Effect of Salinity on the Imbibition Recovery Process of Tight Sandstone Reservoirs
Abstract
:1. Introduction
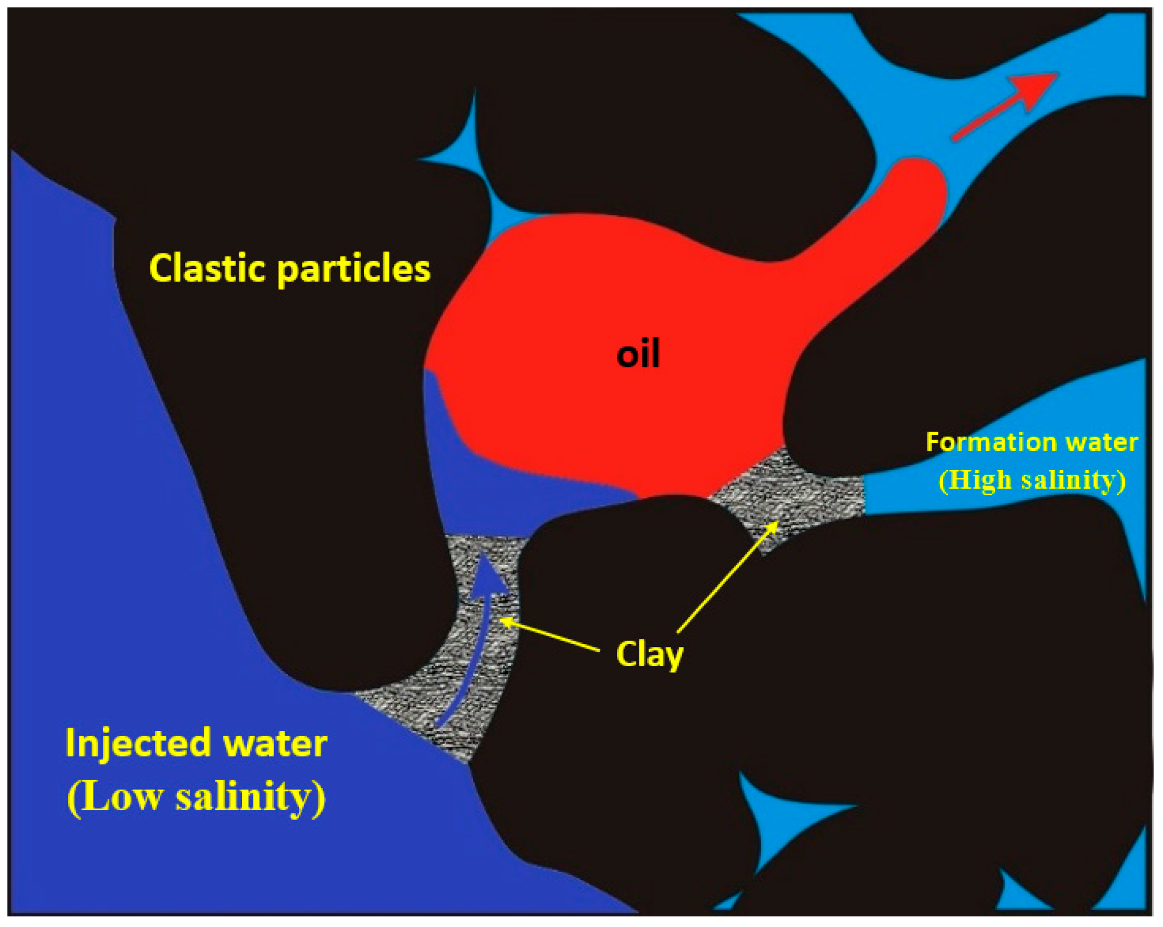
2. Mechanism of Mineralization
2.1. The Influence of Salinity on Interfacial Tension
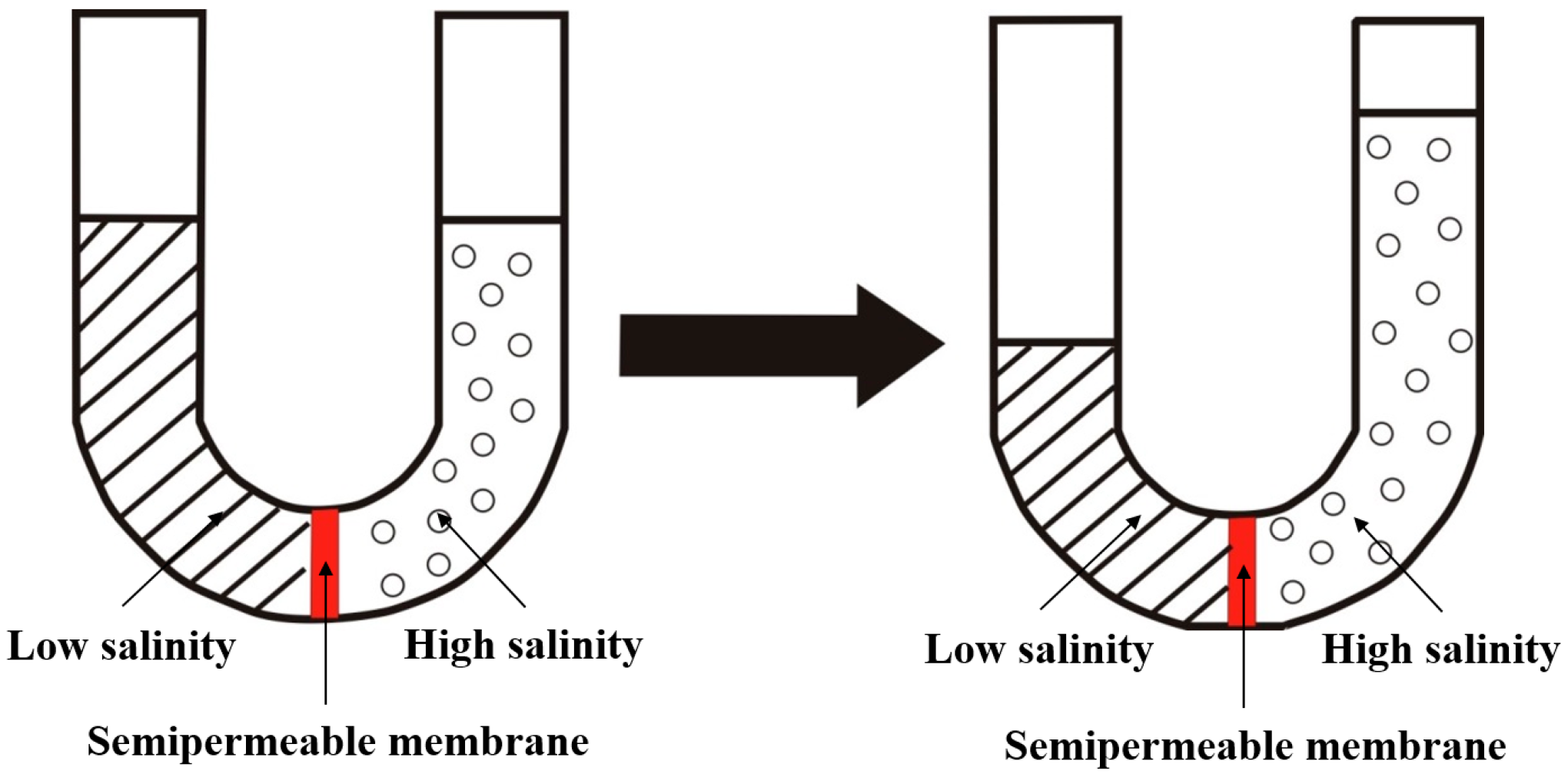
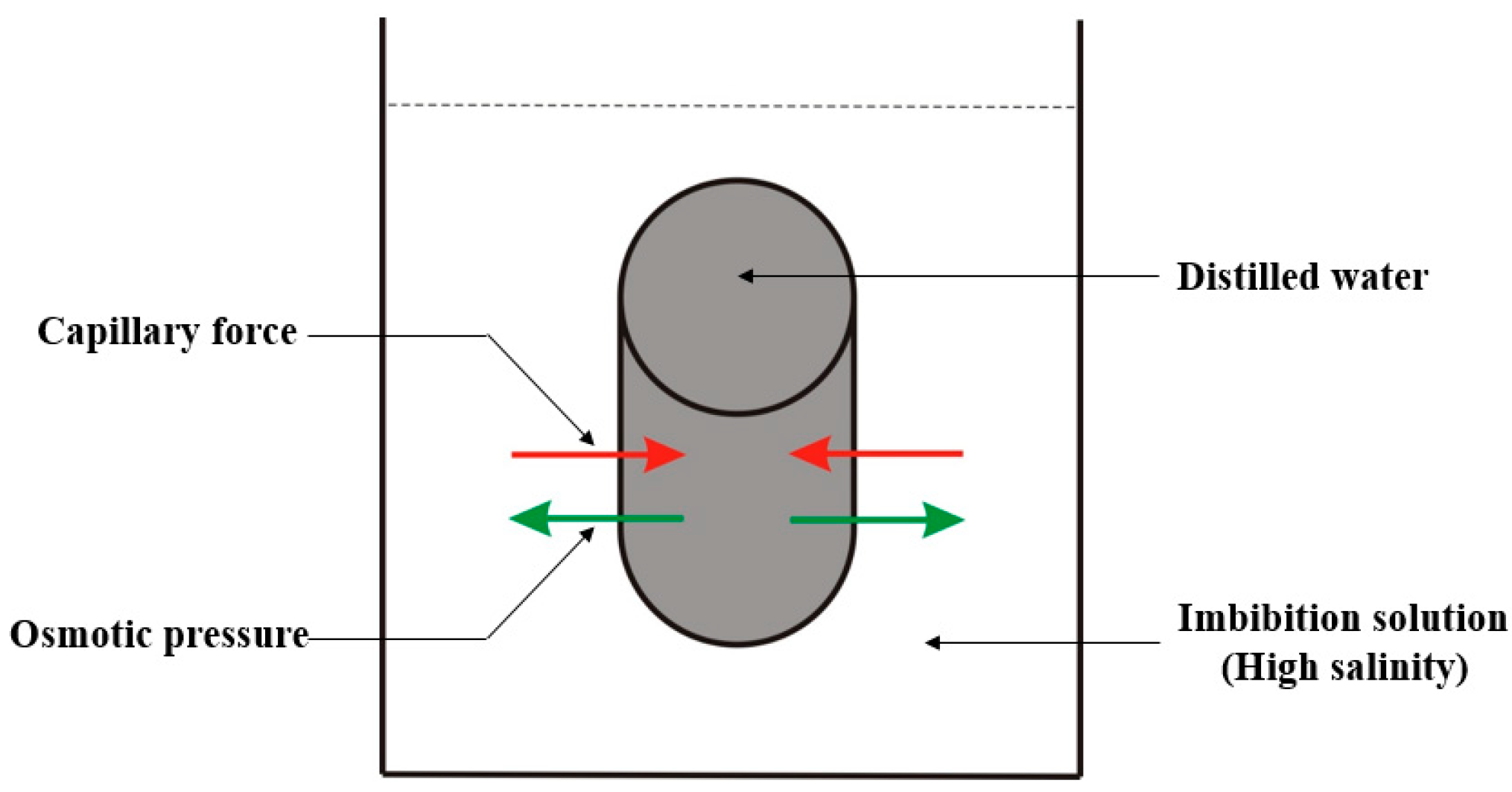
2.2. The Mechanism of Osmotic Pressure
3. Experimental Process
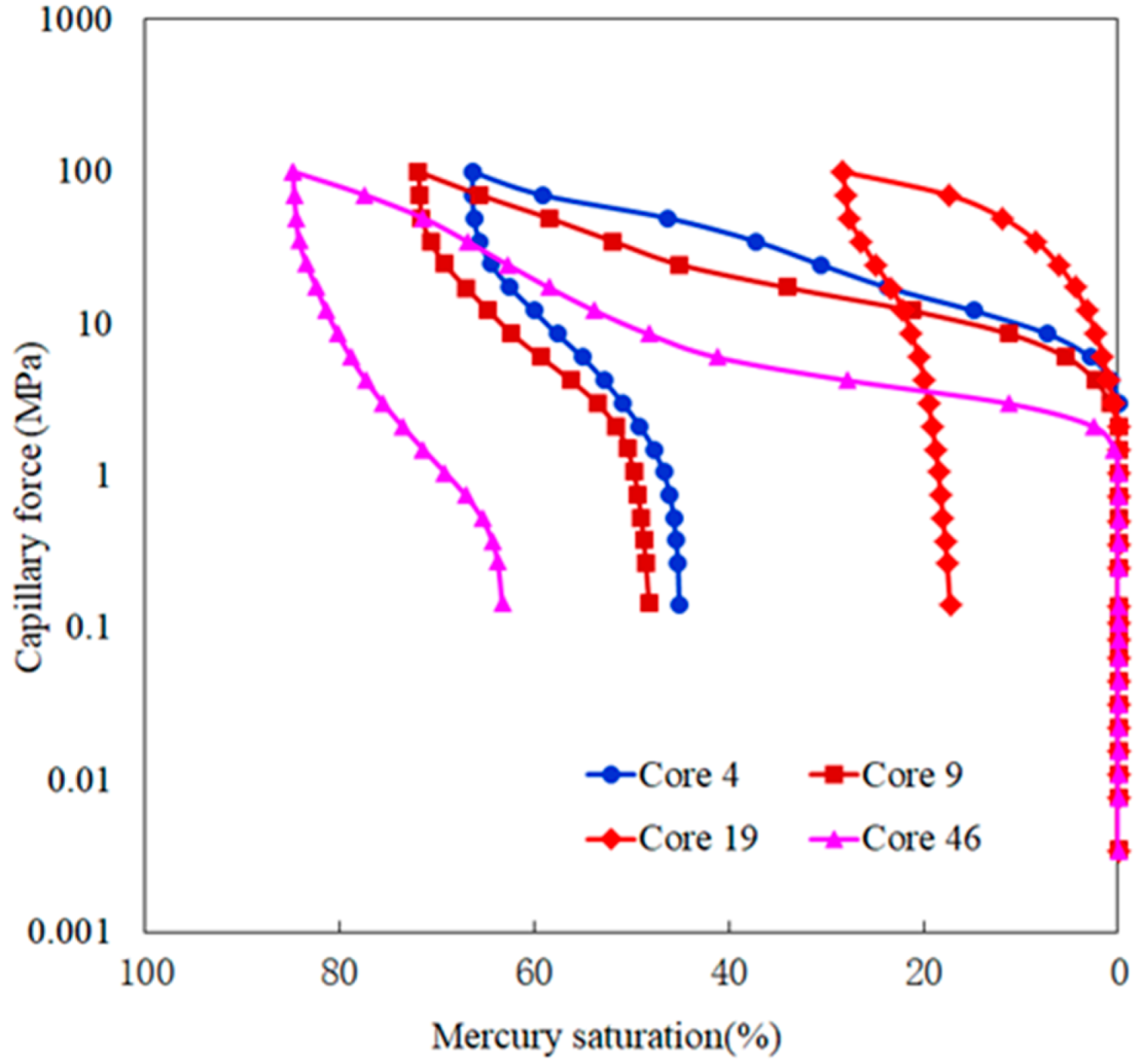
4. Result Analysis
5. Discussion
6. Conclusions
- (1)
- The influence of salinity on the imbibition process of tight sandstone reservoirs is mainly acted through two ways: one is to reduce the fluid interfacial tension, and the other is to construct an osmotic pressure displacement model;
- (2)
- Salinity has significant effects on interfacial tension. The interfacial tension of low-salinity brine is only 1/5 of that of distilled water. However, in the presence of high-efficiency surfactants, the effect of salinity on interfacial tension is not obvious;
- (3)
- The imbibition process of tight sandstone cores is heavily influenced by the degree of salinity. The greater the difference in salt concentration, the greater the permeability, and the greater the influence of salinity on the process of imbibition oil recovery in tight sandstone reservoirs;
- (4)
- At the initial stage of imbibition, the effect of salinity is less important than that of capillary force. At the mid stage of imbibition, the effect of salinity is much more important than that of capillary force and the imbibition curve shows a downward trend. At the end of imbibition, the fluid tends toward imbibition equilibrium, and the effects of capillary force and salinity are not obvious.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- You, Q.; Wang, H.; Zhang, Y.; Liu, Y.; Fang, J.; Dai, C. Experimental study on spontaneous imbibition of recycled fracturing flow-back fluid to enhance oil recovery in low permeability sandstone reservoirs. J. Pet. Sci. Eng. 2018, 166, 375–380. [Google Scholar] [CrossRef]
- Gao, H.; Wang, Y.; Xie, Y.; Ni, J.; Li, T.; Wang, C.; Xue, J. Imbibition and Oil Recovery Mechanism of Fracturing Fluids in Tight Sandstone Reservoirs. ACS Omega 2021, 6, 1991–2000. [Google Scholar] [CrossRef] [PubMed]
- Mejia, L.; Tagavifar, M.; Xu, K.; Mejia, M.; Du, Y.; Balhoff, M. Surfactant flooding in oil-wet micromodels with high permeability fractures. Fuel 2019, 241, 1117–1128. [Google Scholar] [CrossRef]
- Liu, S.; Ni, J.; Wen, X.; Liu, X.; Huang, Z.; Zhou, D.; Ren, P. A dual-porous and dual-permeable media model for imbibition in tight sandstone reservoirs. J. Pet. Sci. Eng. 2020, 194, 107477. [Google Scholar]
- Cheng, Z.; Ning, Z.; Yu, X.; Wang, Q.; Zhang, W. New insights into spontaneous imbibition in tight oil sandstones with NMR. J. Pet. Sci. Eng. 2019, 179, 455–464. [Google Scholar] [CrossRef]
- Liu, D.; Ren, D.; Du, K.; Qi, Y.; Ye, F. Impacts of mineral composition and pore structure on spontaneous imbibition in tight sandstone. J. Pet. Sci. Eng. 2021, 201, 108397. [Google Scholar] [CrossRef]
- Katende, A.; Sagala, F. A Critical review of Low Salinity Water Flooding: Mechanism, Laboratory and Field Application. J. Mol. Liq. 2019, 278, 627–649. [Google Scholar] [CrossRef]
- Mohammadi, S.; Kord, S.; Moghadasi, J. An experimental investigation into the spontaneous imbibition of surfactant assisted low salinity water in carbonate rocks. Fuel 2019, 243, 142–154. [Google Scholar] [CrossRef]
- Tetteh, J.; Janjang, N.M.; Barati, R. Wettability Alteration and Enhanced Oil Recovery Using Low Salinity Waterflooding in Limestone Rocks: A Mechanistic Study. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 23–26 April 2018. [Google Scholar]
- Song, J.; Wang, Q.; Shaik, I.; Puerto, M.; Bikkina, P.; Aichele, C.; Biswal, S.L.; Hirasaki, G.J. Effect of salinity, Mg2+ and SO42− on “smart water”-induced carbonate wettability alteration in a model oil system. J. Colloid Interface Sci. 2019, 563, 145–155. [Google Scholar] [CrossRef]
- Hidayat, F.; Erfando, T.; Maulana, B.F. Spontaneous Imbibition Test of Low Salinity Injection at Low Saline Waxy Crude Carbonate. J. Earth Energy Eng. 2018, 7, 14. [Google Scholar] [CrossRef]
- Ghandi, E.; Parsaei, R.; Riazi, M. Enhancing the spontaneous imbibition rate of water in oil-wet dolomite rocks through boosting a wettability alteration process using carbonated smart brines. Pet. Sci. 2019, 16, 127–139. [Google Scholar] [CrossRef] [Green Version]
- Zaeri, M.R.; Hashemi, R.; Shahverdi, H.; Sadeghi, M. Enhanced oil recovery from carbonate reservoirs by spontaneous imbibition of low salinity water. Pet. Sci. 2018, 15, 564–576. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Li, C.; Song, K.; Zou, S.; Yang, Z.; Shen, Y.; Meng, Q.; Liu, Y. The influence of salinity and mineral components on spontaneous imbibition in tight sandstone. Fuel 2020, 269, 117087. [Google Scholar] [CrossRef]
- Wei, B.; Wang, L.; Song, T.; Zhong, M.; Varfolomeev, M.A. Enhanced oil recovery by low-salinity water spontaneous imbibition (LSW-SI) in a typical tight sandstone formation of ma hu sag from core scale to field scale—Science Direct. Petroleum 2020, 7, 272–281. [Google Scholar] [CrossRef]
- Ren, X.; Li, A.; Memon, A.; Fu, S.; Wang, G.; He, B. Experimental Simulation on Imbibition of the Residual Fracturing Fluid in Tight Sandstone Reservoirs. J. Energy Resour. Technol. 2019, 141, 082905.1–082905.9. [Google Scholar] [CrossRef]
- Chavan, M.; Dandekar, A.; Patil, S.; Khataniar, S. Low-salinity-based enhanced oil recovery literature review and associated screening criteria. Pet. Sci. 2019, 16, 1344–1360. [Google Scholar] [CrossRef] [Green Version]
- Hamad, W. Low Salinity Water Flooding by Spontaneous Imbibition. Ph.D. Thesis, University of Leoben, Leoben, Austria, 2020. [Google Scholar]
- Feldmann, F. An Experimental and Numerical Study of Low Salinity Effects on the Oil Recovery of Carbonate Limestone Samples; Cuvillier Verlag: Göttingen, Germany, 2020. [Google Scholar]
- Behera, U.S.; Sangwai, J.S. Nanofluids of Silica Nanoparticles in Low Salinity Water with Surfactant and Polymer (SMART LowSal) for Enhanced Oil Recovery. J. Mol. Liq. 2021, 342, 117388. [Google Scholar] [CrossRef]
- Yuan, L.; Habibi, A.; Dehghanpour, H. Liquid Imbibition in Tight Rocks: The Role of Disjoining Pressure. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127037. [Google Scholar] [CrossRef]
- Al-Saedi, H.N.; Flori, R.E.; Brady, P.V. Effect of divalent cations in formation water on wettability alteration during low salinity water flooding in sandstone reservoirs: Oil recovery analyses, surface reactivity tests, contact angle, and spontaneous imbibition experiments. J. Mol. Liq. 2019, 275, 163–172. [Google Scholar] [CrossRef]
- Zhu, D.; Li, B.; Li, H.; Li, B.; Cao, Y.; Li, Z. Effects of low-salinity water on the interface characteristics and imbibition process. J. Pet. Sci. Eng. 2021, 208, 109564. [Google Scholar] [CrossRef]
- Guo, J.; Li, M.; Chen, C.; Tao, L.; Liu, Z.; Zhou, D. Experimental investigation of spontaneous imbibition in tight sandstone reservoirs. J. Pet. Sci. Eng. 2020, 193, 107395. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, D.; Yan, L.; Liu, S.; Liu, Y. On the Imbibition Model for Oil-Water Replacement of Tight Sandstone Oil Reservoirs. Geofluids 2021, 2021, 8846132. [Google Scholar] [CrossRef]







| Number | Porosity/% | Average Air Permeability/10−3 μm | Quartz/% | Feldspar/% | Carbonate Interstitial/% | Intergranular Pores/% | Total Dissolved Pores/% | Absolute Clay Content/% |
|---|---|---|---|---|---|---|---|---|
| 4 | 3.53 | 0.0413 | 23.0 | 51.0 | 0.0 | 0.0 | 0.0 | 3.24 |
| 9 | 7.39 | 0.0840 | 21.0 | 50.0 | 9.0 | 0.1 | 1.0 | 5.79 |
| 19 | 4.27 | 0.0280 | 22.0 | 39.0 | 0.0 | 0.0 | 0.0 | 10.05 |
| 46 | 8.16 | 0.1366 | 33.0 | 35.0 | 12.0 | 0.5 | 0.0 | 2.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yan, L.; Gao, Q.; Liu, Y.; Huang, H.; Liu, S. Effect of Salinity on the Imbibition Recovery Process of Tight Sandstone Reservoirs. Processes 2022, 10, 228. https://doi.org/10.3390/pr10020228
Liu X, Yan L, Gao Q, Liu Y, Huang H, Liu S. Effect of Salinity on the Imbibition Recovery Process of Tight Sandstone Reservoirs. Processes. 2022; 10(2):228. https://doi.org/10.3390/pr10020228
Chicago/Turabian StyleLiu, Xiong, Le Yan, Qian Gao, Yafei Liu, Hai Huang, and Shun Liu. 2022. "Effect of Salinity on the Imbibition Recovery Process of Tight Sandstone Reservoirs" Processes 10, no. 2: 228. https://doi.org/10.3390/pr10020228





