Enhancing Single- and Two-Stage Anaerobic Digestion of Thickened Waste-Activated Sludge through FNA-Heat Pretreatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Substrate and Inoculum
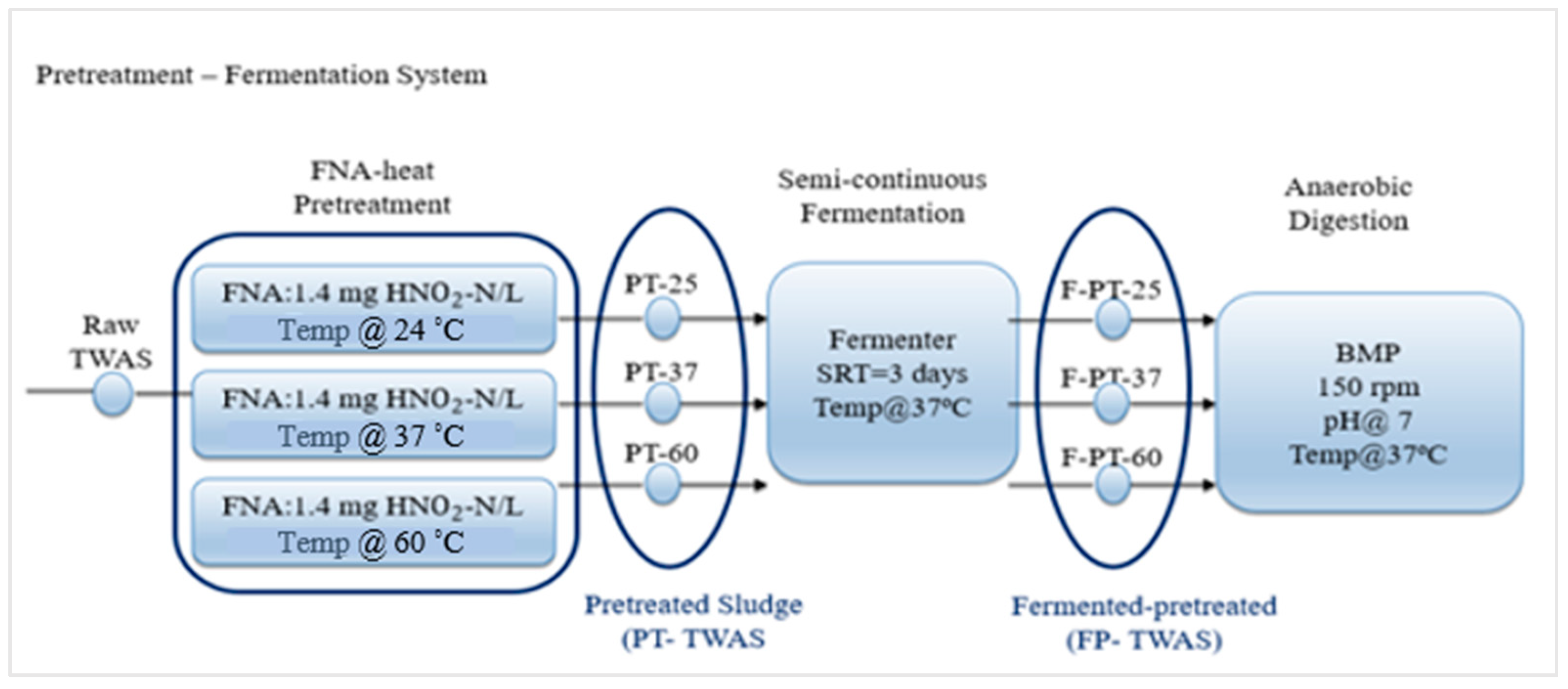
2.3. Pretreatment
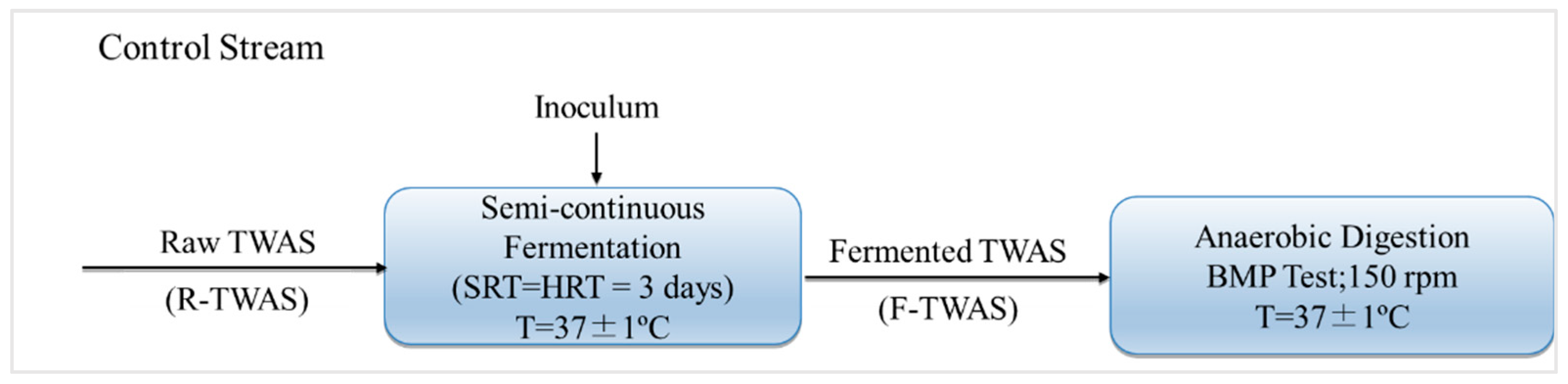
2.4. Hydrolysis/Acidification Experiment–Semi-Continuous
2.5. BMP
2.6. Analytical Methods
2.7. Performance Analysis
3. Results and Discussion
3.1. Disintegration of TWAs through FNA Pretreatment and Fermentation Process
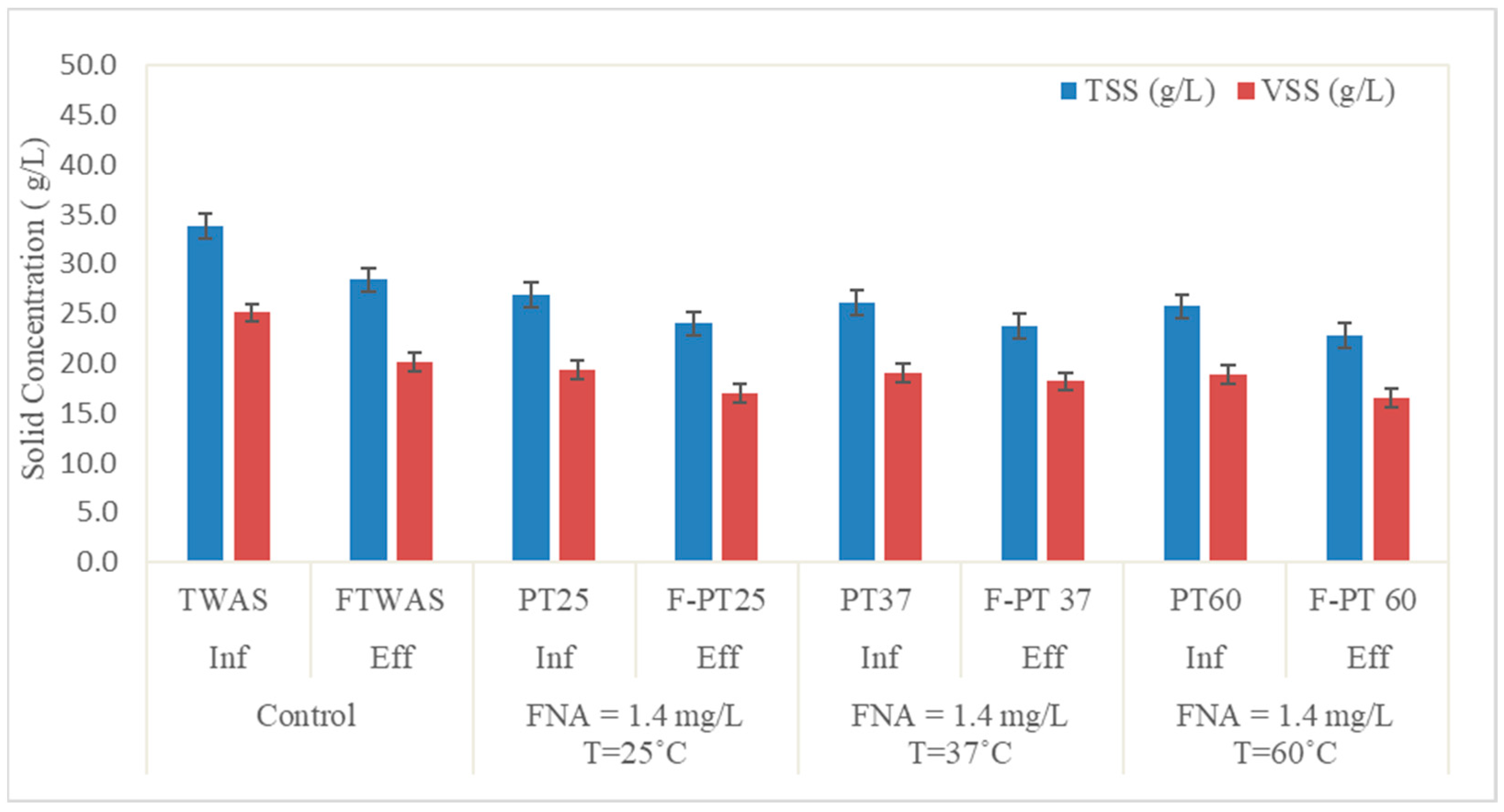
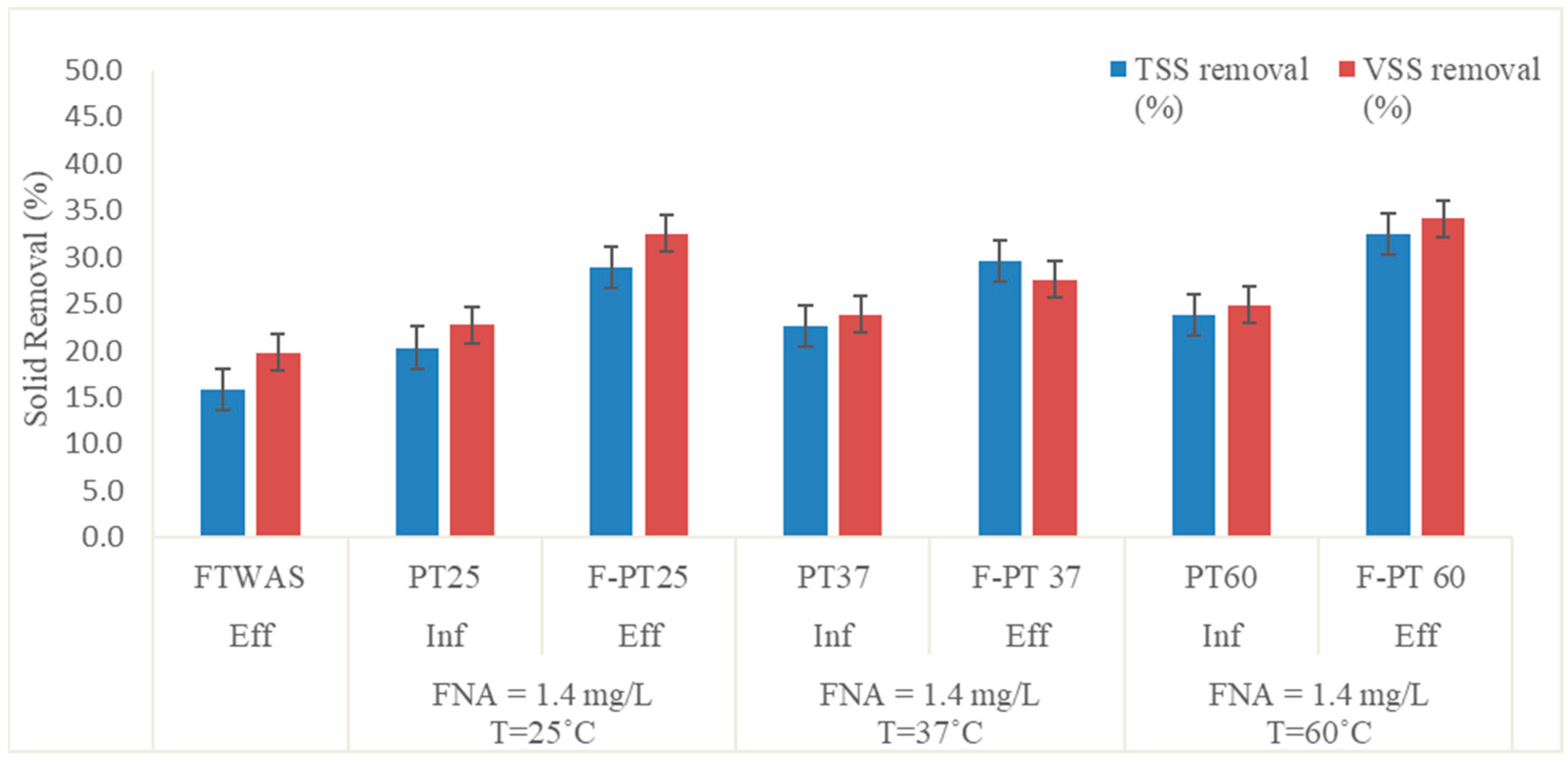
3.1.1. Suspended Solid Reduction
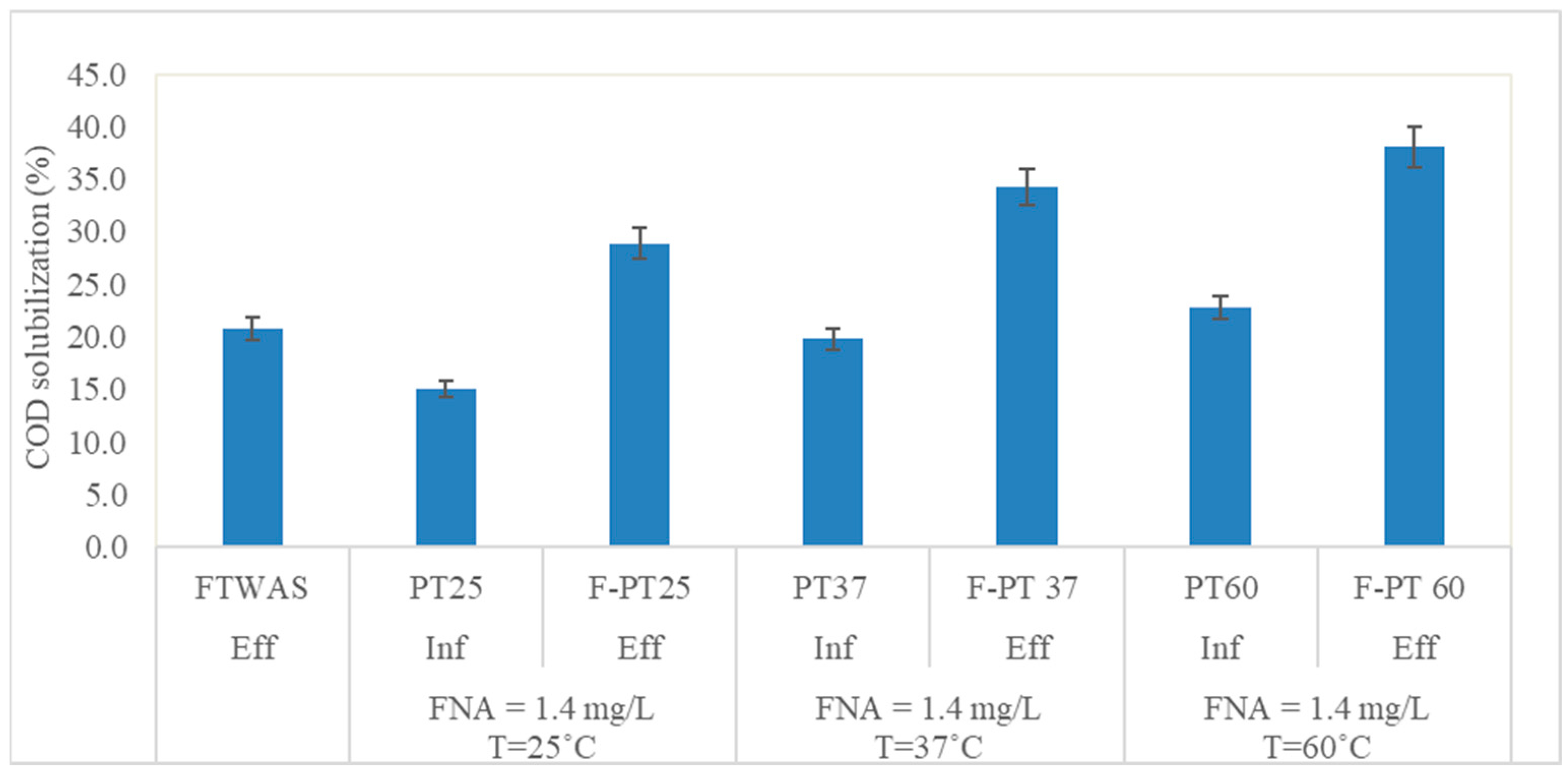
3.1.2. COD Solubilization
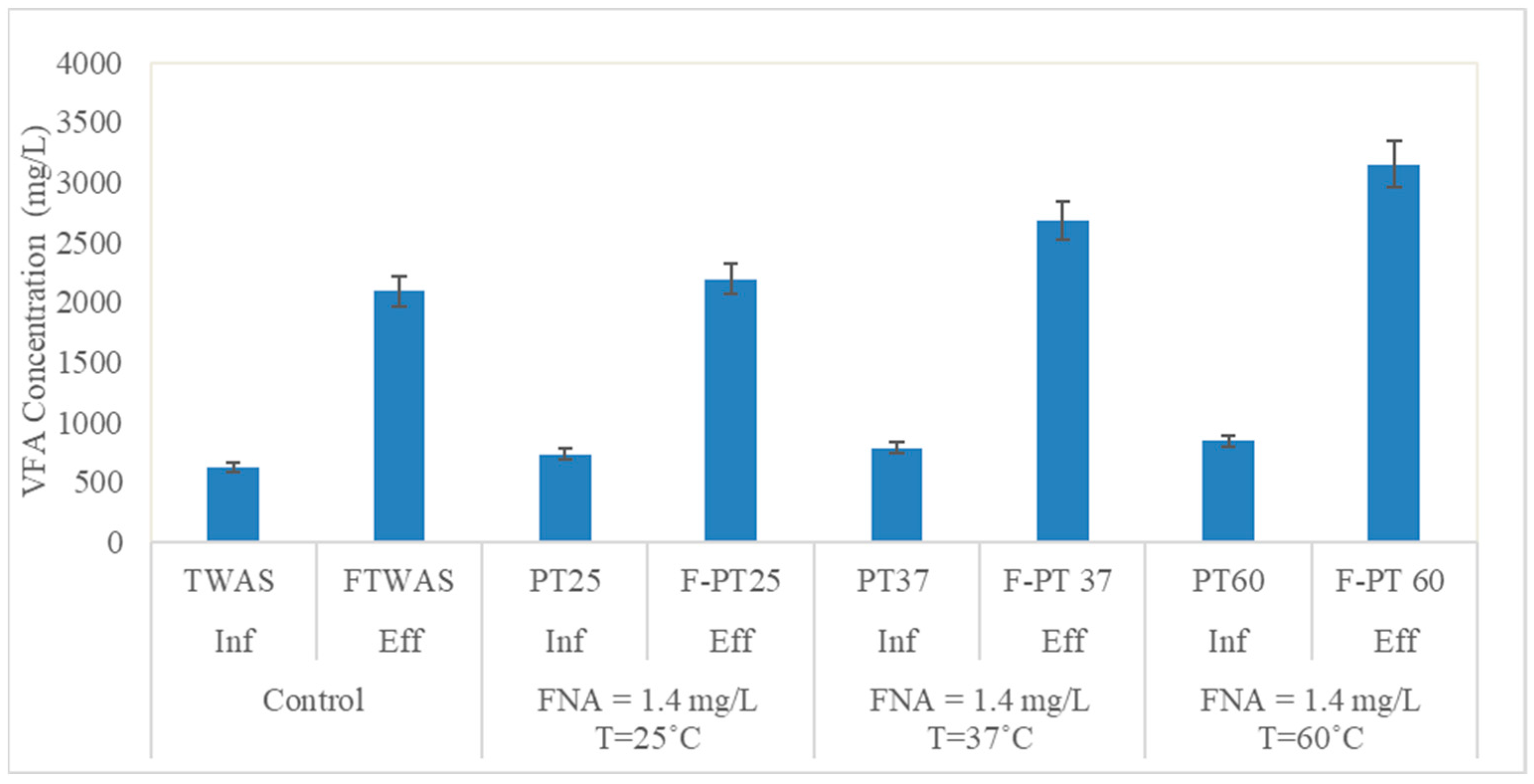
3.1.3. VFAs Production
3.2. Anaerobic Digestion
3.2.1. Methane Production Yield
3.2.2. Anaerobic Biodegradability
3.2.3. COD Mass Balance
3.2.4. Kinetic Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sarwar, R.; Elbeshbishy, E.; Parker, W.J. Codigestion of high pressure thermal hydrolysis-treated thickened waste activated sludge with primary sludge in two-stage anaerobic digestion. Environ. Prog. Sustain. Energy 2018, 37, 425–433. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, C.; Wang, D.; Yang, Y.; Fan, L.; Xu, S.; Zhuang, X.J. Quorum sensing responses of activated sludge to free nitrous acid: Zoogloea deformation, AHL redistribution, and microbiota acclimatization. Water Res. 2023, 238, 119993. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Liu, L.; Duan, H.; Li, B.; Tang, X.; Wu, X.; Liu, G.; Zhang, L.J.S. Improving sludge dewaterability by free nitrous acid and lysozyme pretreatment: Performances and mechanisms. Sci. Total Environ. 2023, 855, 158648. [Google Scholar] [CrossRef] [PubMed]
- Biswal, B.K.; Huang, H.; Dai, J.; Chen, G.-H.; Wu, D. Impact of low-thermal pretreatment on physicochemical properties of saline waste activated sludge, hydrolysis of organics and methane yield in anaerobic digestion. Bioresour. Technol. 2020, 297, 122423. [Google Scholar]
- Manzanares, P. The role of biorefinering research in the development of a modern bioeconomy. Acta Innov. 2020, 37, 47–56. [Google Scholar] [CrossRef]
- Lopes, T.F.; Łukasik, R.M. Economic, social and environmental impacts attained by the use of the effluents generated within a small-scale biorefinery concept. Acta Innov. 2020, 36, 57–63. [Google Scholar] [CrossRef]
- Ximenes, J.; Siqueira, A.; Kochańska, E.; Łukasik, R.M. Valorisation of agri-and aquaculture residues via biogas production for enhanced industrial application. Energies 2021, 14, 2519. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Chen, Z.; Wei, W.; Chen, X.; Mannina, G.; Ni, B.-J. A novel strategy for efficiently transforming waste activated sludge into medium-chain fatty acid using free nitrous acid. Sci. Total Environ. 2023, 862, 160826. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, A.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, G.; Ye, L.; Yuan, Z. Enhancing methane production from waste activated sludge using combined free nitrous acid and heat pre-treatment. Water Res. 2014, 63, 71–80. [Google Scholar] [CrossRef]
- Pijuan, M.; Wang, Q.; Ye, L.; Yuan, Z. Improving secondary sludge biodegradability using free nitrous acid treatment. Bioresour. Technol. 2012, 116, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, Z.; Ye, X.; Huang, F.; Chen, S. Performance and mechanism of free nitrous acid on the solubilization of waste activated sludge. RSC Adv. 2018, 8, 15897–15905. [Google Scholar] [CrossRef] [PubMed]
- Zupančič, G.D.; Roš, M. Heat and energy requirements in thermophilic anaerobic sludge digestion. Energy 2003, 28, 2255–2267. [Google Scholar] [CrossRef]
- Azizi, A.; Koupaie, E.H.; Hafez, H.; Elbeshbishy, E. Improving single-and two-stage anaerobic digestion of source separated organics by hydrothermal pretreatment. Biochem. Eng. J. 2019, 148, 77–86. [Google Scholar] [CrossRef]
- Jiang, W.; Tao, J.; Luo, J.; Xie, W.; Zhou, X.; Cheng, B.; Guo, G.; Ngo, H.H.; Guo, W.; Cai, H.J.C. Pilot-scale two-phase anaerobic digestion of deoiled food waste and waste activated sludge: Effects of mixing ratios and functional analysis. Chemosphere 2023, 329, 138653. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Sun, H.; Chen, P.; Zheng, Y.; Guo, J.; Dong, R.J.B.T. Two-step anaerobic digestion of rice straw with nanobubble water. Bioresour. Technol. 2023, 376, 128928. [Google Scholar] [CrossRef] [PubMed]
- Chegini, S.; Okoye, F.; Elbeshbishy, E. Chemical pretreatment of thickened waste activated sludge (TWAS) to improve biogas production. In Residuals and Biosolids Conference 2020; Water Environment Federation: Alexandria, VA, USA, 2020. [Google Scholar]
- Zhang, T.; Wang, Q.; Ye, L.; Batstone, D.; Yuan, Z. Combined free nitrous acid and hydrogen peroxide pre-treatment of waste activated sludge enhances methane production via organic molecule breakdown. Sci. Rep. 2015, 5, 16631. [Google Scholar] [CrossRef]
- Angelakis, A.N.; Snyder, S.A. Wastewater Treatment and Reuse: Past, Present, and Future; Multidisciplinary Digital Publishing Institute: Basel, Switzerland, 2015. [Google Scholar]
- Angelidaki, I.; Alves, M.; Bolzonella, D.; Borzacconi, L.; Campos, J.; Guwy, A.; Kalyuzhnyi, S.; Jenicek, P.; Van Lier, J.B. Defining the biomethane potential (BMP) of solid organic wastes and energy crops: A proposed protocol for batch assays. Water Sci. Technol. 2009, 59, 927–934. [Google Scholar] [CrossRef]
- Van Lier, J.B.; Mahmoud, N.; Zeeman, G. Anaerobics for Wastewater Treatment. Paper Presented at The 3rd International Meeting on Environmental Biotechnology and Engineering. 2008, pp. 415–456. Available online: https://research.wur.nl/en/publications/anaerobics-for-wastewater-treatment (accessed on 23 January 2024).
- Wei, W.; Wang, Q.; Zhang, L.; Laloo, A.; Duan, H.; Batstone, D.J.; Yuan, Z. Free nitrous acid pre-treatment of waste activated sludge enhances volatile solids destruction and improves sludge dewaterability in continuous anaerobic digestion. Water Res. 2018, 130, 13–19. [Google Scholar] [CrossRef]
- Bonilla, S.; Choolaei, Z.; Meyer, T.; Edwards, E.A.; Yakunin, A.F.; Allen, D.G. Evaluating the effect of enzymatic pretreatment on the anaerobic digestibility of pulp and paper biosludge. Biotechnol. Rep. 2018, 17, 77–85. [Google Scholar] [CrossRef]
- Choi, J.-M.; Han, S.-K.; Lee, C.-Y. Enhancement of methane production in anaerobic digestion of sewage sludge by thermal hydrolysis pretreatment. Bioresour. Technol. 2018, 259, 207–213. [Google Scholar] [CrossRef]
- Ohimain, E.I.; Izah, S.C.J.R.; Reviews, S.E. A review of biogas production from palm oil mill effluents using different configurations of bioreactors. Renew. Sustain. Energy Rev. 2017, 70, 242–253. [Google Scholar] [CrossRef]
- Zahedi, S.; Romero-Güiza, M.; Icaran, P.; Yuan, Z.; Pijuan, M. Optimization of free nitrous acid pre-treatment on waste activated sludge. Bioresour. Technol. 2018, 252, 216–220. [Google Scholar] [CrossRef]
- Atelge, M.; Krisa, D.; Kumar, G.; Eskicioglu, C.; Nguyen, D.D.; Chang, S.W.; Atabani, A.; Al-Muhtaseb, A.H.; Unalan, S. Biogas production from organic waste: Recent progress and perspectives. Waste Biomass Valoriz. 2020, 11, 1019–1040. [Google Scholar] [CrossRef]
- Saha, M.; Eskicioglu, C.; Marin, J. Microwave, ultrasonic and chemo-mechanical pretreatments for enhancing methane potential of pulp mill wastewater treatment sludge. Bioresour. Technol. 2011, 102, 7815–7826. [Google Scholar] [CrossRef] [PubMed]
- Atelge, M.; Atabani, A.; Banu, J.R.; Krisa, D.; Kaya, M.; Eskicioglu, C.; Kumar, G.; Lee, C.; Yildiz, Y.; Unalan, S.J.F. A critical review of pretreatment technologies to enhance anaerobic digestion and energy recovery. Fuel 2020, 270, 117494. [Google Scholar] [CrossRef]
- Uthirakrishnan, U.; Sharmila, V.G.; Merrylin, J.; Kumar, S.A.; Dharmadhas, J.S.; Varjani, S.; Banu, J.R. Current advances and future outlook on pretreatment techniques to enhance biosolids disintegration and anaerobic digestion: A critical review. Chemosphere 2022, 288, 132553. [Google Scholar] [CrossRef]
- Apollo, S.; Onyango, M.S.; Ochieng, A.J.C.E.J. Integrated UV photodegradation and anaerobic digestion of textile dye for efficient biogas production using zeolite. Chem. Eng. J. 2014, 245, 241–247. [Google Scholar] [CrossRef]
- Akgul, D.; Cella, M.A.; Eskicioglu, C. Influences of low-energy input microwave and ultrasonic pretreatments on single-stage and temperature-phased anaerobic digestion (TPAD) of municipal wastewater sludge. Energy 2017, 123, 271–282. [Google Scholar] [CrossRef]
- Bao, H.; Yang, H.; Zhang, H.; Liu, Y.; Su, H.; Shen, M.J.E.R. Improving methane productivity of waste activated sludge by ultrasound and alkali pretreatment in microbial electrolysis cell and anaerobic digestion coupled system. Environ. Res. 2020, 180, 108863. [Google Scholar] [CrossRef]
- Kim, J.; Yu, Y.; Lee, C. Thermo-alkaline pretreatment of waste activated sludge at low-temperatures: Effects on sludge disintegration, methane production, and methanogen community structure. Bioresour. Technol. 2013, 144, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Peng, Y.; Wei, Y.; Li, B.; Bao, P.; Wang, Y. Free nitrous acid pretreatment of wasted activated sludge to exploit internal carbon source for enhanced denitrification. Bioresour. Technol. 2015, 179, 20–25. [Google Scholar] [CrossRef] [PubMed]
- De Gioannis, G.; Muntoni, A.; Polettini, A.; Pomi, R.; Spiga, D.J.W.M. Energy recovery from one-and two-stage anaerobic digestion of food waste. Waste Manag. 2017, 68, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Duan, H.; Gao, S.; Li, X.; Ab Hamid, N.H.; Jiang, G.; Zheng, M.; Bai, X.; Bond, P.L.; Lu, X.; Chislett, M.M. Improving wastewater management using free nitrous acid (FNA). Water Res. 2020, 171, 115382. [Google Scholar] [CrossRef]
- Zhang, L.; Duan, H.; Ye, L.; Liu, L.; Batstone, D.J.; Yuan, Z. Increasing capacity of an anaerobic sludge digester through FNA pre-treatment of thickened waste activated sludge. Water Res. 2019, 149, 406–413. [Google Scholar] [CrossRef]
- Zhang, T. FNA-Based Pre-Treatment of Primary, Secondary and Digested Sludge. Ph.D. Thesis, The University of Queensland, St Lucia, QLD, Australia, 2016. [Google Scholar]
- Alpaslan Kocamemi, B.; Çelik, S.; Senol, A.B.; Kurt, H.; Erken, E. Paradigm Shift in Domestic Wastewater Treatment: Toward Energy Minimization, Greenhouse Gas Emission Reduction, and Resources Recovery. In Wastewater Management and Technologies; Springer: Berlin/Heidelberg, Germany, 2023; pp. 211–237. [Google Scholar]
- Ghosh, A.; Ray, A.; Goswami, A.; Ali, O.A.; Singh, P.K.; Pattnaik, R. Recent Development and Innovations in Integrated Biogas-Wastewater Treatment. In Biorefinery for Water and Wastewater Treatment; Springer: Berlin/Heidelberg, Germany, 2023; pp. 271–297. [Google Scholar]
- Zhao, Q.; Ying, H.; Liu, Y.; Wang, H.; Xu, J.; Wang, W.; Ren, J.; Meng, S.; Wang, N.; Mu, R. Towards low energy-carbon footprint: Current versus potential P recovery paths in domestic wastewater treatment plants. J. Environ. Manag. 2023, 344, 118653. [Google Scholar] [CrossRef]



| Sample ID# | ~FNA (mg HNO2-N/L) | Temperature (°C) | pH |
|---|---|---|---|
| Raw TWAS (Control) | 0 | 24 ± 1 | 6.7 ± 0.1 |
| PT-25 | 1.4 | 24 ± 1 | 5.5 ± 0.1 |
| PT-37 | 1.4 | 37 ± 1 | 5.5 ± 0.1 |
| PT-60 | 1.4 | 60 ± 1 | 5.5 ± 0.1 |
| AD | Gompertz Kinetic Model | |||||
|---|---|---|---|---|---|---|
| Exp.ID# | MMPR * (mL/d) | Cumulative CH4 (mL) | P (mL CH4) | Rm (mL CH4/d) | Lam (d) | R2 |
| Control | 21 ± 1.0 | 228 ± 9 | 222 | 16 | 0.5 | 0.999 |
| F-TWAS | 20 ± 0.8 | 274 ± 11 | 267 | 20 | 0.9 | 0.999 |
| PT-25 | 23 ± 1.1 | 278 ± 14 | 272 | 21 | 1.1 | 0.999 |
| F-PT-25 | 21 ± 0.6 | 274 ± 9 | 278 | 20 | 0.9 | 0.999 |
| PT-37 | 23 ± 0.2 | 286 ± 15 | 276 | 20 | 0.1 | 0.999 |
| F-PT-37 | 24 ± 0.5 | 297 ± 23 | 291 | 21 | 0.9 | 0.999 |
| PT-60 | 23 ± 0.4 | 302 ± 21 | 276 | 19 | 0.5 | 0.999 |
| F-PT-60 | 27 ± 0.5 | 311 ± 24 | 299 | 23 | 0.7 | 0.999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chegini, S.; Elbeshbishy, E. Enhancing Single- and Two-Stage Anaerobic Digestion of Thickened Waste-Activated Sludge through FNA-Heat Pretreatment. Processes 2024, 12, 345. https://doi.org/10.3390/pr12020345
Chegini S, Elbeshbishy E. Enhancing Single- and Two-Stage Anaerobic Digestion of Thickened Waste-Activated Sludge through FNA-Heat Pretreatment. Processes. 2024; 12(2):345. https://doi.org/10.3390/pr12020345
Chicago/Turabian StyleChegini, Salomeh, and Elsayed Elbeshbishy. 2024. "Enhancing Single- and Two-Stage Anaerobic Digestion of Thickened Waste-Activated Sludge through FNA-Heat Pretreatment" Processes 12, no. 2: 345. https://doi.org/10.3390/pr12020345





