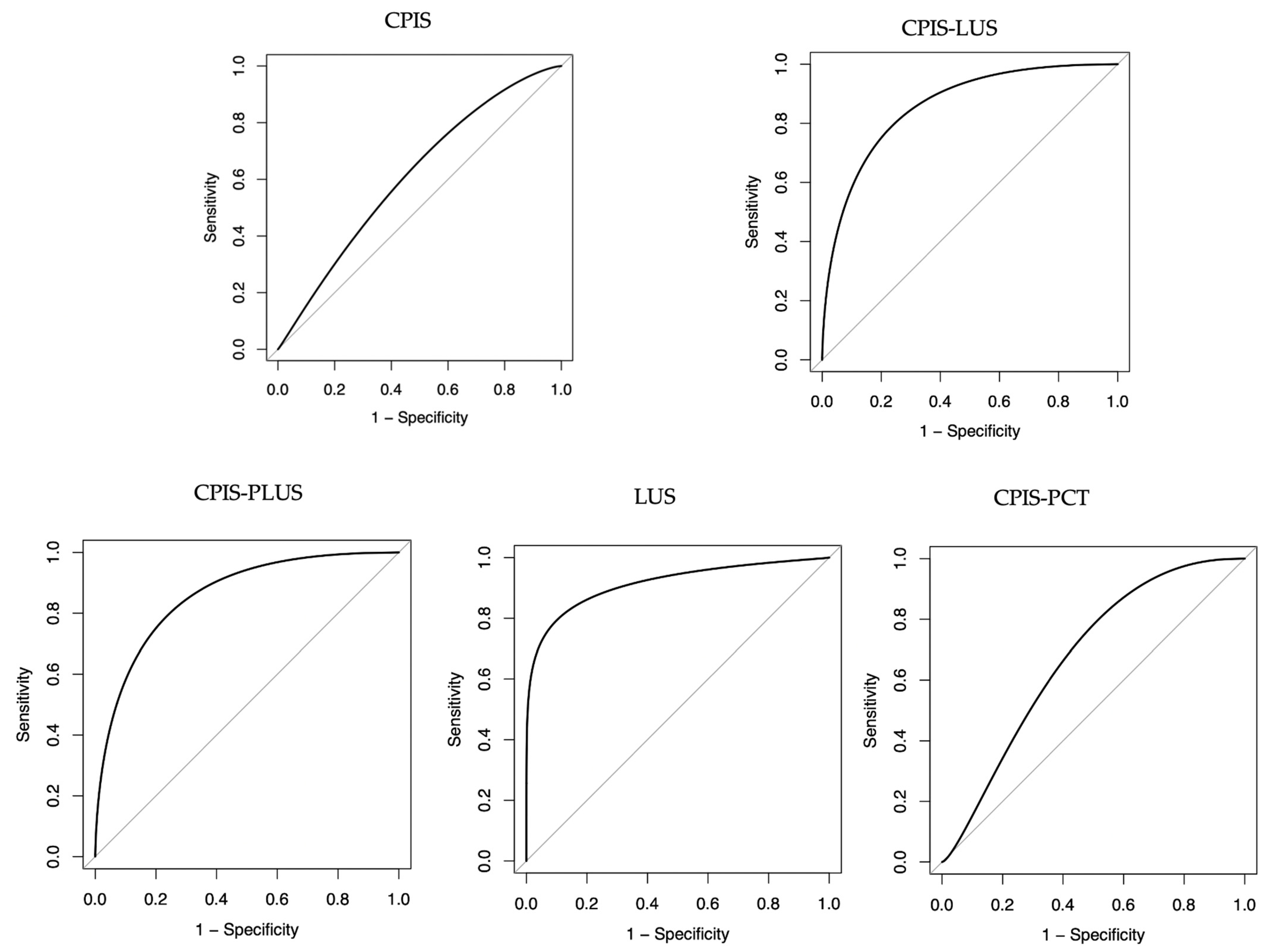
| AUC | Sensibility (%) | Specificity (%) | PPV (%) | NPV (%) | |
|---|---|---|---|---|---|
| CPIS > 6 | 0.61 (0.50–0.75) | 78 (63–88) | 46 (29–63) | 67 (53–78) | 59 (39–77) |
| CPIS-LUS ≥ 12 | 0.86 (0.76–0.92) | 76 (61–86) | 77 (59–89) | 82 (67–91) | 69 (52–82) |
| CPIS-PLUS ≥ 12 | 0.86 (0.76–0.92) | 80 (65–89) | 73 (54–86) | 81 (67–91) | 71 (52–84) |
| LUS ≥ 7 | 0.91 (0.78–0.95) | 81 (67–91) | 74 (56–87) | 82 (67–91) | 74 (56–87) |
| CPIS-PCT ≥ 7 | 0.67 (0.57–0.78) | 60 (45–73) | 60 (45–73) | 60 (45–73) | 60 (45–73) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becerra-Hervás, J.; Guitart, C.; Covas, A.; Bobillo-Pérez, S.; Rodríguez-Fanjul, J.; Carrasco-Jordan, J.L.; Cambra Lasaosa, F.J.; Jordan, I.; Balaguer, M. The Clinical Pulmonary Infection Score Combined with Procalcitonin and Lung Ultrasound (CPIS-PLUS), a Good Tool for Ventilator Associated Pneumonia Early Diagnosis in Pediatrics. Children 2024, 11, 592. https://doi.org/10.3390/children11050592
Becerra-Hervás J, Guitart C, Covas A, Bobillo-Pérez S, Rodríguez-Fanjul J, Carrasco-Jordan JL, Cambra Lasaosa FJ, Jordan I, Balaguer M. The Clinical Pulmonary Infection Score Combined with Procalcitonin and Lung Ultrasound (CPIS-PLUS), a Good Tool for Ventilator Associated Pneumonia Early Diagnosis in Pediatrics. Children. 2024; 11(5):592. https://doi.org/10.3390/children11050592
Chicago/Turabian StyleBecerra-Hervás, Judit, Carmina Guitart, Aina Covas, Sara Bobillo-Pérez, Javier Rodríguez-Fanjul, Josep L. Carrasco-Jordan, Francisco José Cambra Lasaosa, Iolanda Jordan, and Mònica Balaguer. 2024. "The Clinical Pulmonary Infection Score Combined with Procalcitonin and Lung Ultrasound (CPIS-PLUS), a Good Tool for Ventilator Associated Pneumonia Early Diagnosis in Pediatrics" Children 11, no. 5: 592. https://doi.org/10.3390/children11050592
APA StyleBecerra-Hervás, J., Guitart, C., Covas, A., Bobillo-Pérez, S., Rodríguez-Fanjul, J., Carrasco-Jordan, J. L., Cambra Lasaosa, F. J., Jordan, I., & Balaguer, M. (2024). The Clinical Pulmonary Infection Score Combined with Procalcitonin and Lung Ultrasound (CPIS-PLUS), a Good Tool for Ventilator Associated Pneumonia Early Diagnosis in Pediatrics. Children, 11(5), 592. https://doi.org/10.3390/children11050592





