Degradation of Diazepam with Gamma Radiation, High Frequency Ultrasound and UV Radiation Intensified with H2O2 and Fenton Reagent
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Effect of Operational Conditions on the Degradation of DZP by Radiolysis, Sonolysis and Photolysis
3.2. Initial Effect of pH on Degradation of DZP
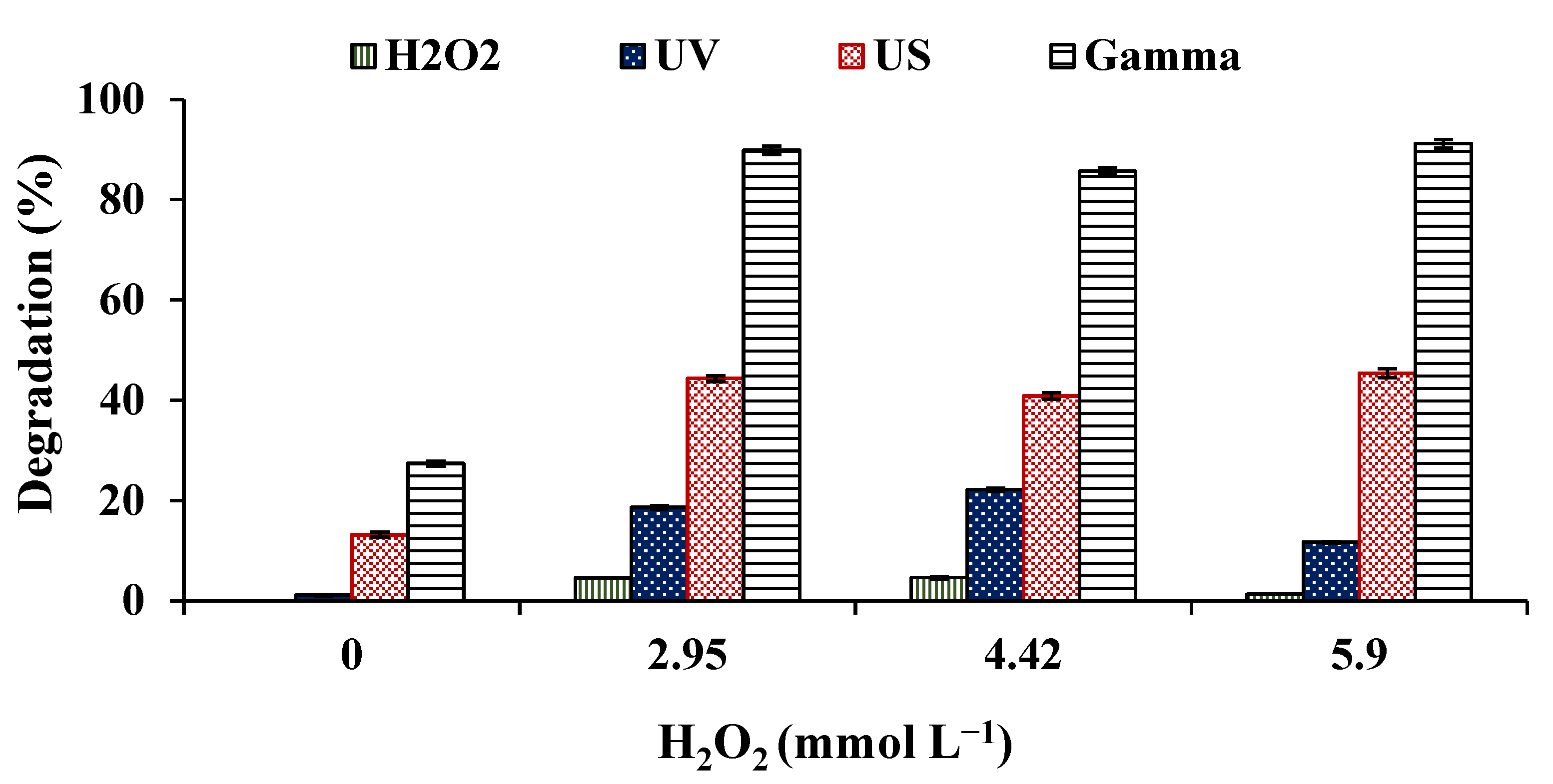
3.3. Effect of Hydrogen Peroxide on the Degradation of DZP by Sonolysis, Radiolysis and Photolysis
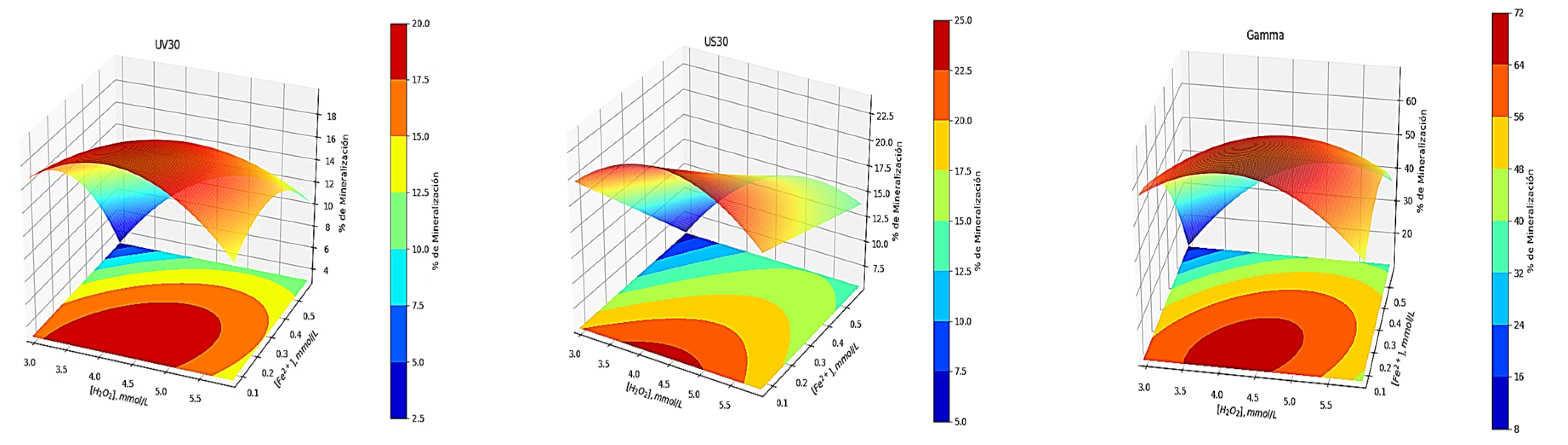
3.4. Effect of the Fenton Reagent on the Degradation of DZP by Sonolysis, Radiolysis and Photolysis
3.5. Evaluation of Energy Efficiency in the Degradation of DZP with AOPs
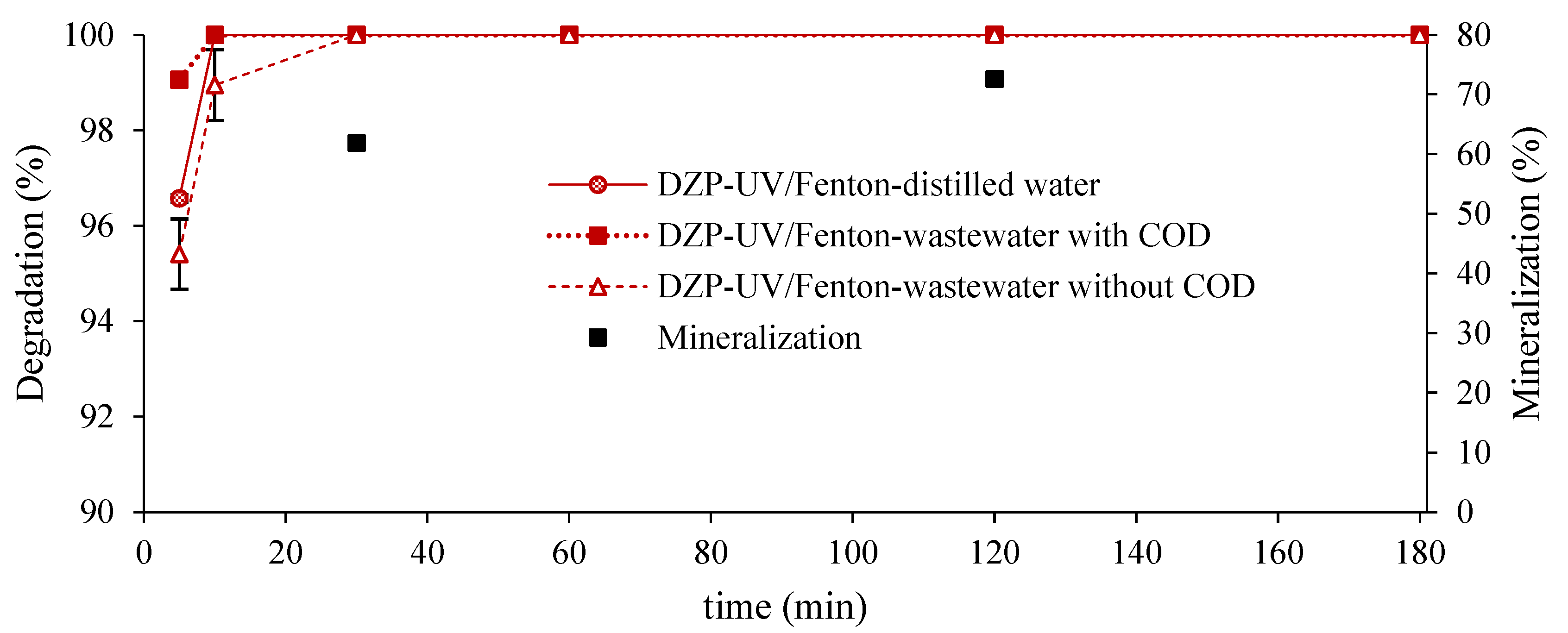
3.6. Study of the Photo-Fenton Process with Solar Radiation in a Real Wastewater
4. Conclusions
- The three processes of advanced oxidation, sonolysis, radiolysis, and photolysis combined with the Fenton reagent guarantee the total degradation of diazepam in synthetic matrices and more than 80% in a real matrix with partial mineralization.
- Diazepam sonolysis guarantees 28% degradation of the drug at 30.6 W and 862 kHz at 3 h of experimentation, while with photolysis 38% is achieved after 5 h. With the increase of the dose of irradiation increases the degradation of the drug for doses greater than 500 Gy, reaching the total degradation for a dose of 2500 Gy. In all cases, the process is favored in acidic conditions, with the best results for the ultrasonic and photolytic study at pH 2.5 and for the radiolytic at pH 3.
- The combination of the processes with hydrogen peroxide guarantees the intensification of the same to the 10 min with an increase in more than 30%, 63%, and 21% for the sonolytic, radiolytic, and photochemical degradation, respectively.
- Integration of the processes with Fenton reagent guarantees the total elimination of the drugs at 10 min in a synthetic matrix, and an increase in mineralization by more than 16%, 60%, and 12% for sonolytic, radiolytic degradation, and photochemistry, respectively.
- All of the criteria for the evaluation of the energy consumption agree that the photo-Fenton process constitutes the one with the lowest energy consumption for the degradation of diazepam in the water matrix.
- The degradation of the DZP in real residual water gave the best results in the experiments where the COD was taken into account to adjust the H2O2, and Fe2+ concentrations. The photo-Fenton process guarantees total degradation using solar radiation as a source of energy after 10 min. A decrease in COD, and BOD5 of waste water was achieved below the limits required by NC-27-2012 for classification B. The gamma-Fenton process guarantees maximum efficiency in decreasing COD, BOD5, and TOC with 89.2%, 82.1%, and 88.1%, respectively.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serna-Galvis, E.A.; Silva-Agredo, J.; Botero-Coy, A.M.; Moncayo-Lasso, A.; Hernández, F.; Torres-Palma, R.A. Effective elimination of fifteen relevant pharmaceuticals in hospital wastewater from Colombia by combination of a biological system with a sonochemical process. Sci. Total Environ. 2019, 670, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, A.B.; Bowers, I.; Subedi, B. Trends in Substance Use in Two United States Communities during Early COVID-19 Lockdowns Based on Wastewater Analysis. Environ. Sci. Technol. Lett. 2021, 8, 890–896. [Google Scholar] [CrossRef]
- Nieto-Juárez, J.I.; Torres-Palma, R.A.; Botero-Coy, A.M.; Hernández, F. Pharmaceuticals and environmental risk assessment in municipal wastewater treatment plants and rivers from Peru. Environ. Int. 2021, 155, 106674. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Hu, W.; Zhang, H.; Wang, H.; Sun, P. Enhanced Degradation of Sulfonamide Antibiotics by UV Irradiation Combined with Persulfate. Processes 2021, 9, 226. [Google Scholar] [CrossRef]
- Tejeda, C.; Quiñonez, E.; Peña, M. Contaminantes emergentes en aguas: Metabolitos de fármacos. Rev. Fac. Cienc. Básicas 2014, 10, 80–101. [Google Scholar] [CrossRef] [Green Version]
- Ellis, J.B. Pharmaceutical and personal care products (PPCPs) in urban receiving waters. Environ. Pollut. 2006, 144, 184–189. [Google Scholar] [CrossRef]
- Bade, R.; Ghetia, M.; White, J.M.; Gerber, C. Determination of prescribed and designer benzodiazepines and metabolites in influent wastewater. Anal. Methods 2020, 12, 3637–3644. [Google Scholar] [CrossRef]
- de Araujo, F.G.; Bauerfeldt, G.F.; Marques, M.; Martins, E.M. Development and Validation of an Analytical Method for the Detection and Quantification of Bromazepam, Clonazepam and Diazepam by UPLC-MS/MS in Surface Water. Bull. Environ. Contam. Toxicol. 2019, 103, 362–366. [Google Scholar] [CrossRef]
- Fernández-Rubio, J.; Rodríguez-Gil, J.L.; Postigo, C.; Mastroianni, N.; de Alda, M.L.; Barceló, D.; Valcárcel, Y. Psychoactive pharmaceuticals and illicit drugs in coastal waters of North-Western Spain: Environmental exposure and risk assessment. Chemosphere 2019, 224, 379–389. [Google Scholar] [CrossRef]
- Zeyuan, W.; Siyue, G.; Qingying, D.; Meirong, Z.; Fangixing, Y. Occurrence and risk assessment of psychoactive substances in tap water from China. Environ. Pollut. 2020, 261, 114163. [Google Scholar] [CrossRef]
- Lei, H.-J.; Yang, B.; Ye, P.; Yang, Y.-Y.; Zhao, J.-L.; Liu, Y.-S.; Xie, L.; Ying, G.-G. Occurrence, fate and mass loading of benzodiazepines and their transformation products in eleven wastewater treatment plants in Guangdong province, China. Sci. Total Environ. 2021, 755, 142648. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; You, W.-D.; Yang, B.; Chen, Y.; Wang, L.-G.; Zhao, J.-L.; Ying, G.-G. Pollution characteristics and removal of typical pharmaceuticals in hospital wastewater and municipal wastewater treatment plants. Huanjing Kexue/Environ. Sci. 2021, 42, 2928–2936. [Google Scholar]
- Lebreton, M.; Malgouyres, J.-M.; Carayon, J.-L.; Bonnafé, E.; Géret, F. Effects of the anxiolytic benzodiazepine oxazepam on freshwater gastropod reproduction: A prospective study. Ecotoxicology 2021, 30, 1880–1892. [Google Scholar] [CrossRef] [PubMed]
- Subedi, B.; Anderson, S.; Croft, T.L.; Rouchka, E.C.; Zhang, M.; Hammond-Weinberger, D.R. Gene alteration in zebrafish exposed to a mixture of substances of abuse. Environ. Pollut. 2021, 278, 116777. [Google Scholar] [CrossRef] [PubMed]
- INCB. Psychotropic Substances: Statistics for 2013; Assessments of Annual Medical and Scientific Requirements for Substances in Schedules II, III and IV of the Convention on Psychotropic Substances of 19711 (E/INCB/2014/3) United Nations; INCB: New York, NY, USA, 2014. [Google Scholar]
- Chaichi, M.J.; Alijanpour, S.O. A new chemiluminescence method for determination of clonazepam and diazepam based on 1-ethyl-3-methylimidazolium ethylsulfate/copper as catalyst. Spectrochimica Acta Part A. Mol. Biomol. Spectrosc. 2014, 118, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Caban, M.; Kumirska, J.; Bialk-Bielińska, A.; Stepnowski, P. Current issues in pharmaceutical residues in drinking water. Curr. Anal. Chem. 2015, 12, 249–257. [Google Scholar] [CrossRef]
- Cunha, D.L.; da Silva, A.S.; Coutinho, R.; Marques, M. Optimization of Ozonation Process to Remove Psychoactive Drugs from Two Municipal Wastewater Treatment Plants. Water Air Soil Pollut. 2022, 233, 67. [Google Scholar] [CrossRef]
- Mergenbayeva, S.P.; Poulopoulos, S.G. Comparative Study on UV-AOPs for Efficient Continuous Flow Removal of 4-tert-Butylphenol. Processes 2022, 10, 8. [Google Scholar] [CrossRef]
- Serna-Galvis, E.A.; Porras, J.; Torres-Palma, R.A. A critical review on the sonochemical degradation of organic pollutants in urine, seawater, and mineral water. Ultrason. Sonochem. 2022, 82, 105861. [Google Scholar] [CrossRef]
- Wang, J.; Chu, L. Irradiation treatment of pharmaceutical and personal care products (PPCPs) in water and wastewater: An overview. Radiat. Phys. Chem. 2016, 125, 56–64. [Google Scholar] [CrossRef]
- Bagheri, H.; Akhami, A.; Noroozi, A. Removal of pharmaceutical compounds from hospital wastewaters using nanomaterials. A Review. Anal. Bioanal. Chem. Res. 2016, 3, 1–18. [Google Scholar]
- Domènech, X.; Jardim, W.F.; Litter, M.I. Procesos avanzados de oxidación para la eliminación de contaminantes. In Eliminación de Contaminantes por Fotocatálisis Heterogénea; CYTED: La Plata, Argentina, 2001. [Google Scholar]
- You, W.-D.; Ye, P.; Yang, B.; Luo, X.; Fang, J.; Mai, Z.-T.; Sun, J.-L. Degradation of 17 benzodiazepines by the UV/H2O2 Treatment. Front. Environ. Sci. 2021, 9, 764841. [Google Scholar] [CrossRef]
- Ribeiro, A.R.; Nunes, O.C.; Pereira, M.; Silva, A. An overview on the advanced oxidation processes applied for the treatment of water pollutants defined in the recently launched Directive 2013/39/EU. Environ. Int. 2015, 75, 3351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Expósito, A.J.; Patterson, D.A.; Monteagudo, J.M.; Durán, A. Sono-photodegradation of carbamazepine in a thin falling film reactor: Operation costs in pilot plant. Ultrason. Sonochem. 2017, 34, 496–503. [Google Scholar] [CrossRef] [Green Version]
- Alalm, M.G.; Tawfik, A.; Ookawara, S. Degradation of four pharmaceuticals by solar photo-Fenton process: Kinetics and costs estimation. J. Environ. Chem. Eng. 2015, 3, 46–51. [Google Scholar] [CrossRef]
- Gong, Y.; Li, J.; Zhang, Y.; Zhang, M.; Tian, X.; Wang, A. Partial degradation of levofloxacin for biodegradability improvement by electro-Fenton process using an activated carbon fiber felt cathode. J. Hazard. Mater. 2016, 304, 320328. [Google Scholar] [CrossRef]
- Xu, L. Degradation of Refractory Contaminants in Water by Chemical-Free Radicals Generated by Ultrasound and UV Irradiation. Ph.D. Thesis, The Hong Kong Polytechnic University, Hongkong, China, 2014. [Google Scholar]
- Kim, H.Y.; Lee, O.M.; Kim, T.H.; Yu, S. Enhanced biodegradability of pharmaceuticals and personal care products by ionizing radiation. Water Environ. Res. 2015, 87, 321–325. [Google Scholar] [CrossRef]
- Rivas, I.O.; Cruz, G.G.; Lastre, A.A.; Manduca, M.A.; Rapado, M.P.; Chávez, A.A.; Silva, A.C.; Jáuregui, U.H. Optimization of radiolytic degradation of sulfadiazine by combining Fenton and gamma irradiation processes. J. Radioanal. Nucl. Chem. 2017, 314, 2597–2607. [Google Scholar] [CrossRef]
- Lastre, A.M.; Cruz, G.G.; Nuevas, P.L.; Jáuregui, H.U.; Silva, A.C. Ultrasonic degradation of sulfadiazine in aqueous solutions. Environ. Sci. Pollut. Res. 2015, 22, 918–925. [Google Scholar] [CrossRef]
- Durán, A.; Monteagudo, J.A.; Martín, I.S. Operation costs of the solar photo-catalytic degradation of pharmaceuticals in water: A mini-review. Chemosphere 2018, 211, 482–488. [Google Scholar] [CrossRef]
- Kanakaraju, D.; Glass, B.D.; Oelgemoller, M. Advanced oxidation process-mediated removal of pharmaceuticals from water: A review. J. Environ. Manag. 2018, 219, 189–207. [Google Scholar] [CrossRef] [PubMed]
- Torun, M.; Gültekin, Ö.; Solpan, D.; Güven, O. Mineralization of paracetamol in aqueous solution with advanced oxidation processes. Environ. Technol. 2014, 36, 970–982. [Google Scholar] [CrossRef] [PubMed]
- Quijano, P.D.; Orozco, D.J.; Holguín, H. Conocimientos y prácticas de pacientes sobre disposición de medicamentos no consumidos. Aproximación a la ecofarmacovigilancia. Salud Pública 2016, 18, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rizzo, L.; Della, A.; Fiorentino, A.; Li Puma, G. Disinfection of urban wastewater by solar driven and UV lamp e TiO2 photocatalysis: Effect on a multi drug resistant Escherichia coli strain. Water Res. 2014, 53, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Rossi-Bautitz, I.; Pupo-Nogueira, R. Photodegradation of lincomycin and diazepam in sewage treatment plant effluent by photo-Fenton process. Catal. Today 2010, 151, 94–99. [Google Scholar] [CrossRef]
- Monteiro, M.A.; Spisso, B.F.; dos Santos JR, M.P.; da Costa, R.P.; Ferreira, R.G.; Pereira, M.U.; Miranda, T.S.; de Andrade BR, G.; d’Avila, L.A. Occurrence of antimicrobials in river water samples from rural region of the state of Rio de Janeiro, Brazil. J. Environ. Prot. 2016, 7, 230–241. [Google Scholar] [CrossRef] [Green Version]
- Matongo, S.; Birungi, G.; Moodley, B.; Ndungu, P. Pharmaceuticals residues in water and sediment of Msunduzi river, KwaZulu-Natal, South Africa. Chemosphere 2015, 134, 133–140. [Google Scholar] [CrossRef]
- Roudi, A.M.; Salem, S.; Abedini, M.; Maslahati, A.; Imran, M. Response Surface Methodology (RSM)-Based Prediction and Optimization of the Fenton Process in Landfill Leachate Decolorization. Processes 2021, 9, 2284. [Google Scholar] [CrossRef]
- Zhang, Y.; Xiao, Y.; Zhang, J.; Chang, V.; Lim, T.T. Degradation of cyclophosphamide and 5-fluorouracil in water using UV and UV/H2O2: Kinetics investigation, pathways and energetic analysis. J. Environ. Chem. Eng. 2017, 5, 1133–1139. [Google Scholar] [CrossRef]


| Run | H2O2 (mmol L−1) | Fe2+ (mmol L−1) | Fenton | Sono-Fenton | Gamma-Fenton | Photo-Fenton | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| % D | % M | % D | % M | % D | % M | % D | % M | |||
| 1 | - | - | 13.2 | 27.4 | 1.1 | - | - | - | 13.2 | 27.4 |
| 2 | 5.90 | 0.59 | 98.0 | 7.3 | 100 | 14.3 | 100 | 37.5 | 100 | 10.6 |
| 3 | 5.90 | 0.20 | 78.2 | 4.3 | 80.8 | 17.9 | 94.9 | 51.2 | 86.7 | 14.8 |
| 4 | 5.90 | 0.12 | 71.1 | 2.8 | 74.8 | 17.4 | 92.8 | 50.1 | 46.5 | 14.5 |
| 5 | 2.95 | 0.29 | 89.6 | 1.9 | 100 | 14.4 | 96.1 | 48.7 | 79.9 | 14.0 |
| 6 | 2.95 | 0.10 | 73.2 | 2.1 | 74.3 | 19.4 | 96.3 | 58.4 | 64.3 | 16.9 |
| 7 | 2.95 | 0.06 | 51.3 | 3.0 | 58.7 | 18.3 | 96.4 | 56.7 | 35.8 | 16.4 |
| 8 | 4.42 | 0.44 | 97.2 | 9.6 | 100 | 15.0 | 96.5 | 53.0 | 98.1 | 15.2 |
| 9 | 4.42 | 0.15 | 71.6 ± 2.5 | 7.6 ± 0.8 | 65.9 ± 2.3 | 23.6 ± 1.1 | 95.3 ± 1.8 | 68.3 ± 2.7 | 47.7 ± 1.9 | 19.7 ± 0.7 |
| 10 | 4.42 | 0.09 | 69.1 | 4.9 | 59.0 | 22.5 | 95.3 | 66.0 | 37.0 | 19.3 |
| Process | Time (min) | EE/O (kWh L−1) | DW (kWh mg−1) | * (EE/O) (kWh L−1) | * (DW) (kWh mg−1) |
|---|---|---|---|---|---|
| Gamma | 40 | 1.2 | NA | NA | |
| Gamma-H2O2 | 12 | 1.7 | NA | NA | |
| Gamma-Fenton | 12 | 17.8 | 1.0 | ||
| Sonolysis | 180 | 2.5 | NA | NA | |
| Sono-H2O2 | 60 | NA | NA | ||
| Sono-Fenton | 10 | 0.5 | |||
| Photolysis | 300 | 1.1 | NA | NA | |
| Foto-H2O2 | 300 | NA | NA | ||
| Foto-Fenton | 10 | 0.2 |
| Parameters | Waste Water | Treated Water | Efficiency (%) | NC 27: 2012 |
|---|---|---|---|---|
| Temperature (°C) | 27 | 26 | - | <40 |
| pH | 7.65 | 7.32 | - | 6–9 |
| Conductivity (µS cm−1) | 1085 ± 1 | 87 ± 1 | 91.9 | <2000 |
| CO (mg of O2 L−1) | 188 ± 30 | 61.4 ± 6 | 67.3 | <90 |
| BOD5 (mg of O2 L−1) | 91 ± 1 | 36.5 ± 1 | 59.8 | <40 |
| Settleable solids (mL L−1) | 2.5 ± 0.1 | 0 | 100 | <2 |
| Floating material | present | absent | - | - |
| Iron (mg L−1) | 0.91 | 0 | 100 | - |
| TOC (mg de C L−1) | 86.4 | 32.9 * 23.7 ** | 61.9 72.6 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manduca Artiles, M.; Gómez González, S.; González Marín, M.A.; Gaspard, S.; Jauregui Haza, U.J. Degradation of Diazepam with Gamma Radiation, High Frequency Ultrasound and UV Radiation Intensified with H2O2 and Fenton Reagent. Processes 2022, 10, 1263. https://doi.org/10.3390/pr10071263
Manduca Artiles M, Gómez González S, González Marín MA, Gaspard S, Jauregui Haza UJ. Degradation of Diazepam with Gamma Radiation, High Frequency Ultrasound and UV Radiation Intensified with H2O2 and Fenton Reagent. Processes. 2022; 10(7):1263. https://doi.org/10.3390/pr10071263
Chicago/Turabian StyleManduca Artiles, Michel, Susana Gómez González, María A. González Marín, Sarra Gaspard, and Ulises J. Jauregui Haza. 2022. "Degradation of Diazepam with Gamma Radiation, High Frequency Ultrasound and UV Radiation Intensified with H2O2 and Fenton Reagent" Processes 10, no. 7: 1263. https://doi.org/10.3390/pr10071263






