Apple Pomace-Derived Cationic Cellulose Nanocrystals for PFAS Removal from Contaminated Water
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
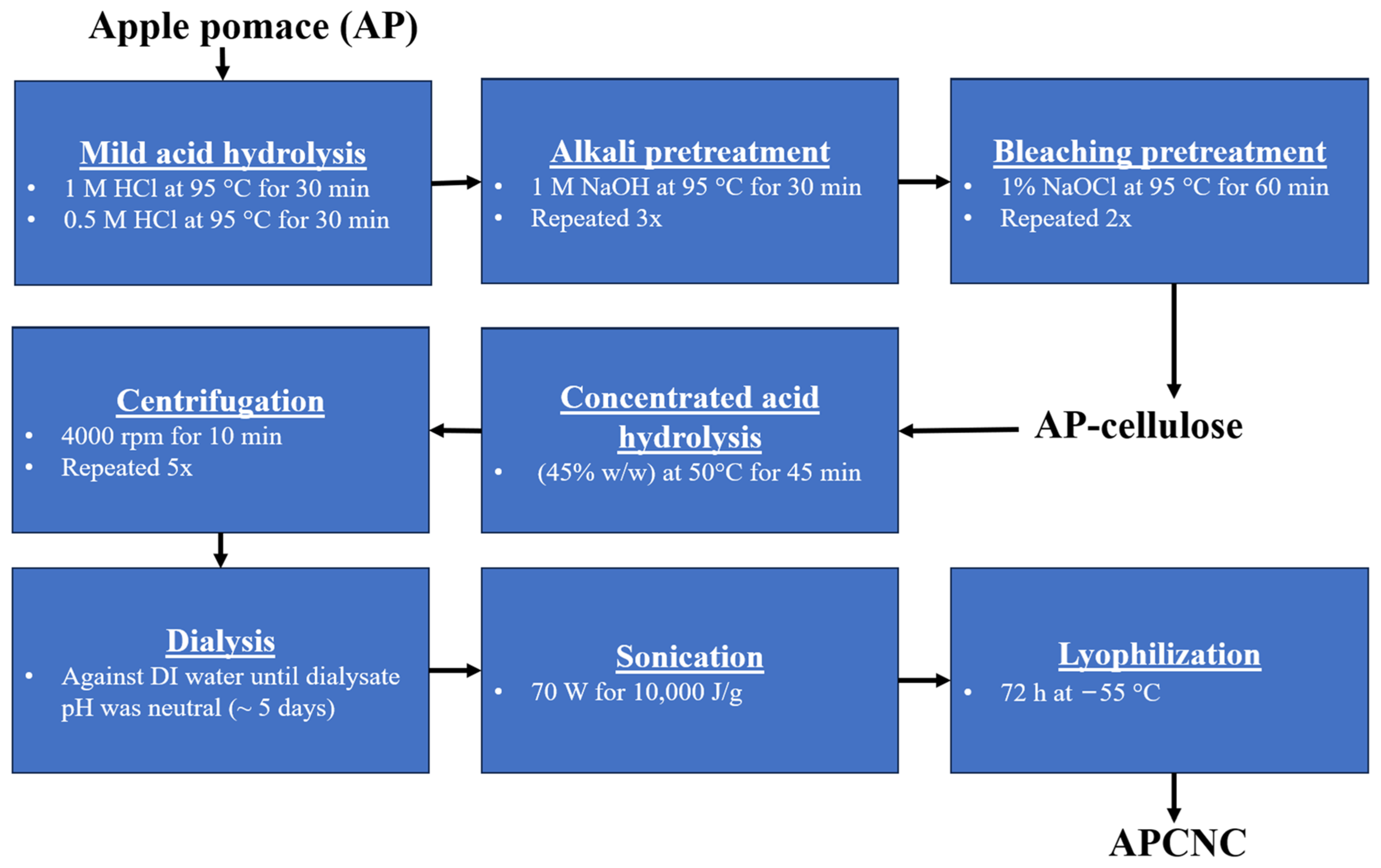
2.2. Isolation of Cellulose from AP
2.3. Synthesis of APCNC from AP-Cellulose
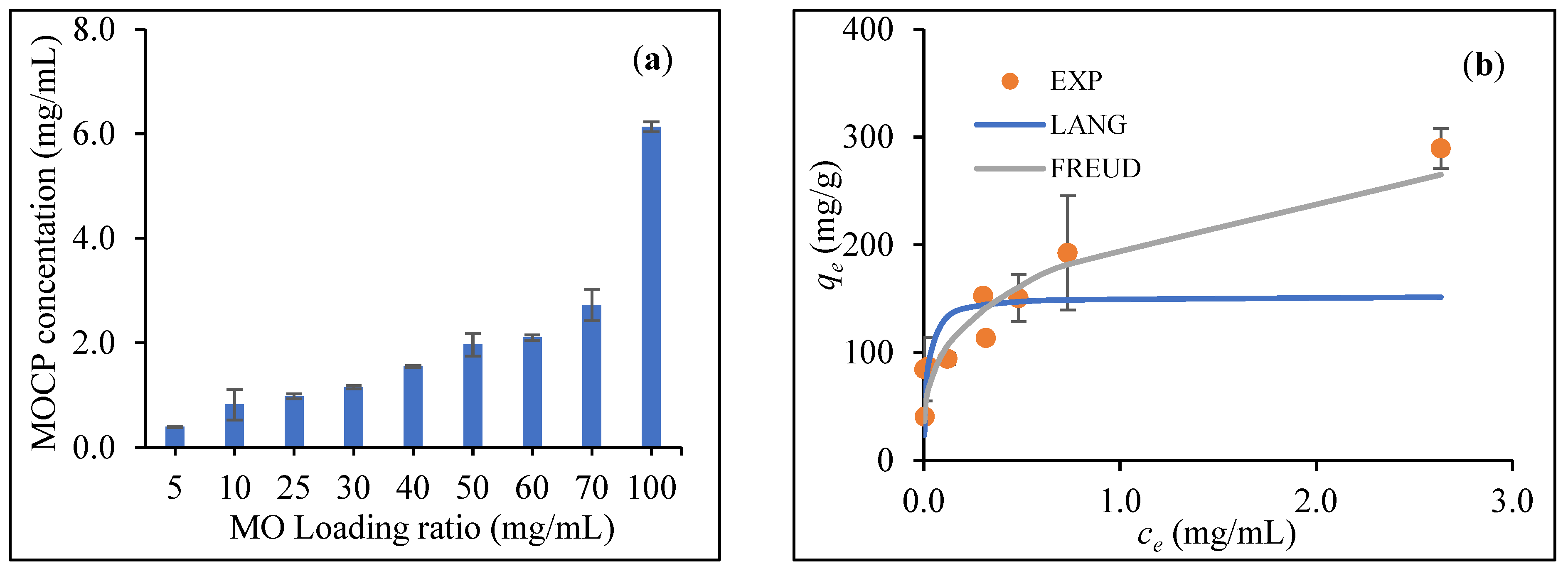
2.4. MOCP Extraction and Coating onto CCNC and APCNC
Analysis of the Equilibrium Uptake of MOCP onto CCNC
2.5. Characterization of AP, AP-Cellulose, APCNC, and MOCP/APCNC
2.5.1. Chemical Composition of AP
2.5.2. HPLC Analysis
2.5.3. Scanning Electron Microscopy (SEM)
2.5.4. Fourier-Transform Infrared Spectroscopy (FTIR)
2.5.5. Powder X-ray Diffraction (PXRD)
2.5.6. Thermogravimetric analysis (TGA)
2.5.7. Brunauer–Emmet–Teller (BET) Surface
2.5.8. Dynamic Light Scattering (DLS) and Zeta Potential Analysis
2.6. Batch Adsorption on PFOA onto APCNC and MOCP/APCNC
2.6.1. Batch PFOA Adsorption Kinetics
2.6.2. Batch PFOA Equilibrium Adsorption and Isotherm Experiments
2.6.3. Effects of Ionic Strength and Dissolved Organic Matter on PFOA Adsorption
3. Results and Discussion
3.1. Synthesis of APCNC from AP
3.2. Cationization of CNC with MOCP Coating
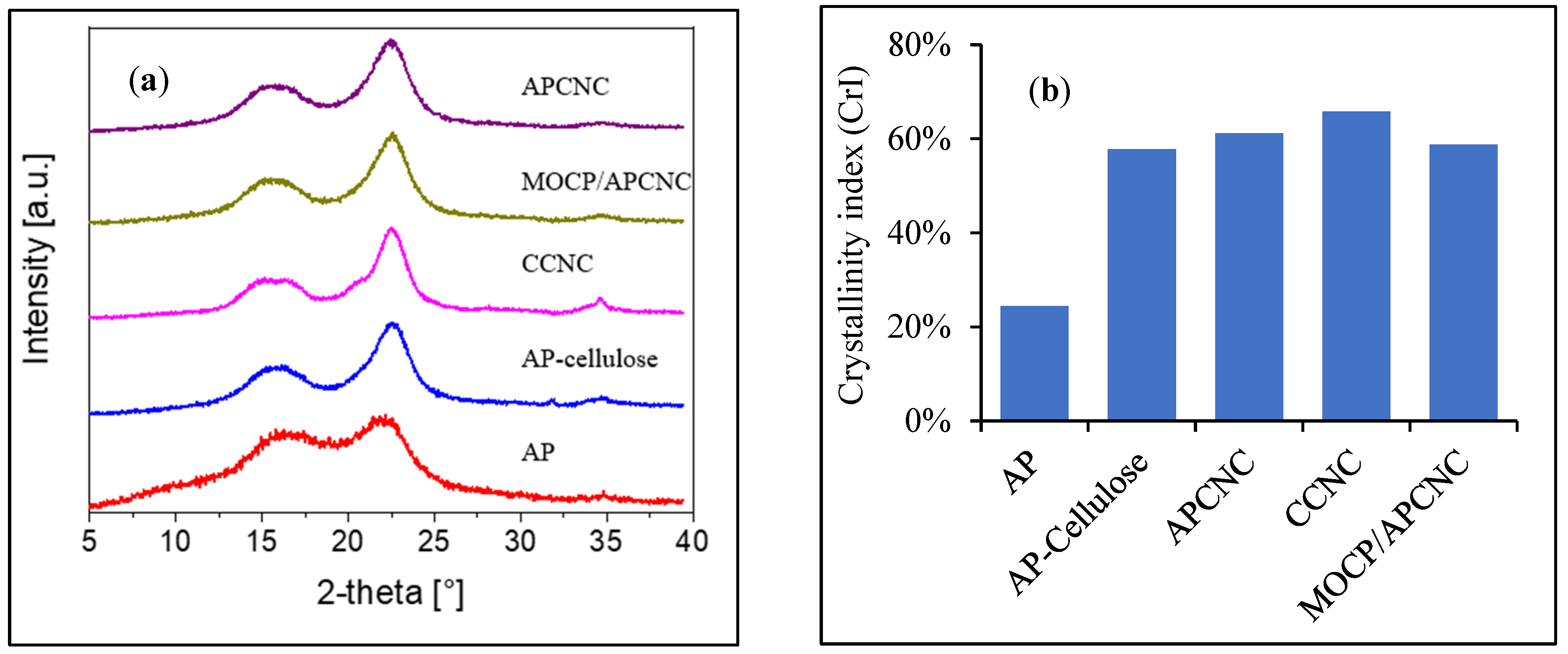
3.3. Characterization of AP, AP-Cellulose, APCNC, and MOCP/APCNC
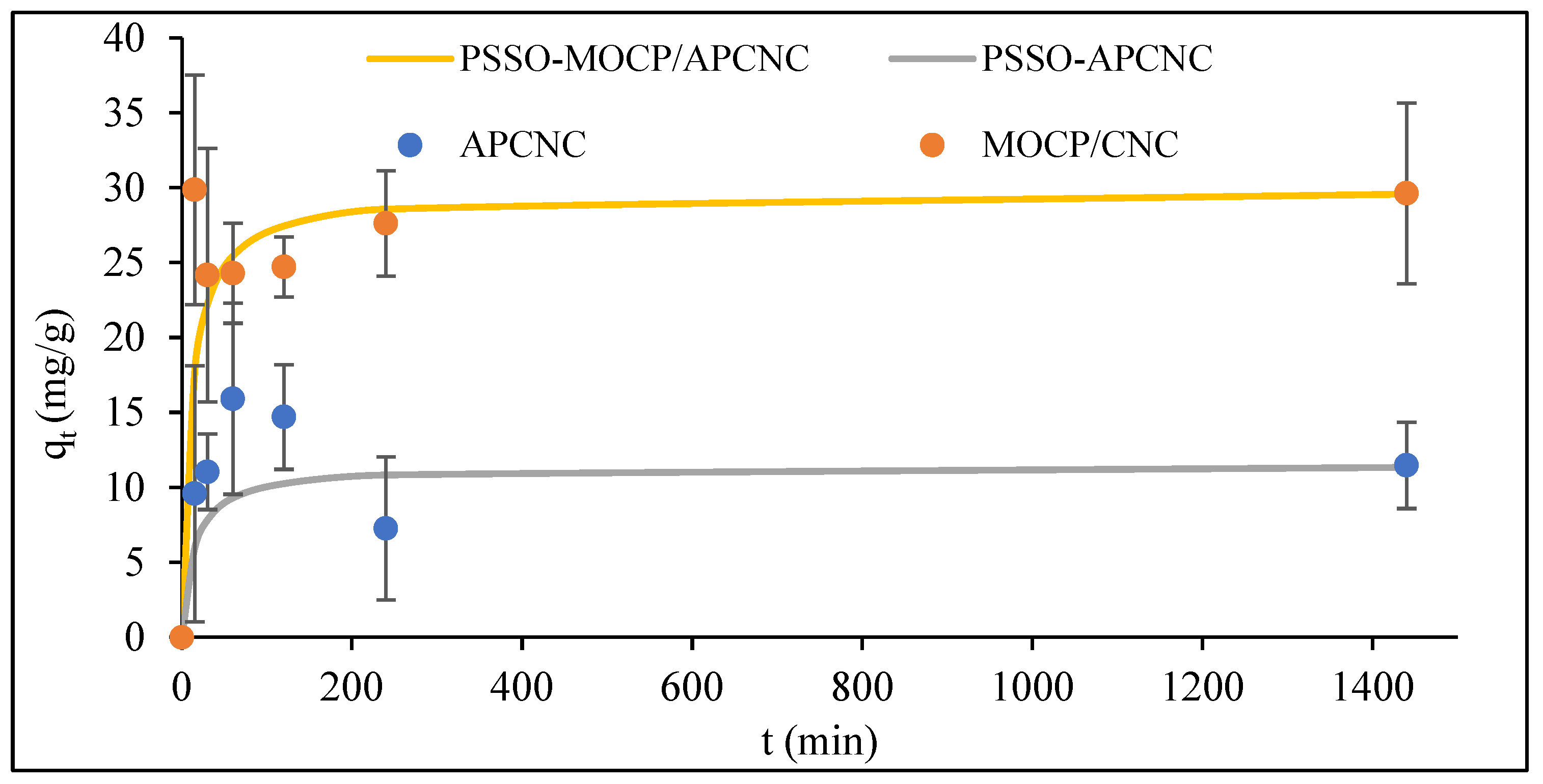
3.4. Batch Adsorption Studies of PFOA onto CNC-Based Adsorbents
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Park, S.; Zenobio, J.E.; Lee, L.S. Perfluorooctane Sulfonate (PFOS) Removal with Pd0/NFe0 Nanoparticles: Adsorption or Aqueous Fe-Complexation, Not Transformation? J. Hazard. Mater. 2018, 342, 20–28. [Google Scholar] [CrossRef]
- Wanninayake, D.M. Comparison of Currently Available PFAS Remediation Technologies in Water: A Review. J. Environ. Manag. 2021, 283, 111977. [Google Scholar] [CrossRef]
- Gomis, M.I.; Vestergren, R.; Borg, D.; Cousins, I.T. Comparing the Toxic Potency in Vivo of Long-Chain Perfluoroalkyl Acids and Fluorinated Alternatives. Environ. Int. 2018, 113, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sunderland, E.M.; Hu, X.; Dassuncao, C. A Review of Pathways of Human Exposure to PFAS. Physiol. Behav. 2019, 173, 665–676. [Google Scholar] [CrossRef]
- Ross, I.; Mcdonough, J.; Miles, J.; Storch, P.; Kochunarayanan, P.T.; Kalve, E.; Hurst, J.; Dasgupta, S.S.; Burdick, J. A Review of Emerging Technologies for Remediation of PFASs. Remediat. J. 2018, 28, 101–126. [Google Scholar] [CrossRef]
- Xu, B.; Liu, S.; Zhou, J.L.; Zheng, C.; Jin, W.; Chen, B.; Zhang, T.; Qiu, W. PFAS and Their Substitutes in Groundwater: Occurrence, Transformation and Remediation. J. Hazard. Mater. 2021, 412, 125159. [Google Scholar] [CrossRef] [PubMed]
- Vu, C.T.; Wu, T. Recent Progress in Adsorptive Removal of Per- and Poly-Fluoroalkyl Substances (PFAS) from Water/Wastewater. Crit. Rev. Environ. Sci. Technol. 2022, 52, 90–129. [Google Scholar] [CrossRef]
- Pauletto, P.S.; Bandosz, T.J. Activated Carbon versus Metal-Organic Frameworks: A Review of Their PFAS Adsorption Performance. J. Hazard. Mater. 2022, 425, 127810. [Google Scholar] [CrossRef] [PubMed]
- Militao, I.M.; Roddick, F.A.; Bergamasco, R.; Fan, L. Removing PFAS from Aquatic Systems Using Natural and Renewable Material-Based Adsorbents: A Review. J. Environ. Chem. Eng. 2021, 9, 105271. [Google Scholar] [CrossRef]
- Tan, K.B.; Vakili, M.; Horri, B.A.; Poh, P.E.; Abdullah, A.Z.; Salamatinia, B. Adsorption of Dyes by Nanomaterials: Recent Developments and Adsorption Mechanisms. Sep. Purif. Technol. 2015, 150, 229–242. [Google Scholar] [CrossRef]
- Janusz, W.; Kowalska, K.; Skwarek, E. Research on Deposition of Silver Nanoparticles at the Cellulose/NaNO3 Interface. Physicochem. Probl. Miner. Process. 2023, 59, 173554. [Google Scholar] [CrossRef]
- Jia, G.; Wang, H.; Yan, L.; Wang, X.; Pei, R.; Yan, T.; Zhao, Y.; Guo, X. Cytotoxicity of Carbon Nanomaterials: Single-Wall Nanotube, Multi-Wall Nanotube, and Fullerene. Environ. Sci. Technol. 2005, 39, 1378–1383. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, A.W.; De Lannoy, C.F.; Wiesner, M.R. Cellulose Nanomaterials in Water Treatment Technologies. Environ. Sci. Technol. 2015, 49, 5277–5287. [Google Scholar] [CrossRef] [PubMed]
- Kargarzadeh, H.; Mariano, M.; Gopakumar, D.; Ahmad, I.; Thomas, S.; Dufresne, A.; Huang, J.; Lin, N. Advances in Cellulose Nanomaterials; Springer: Dordrecht, The Netherlands, 2018; Volume 25, ISBN 1057001817235. [Google Scholar]
- Vincent, S.; Kandasubramanian, B. Cellulose Nanocrystals from Agricultural Resources: Extraction and Functionalisation. Eur. Polym. J. 2021, 160, 110789. [Google Scholar] [CrossRef]
- Liu, H.; Kumar, V.; Jia, L.; Sarsaiya, S.; Kumar, D.; Juneja, A.; Zhang, Z.; Sindhu, R.; Binod, P.; Bhatia, S.K.; et al. Biopolymer Poly-Hydroxyalkanoates (PHA) Production from Apple Industrial Waste Residues: A Review. Chemosphere 2021, 284, 131427. [Google Scholar] [CrossRef]
- Padhi, S.; Singh, A.; Routray, W. Nanocellulose from Agro-Waste: A Comprehensive Review of Extraction Methods and Applications. Rev. Environ. Sci. Biotechnol. 2023. [Google Scholar] [CrossRef]
- Qin, S.; Shekher Giri, B.; Kumar Patel, A.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Kumar Awasthi, M.; et al. Resource Recovery and Biorefinery Potential of Apple Orchard Waste in the Circular Bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef] [PubMed]
- Skaf, D.W.; Punzi, V.L.; Rolle, J.T.; Cullen, E. Impact of Moringa Oleifera Extraction Conditions on Zeta Potential and Coagulation Effectiveness. J. Environ. Chem. Eng. 2021, 9, 104687. [Google Scholar] [CrossRef]
- Szymanska-Chargot, M.; Chylinska, M.; Gdula, K.; Koziol, A.; Zdunek, A. Isolation and Characterization of Cellulose from Different Fruit and Vegetable Pomaces. Polymers 2017, 9, 495. [Google Scholar] [CrossRef]
- Melikoğlu, A.Y.; Bilek, S.E.; Cesur, S. Optimum Alkaline Treatment Parameters for the Extraction of Cellulose and Production of Cellulose Nanocrystals from Apple Pomace. Carbohydr. Polym. 2019, 215, 330–337. [Google Scholar] [CrossRef]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive Removal of Congo Red by Surfactant Modified Cellulose Nanocrystals: A Kinetic, Equilibrium, and Mechanistic Investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Jung, Y.; Jung, Y.; Kwon, M.; Kye, H.; Abrha, Y.W.; Kang, J.W. Evaluation of Moringa Oleifera Seed Extract by Extraction Time: Effect on Coagulation Efficiency and Extract Characteristic. J. Water Health 2018, 16, 904–913. [Google Scholar] [CrossRef]
- Jerri, H.A.; Adolfsen, K.J.; McCullough, L.R.; Velegol, D.; Velegol, S.B. Antimicrobial Sand via Adsorption of Cationic Moringa Oleifera Protein. Langmuir 2012, 28, 2262–2268. [Google Scholar] [CrossRef]
- Nordmark, B.A.; Przybycien, T.M.; Tilton, R.D. Comparative Coagulation Performance Study of Moringa Oleifera Cationic Protein Fractions with Varying Water Hardness. J. Environ. Chem. Eng. 2016, 4, 4690–4698. [Google Scholar] [CrossRef]
- Samineni, L.; Xiong, B.; Chowdhury, R.; Pei, A.; Kuehster, L.; Wang, H.; Dickey, R.; Soto, P.E.; Massenburg, L.; Nguyen, T.H.; et al. 7 Log Virus Removal in a Simple Functionalized Sand Filter. Environ. Sci. Technol. 2019, 53, 12706–12714. [Google Scholar] [CrossRef] [PubMed]
- Abdul Hamid, S.H.; Lananan, F.; Khatoon, H.; Jusoh, A.; Endut, A. A Study of Coagulating Protein of Moringa Oleifera in Microalgae Bio-Flocculation. Int. Biodeterior. Biodegrad. 2016, 113, 310–317. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.O.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Hou, L.; Jia, L.; Morrison, H.M.; Majumder, E.L.-W.; Kumar, D. Enhanced Polyhydroxybutyrate Production from Acid Whey through Determination of Process and Metabolic Limiting Factors. Bioresour. Technol. 2021, 342, 125973. [Google Scholar] [CrossRef]
- Sai Prasanna, N.; Mitra, J. Isolation and Characterization of Cellulose Nanocrystals from Cucumis Sativus Peels. Carbohydr. Polym. 2020, 247, 116706. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, H.; Mu, T.; Li, Q.; Richel, A. Preparation of Cellulose Nanocrystals from Purple Sweet Potato Peels by Ultrasound-Assisted Maleic Acid Hydrolysis. Food Chem. 2023, 403, 134496. [Google Scholar] [CrossRef] [PubMed]
- Foster, E.J.; Moon, R.J.; Agarwal, U.P.; Bortner, M.J.; Bras, J.; Camarero-Espinosa, S.; Chan, K.J.; Clift, M.J.D.D.; Cranston, E.D.; Eichhorn, S.J.; et al. Current Characterization Methods for Cellulose Nanomaterials. Chem. Soc. Rev. 2018, 47, 2609–2679. [Google Scholar] [CrossRef]
- Hossain, S.; Stuart, T.D.; Ramarao, B.V.; VanLeuven, C.C.; Wriedt, M.; Kiemle, D.; Satchwell, M.; Kumar, D. Investigation into Cationic Surfactants and Polyelectrolyte-Coated Β Zeolites for Rapid and High-Capacity Adsorption of Short- and Long-Chain PFAS. Ind. Eng. Chem. Res. 2023, 62, 8373–8384. [Google Scholar] [CrossRef]
- Shoemaker, J.A.; Tettenhorst, D.R. EPA Method 537.1 Determination of Selected per- and Polyfluorinated Alkyl Substances in Drinking Water by Solid Phase Extraction and Liquid Chromatography/Tandem Mass Spectroscopy (LC/MS/MS); US EPA: Washington, DC, USA, 2018; Volume 1.
- Mozaffari Majd, M.; Kordzadeh-Kermani, V.; Ghalandari, V.; Askari, A.; Sillanpää, M. Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010–2020). Sci. Total Environ. 2022, 812, 151334. [Google Scholar] [CrossRef]
- Appleman, T.D.; Dickenson, E.R.V.; Bellona, C.; Higgins, C.P. Nanofiltration and Granular Activated Carbon Treatment of Perfluoroalkyl Acids. J. Hazard. Mater. 2013, 260, 740–746. [Google Scholar] [CrossRef]
- Coelho, C.C.S.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.C.; Teixeira, J.A. Cellulose Nanocrystals from Grape Pomace: Production, Properties and Cytotoxicity Assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Rhim, J.W.; Reddy, J.P.; Luo, X. Isolation of Cellulose Nanocrystals from Onion Skin and Their Utilization for the Preparation of Agar-Based Bio-Nanocomposites Films. Cellulose 2015, 22, 407–420. [Google Scholar] [CrossRef]
- Wijaya, C.J.; Saputra, S.N.; Soetaredjo, F.E.; Putro, J.N.; Lin, C.X.; Kurniawan, A.; Ju, Y.H.; Ismadji, S. Cellulose Nanocrystals from Passion Fruit Peels Waste as Antibiotic Drug Carrier. Carbohydr. Polym. 2017, 175, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Dezfooli, S.M.; Uversky, V.N.; Saleem, M.; Baharudin, F.S.; Hitam, S.M.S.; Bachmann, R.T. A Simplified Method for the Purification of an Intrinsically Disordered Coagulant Protein from Defatted Moringa Oleifera Seeds. Process Biochem. 2016, 51, 1085–1091. [Google Scholar] [CrossRef]
- Saa, R.W.; Fombang, E.N.; Ndjantou, E.B.; Njintang, N.Y. Treatments and Uses of Moringa Oleifera Seeds in Human Nutrition: A Review. Food Sci. Nutr. 2019, 7, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Tukki, O.H.; Barminas, J.T.; Osemeahon, S.A.; Onwuka, J.C.; Donatus, R.A. Adsorption of Colloidal Particles of Moringa Oleifera Seeds on Clay for Water Treatment Applications. J. Water Supply Res. Technol.-AQUA 2016, 65, 75–86. [Google Scholar] [CrossRef]
- Samineni, L.; De Respino, S.; Tu, Y.; Chowdhury, R.; Mohanty, R.P.; Oh, H.; Geitner, M.; Alberg, C.H.; Roman-white, A.; Mckinzie, S.; et al. Effective Pathogen Removal in Sustainable Natural Fiber Moringa Filters. NPJ Clean. Water 2022, 5, 27. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Mariano, M.; El Kissi, N.; Dufresne, A. Cellulose Nanocrystals and Related Nanocomposites: Review of Some Properties and Challenges. J. Polym. Sci. B Polym. Phys. 2014, 52, 791–806. [Google Scholar] [CrossRef]
- Morán, J.I.; Alvarez, V.A.; Cyras, V.P.; Vázquez, A. Extraction of Cellulose and Preparation of Nanocellulose from Sisal Fibers. Cellulose 2008, 15, 149–159. [Google Scholar] [CrossRef]
- Nisha, R.R.; Jegathambal, P.; Parameswari, K.; Kirupa, K. Biocompatible Water Softening System Using Cationic Protein from Moringa Oleifera Extract. Appl. Water Sci. 2017, 7, 2933–2941. [Google Scholar] [CrossRef]
- Yang, H.; Yan, R.; Chen, H.; Lee, D.H.; Zheng, C. Characteristics of Hemicellulose, Cellulose and Lignin Pyrolysis. Fuel 2007, 86, 1781–1788. [Google Scholar] [CrossRef]
- Babaei-Ghazvini, A.; Acharya, B. The Effects of Aspect Ratio of Cellulose Nanocrystals on the Properties of All CNC Films: Tunicate and Wood CNCs. Carbohydr. Polym. Technol. Appl. 2023, 5, 100311. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J.; Lin, S.; Xiao, H.; Yang, Q.; Chen, S.; Yan, B.; Gu, Y. Environmentally Friendly Nanocomposites Based on Cellulose Nanocrystals and Polydopamine for Rapid Removal of Organic Dyes in Aqueous Solution. Cellulose 2020, 27, 2085–2097. [Google Scholar] [CrossRef]
- Brinkmann, A.; Chen, M.; Couillard, M.; Jakubek, Z.J.; Leng, T.; Johnston, L.J. Correlating Cellulose Nanocrystal Particle Size and Surface Area. Langmuir 2016, 32, 6105–6114. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Chhajed, M.; Gupta, P.; Roy, S.; Maji, P.K. Isolation of Cellulose Nanocrystals from Different Waste Bio-Mass Collating Their Liquid Crystal Ordering with Morphological Exploration. Int. J. Biol. Macromol. 2021, 175, 242–253. [Google Scholar] [CrossRef]
- Gicquel, E.; Martin, C.; Heux, L.; Jean, B.; Bras, J. Adsorption versus Grafting of Poly(N-Isopropylacrylamide) in Aqueous Conditions on the Surface of Cellulose Nanocrystals. Carbohydr. Polym. 2019, 210, 100–109. [Google Scholar] [CrossRef]
- Brockman, A.C.; Hubbe, M.A. Charge Reversal System with Cationized Cellulose Nanocrystals to Promote Dewatering of a Cellulosic Fiber Suspension. Cellulose 2017, 24, 4821–4830. [Google Scholar] [CrossRef]
- Nouhi, S.; Kwaambwa, H.M.; Gutfreund, P.; Rennie, A.R. Comparative Study of Flocculation and Adsorption Behaviour of Water Treatment Proteins from Moringa Peregrina and Moringa Oleifera Seeds. Sci. Rep. 2019, 9, 17945. [Google Scholar] [CrossRef] [PubMed]
- Phong Vo, H.N.; Ngo, H.H.; Guo, W.; Hong Nguyen, T.M.; Li, J.; Liang, H.; Deng, L.; Chen, Z.; Hang Nguyen, T.A. Poly-and Perfluoroalkyl Substances in Water and Wastewater: A Comprehensive Review from Sources to Remediation. J. Water Process Eng. 2020, 36, 101393. [Google Scholar] [CrossRef]
- He, J.; Gomeniuc, A.; Olshansky, Y.; Hatton, J.; Abrell, L.; Field, J.A.; Chorover, J.; Sierra-Alvarez, R. Enhanced Removal of Per- and Polyfluoroalkyl Substances by Crosslinked Polyaniline Polymers. Chem. Eng. J. 2022, 446, 137246. [Google Scholar] [CrossRef]
- Qu, Y.; Zhang, C.; Li, F.; Bo, X.; Liu, G.; Zhou, Q. Equilibrium and Kinetics Study on the Adsorption of Perfluorooctanoic Acid from Aqueous Solution onto Powdered Activated Carbon. J. Hazard. Mater. 2009, 169, 146–152. [Google Scholar] [CrossRef]
- Ateia, M.; Attia, M.F.; Maroli, A.; Tharayil, N.; Alexis, F.; Whitehead, D.C.; Karanfil, T. Rapid Removal of Poly- and Perfluorinated Alkyl Substances by Poly(Ethylenimine)-Functionalized Cellulose Microcrystals at Environmentally Relevant Conditions. Environ. Sci. Technol. Lett. 2018, 5, 764–769. [Google Scholar] [CrossRef]
- Köse, K.; Mavlan, M.; Youngblood, J.P. Applications and Impact of Nanocellulose Based Adsorbents. Cellulose 2020, 27, 2967–2990. [Google Scholar] [CrossRef]
- Kostoglou, M.; Karapantsios, T.D. Why Is the Linearized Form of Pseudo-Second Order Adsorption Kinetic Model So Successful in Fitting Batch Adsorption Experimental Data? Colloids Interfaces 2022, 6, 55. [Google Scholar] [CrossRef]
- Zhang, D.; He, Q.; Wang, M.; Zhang, W.; Liang, Y. Sorption of Perfluoroalkylated Substances (PFASs) onto Granular Activated Carbon and Biochar. Environ. Technol. 2021, 42, 1798–1809. [Google Scholar] [CrossRef]
- Liu, L.; Li, D.; Li, C.; Ji, R.; Tian, X. Metal Nanoparticles by Doping Carbon Nanotubes Improved the Sorption of Perfluorooctanoic Acid. J. Hazard. Mater. 2018, 351, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Barisci, S.; Suri, R. Occurrence and Removal of Poly/Perfluoroalkyl Substances (PFAS) in Municipal and Industrial Wastewater Treatment Plants. Water Sci. Technol. 2021, 84, 3442–3468. [Google Scholar] [CrossRef] [PubMed]
- Son, H.; Kim, T.; Yoom, H.; Zhao, D.; An, B. And Polyfluoroalkyl Substances (PFASs) from Surface Water Using Powder-Activated Carbon. Water 2020, 12, 3287. [Google Scholar] [CrossRef]
- Sörengård, M.; Östblom, E.; Köhler, S.; Ahrens, L. Adsorption Behavior of Per- And Polyfluoralkyl Substances (PFASs) to 44 Inorganic and Organic Sorbents and Use of Dyes as Proxies for PFAS Sorption. J. Environ. Chem. Eng. 2020, 8, 103744. [Google Scholar] [CrossRef]
- Militao, I.M.; Roddick, F.; Bergamasco, R.; Fan, L. Rapid Adsorption of PFAS: Application of Moringa Oleifera Seed Powder Encapsulated in Alginate Beads. Environ. Technol. Innov. 2022, 28, 102761. [Google Scholar] [CrossRef]
- Ramos, P.; Singh Kalra, S.; Johnson, N.W.; Khor, C.M.; Borthakur, A.; Cranmer, B.; Dooley, G.; Mohanty, S.K.; Jassby, D.; Blotevogel, J.; et al. Enhanced Removal of Per- and Polyfluoroalkyl Substances in Complex Matrices by PolyDADMAC-Coated Regenerable Granular Activated Carbon. Environ. Pollut. 2022, 294, 118603. [Google Scholar] [CrossRef] [PubMed]
- Kwaambwa, H.M.; Maikokera, R. A Fluorescence Spectroscopic Study of a Coagulating Protein Extracted from Moringa Oleifera Seeds. Colloids Surf. B Biointerfaces 2007, 60, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Gagliano, E.; Sgroi, M.; Falciglia, P.P.; Vagliasindi, F.G.A.; Roccaro, P. Removal of Poly- and Perfluoroalkyl Substances (PFAS) from Water by Adsorption: Role of PFAS Chain Length, Effect of Organic Matter and Challenges in Adsorbent Regeneration. Water Res. 2020, 171, 115381. [Google Scholar] [CrossRef]
- Makowski, M.; Bogunia, M. Influence of Ionic Strength on Hydrophobic Interactions in Water: Dependence on Solute Size and Shape. J. Phys. Chem. B 2020, 124, 10326–10336. [Google Scholar] [CrossRef]
- Qi, Y.; Cao, H.; Pan, W.; Wang, C.; Liang, Y. The Role of Dissolved Organic Matter during Per- and Polyfluorinated Substance (PFAS) Adsorption, Degradation, and Plant Uptake: A Review. J. Hazard. Mater. 2022, 436, 129139. [Google Scholar] [CrossRef]
- Lei, X.; Yao, L.; Lian, Q.; Zhang, X.; Wang, T.; Holmes, W.; Ding, G.; Gang, D.D.; Zappi, M.E. Enhanced Adsorption of Perfluorooctanoate (PFOA) onto Low Oxygen Content Ordered Mesoporous Carbon (OMC): Adsorption Behaviors and Mechanisms. J. Hazard. Mater. 2022, 421, 126810. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.D.; Monteith, D.T.; Cooper, D.M. Long-Term Increases in Surface Water Dissolved Organic Carbon: Observations, Possible Causes and Environmental Impacts. Environ. Pollut. 2005, 137, 55–71. [Google Scholar] [CrossRef] [PubMed]



| Langmuir | Freundlich | ||||
|---|---|---|---|---|---|
| qmax (mg/g) | KL (L/mg) | R2 | Kf (mg/g) (mg/mL)(1/n) | n | R2 |
| 152.54 | 57.98 | 0.9152 | 199.084 | 3.382 | 0.9335 |
| Hydrodynamic Diameter (nm) | Zeta Potential (mV) | |
|---|---|---|
| CCNC | 92.2 | −25.27 |
| APCNC | 230.8 | −15.07 |
| MOCP/APCNC | 427.6 | 7.38 |
| qe (mg/g) | k2 (g/mg/s) | R2 | |
|---|---|---|---|
| APCNC | 11.49 | 2.65 × 10−3 | 0.988 |
| MOCP/APCNC | 29.85 | 5.05 × 10−3 | 1.000 |
| APCNC | MOCP/APCNC | ||
|---|---|---|---|
| Henry | Kh | 2.65 × 10−2 | 4.70 × 10−2 |
| R2 | 0.938 | 0.933 | |
| Freundlich | Kf | 6.30 × 10−3 | 6.78 × 10−2 |
| n | 0.839 | 1.071 | |
| R2 | 0.885 | 0.872 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco, L.A.; Stuart, T.D.; Hossain, M.S.; Ramarao, B.V.; VanLeuven, C.C.; Wriedt, M.; Satchwell, M.; Kumar, D. Apple Pomace-Derived Cationic Cellulose Nanocrystals for PFAS Removal from Contaminated Water. Processes 2024, 12, 297. https://doi.org/10.3390/pr12020297
Franco LA, Stuart TD, Hossain MS, Ramarao BV, VanLeuven CC, Wriedt M, Satchwell M, Kumar D. Apple Pomace-Derived Cationic Cellulose Nanocrystals for PFAS Removal from Contaminated Water. Processes. 2024; 12(2):297. https://doi.org/10.3390/pr12020297
Chicago/Turabian StyleFranco, Luis A., T. Dwyer Stuart, Md Shahadat Hossain, Bandaru V. Ramarao, Charlene C. VanLeuven, Mario Wriedt, Michael Satchwell, and Deepak Kumar. 2024. "Apple Pomace-Derived Cationic Cellulose Nanocrystals for PFAS Removal from Contaminated Water" Processes 12, no. 2: 297. https://doi.org/10.3390/pr12020297







