Physical and Chemical Properties of Pachycymbiola brasiliana Eggshells—From Application to Separative Processes
Abstract
:1. Introduction
2. Results
2.1. Degree of Acetylation, Solubility, and FTIR
2.2. X-ray Diffraction (XRD)
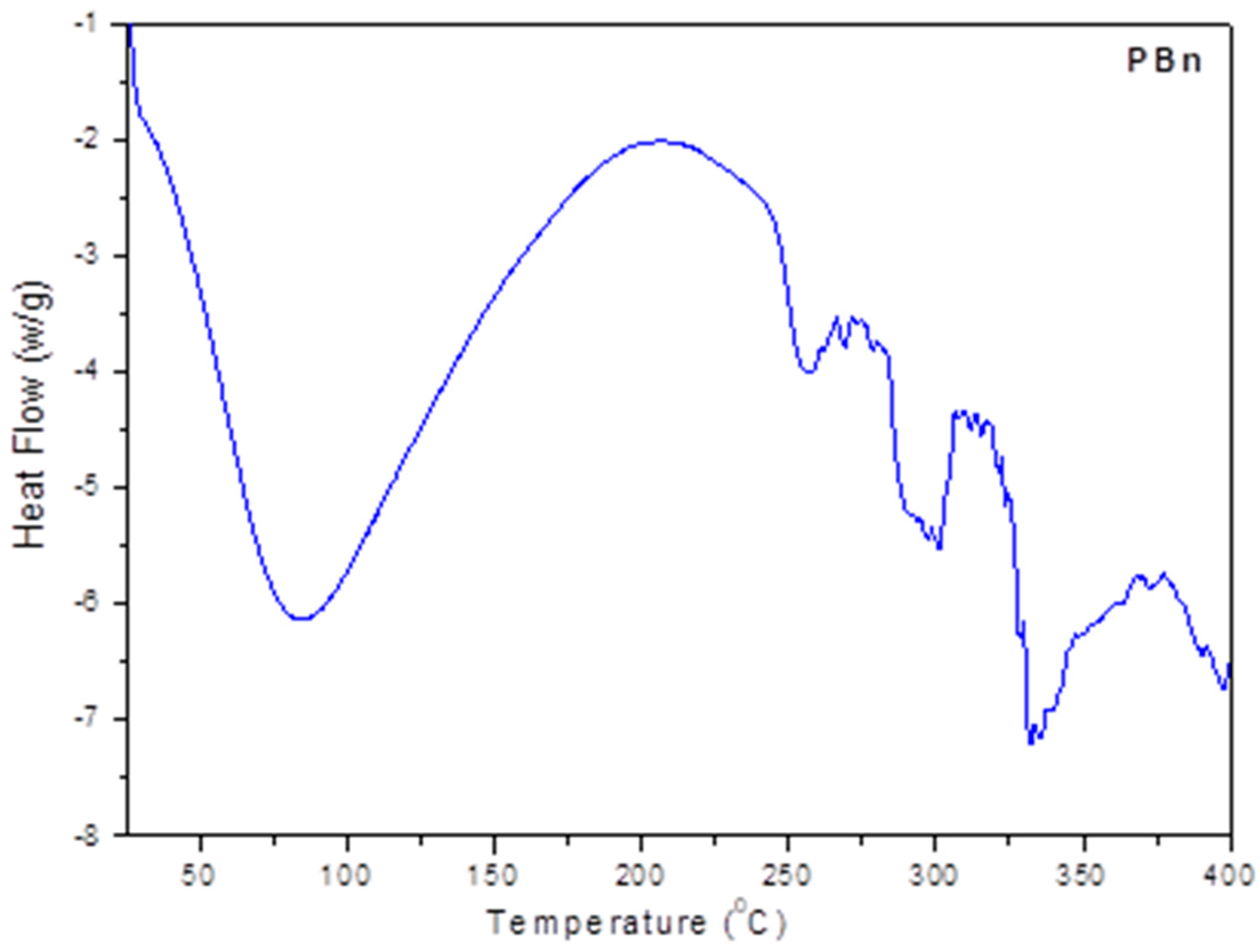
2.3. Differential Scanning Calorimetric Analysis (DSC)
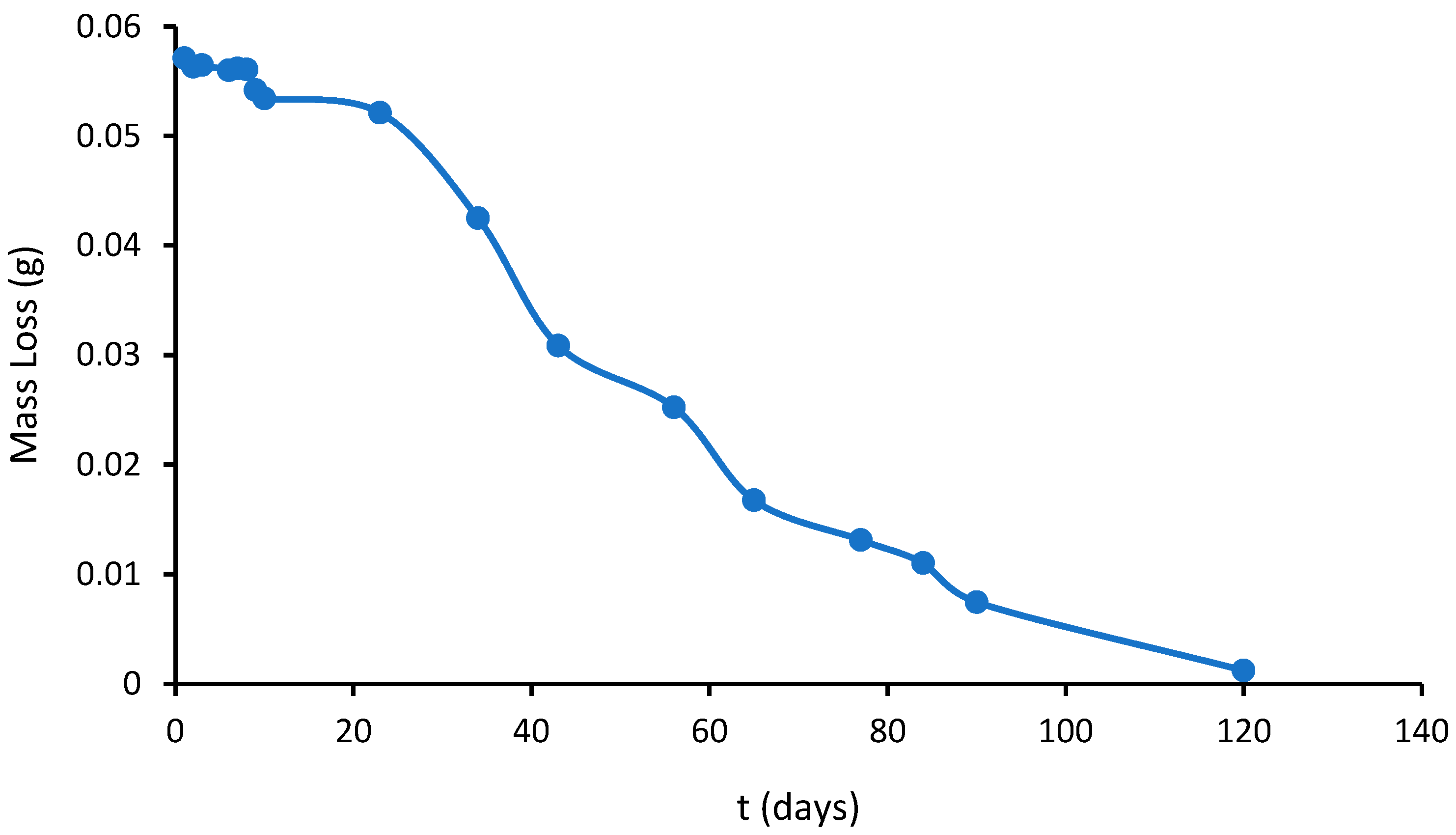
2.4. Thermogravimetric Analysis—Differential Thermal Analysis
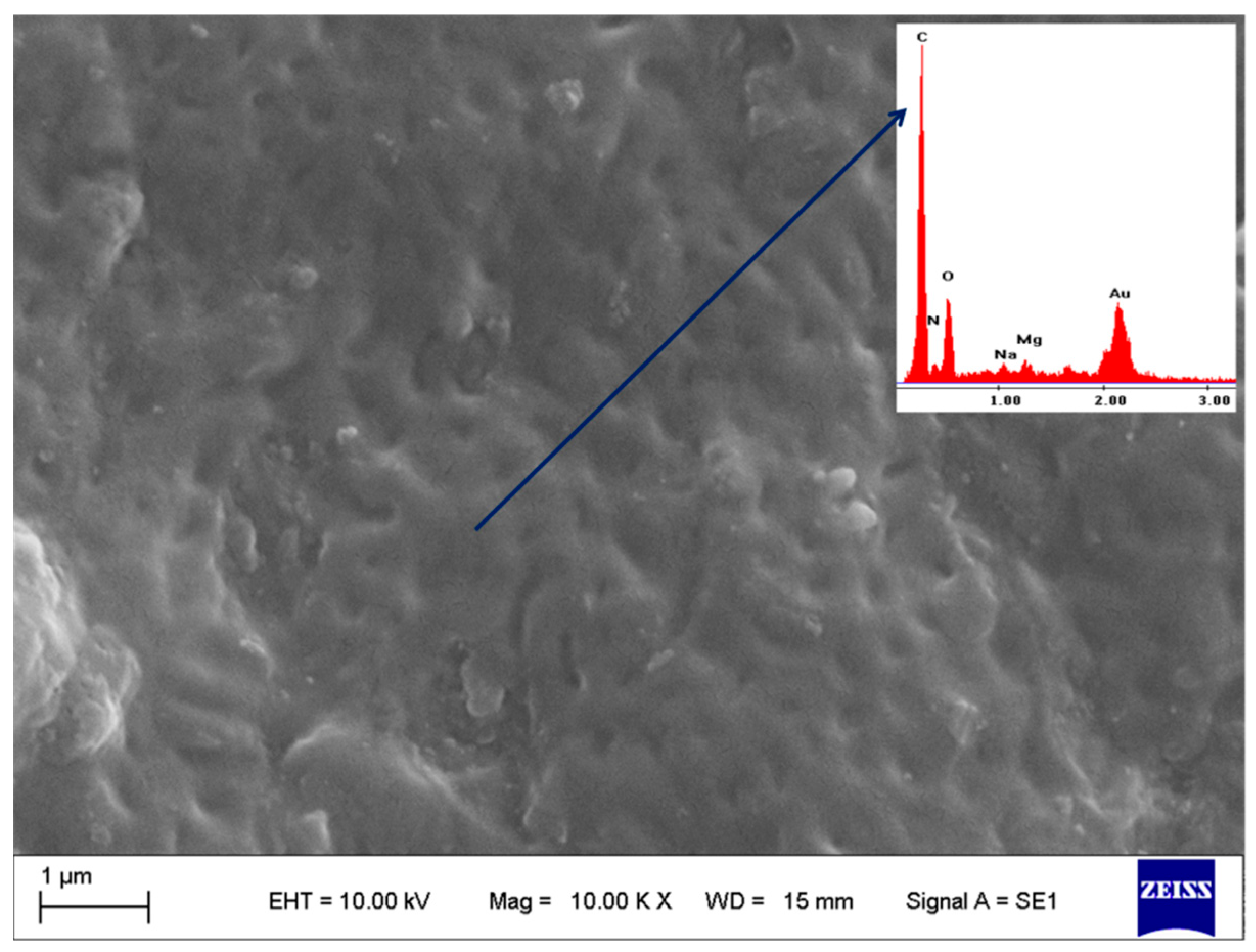
2.5. Scanning Electron Microscopy–Electron and Dispersive X-ray Spectroscopy (SEM-EDX)
2.6. Colorimetry
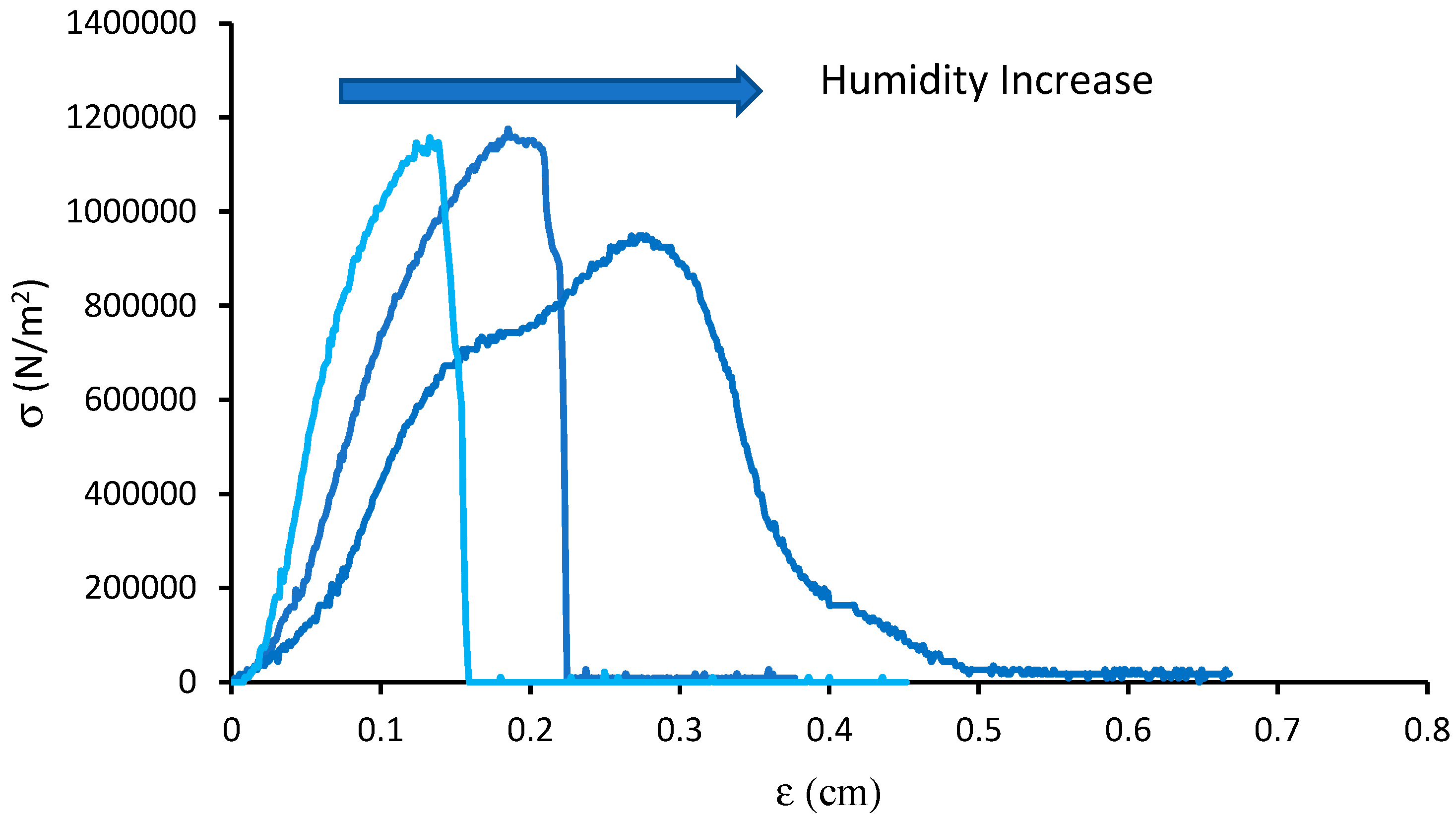
2.7. Mechanical Test
2.8. Contact Angle
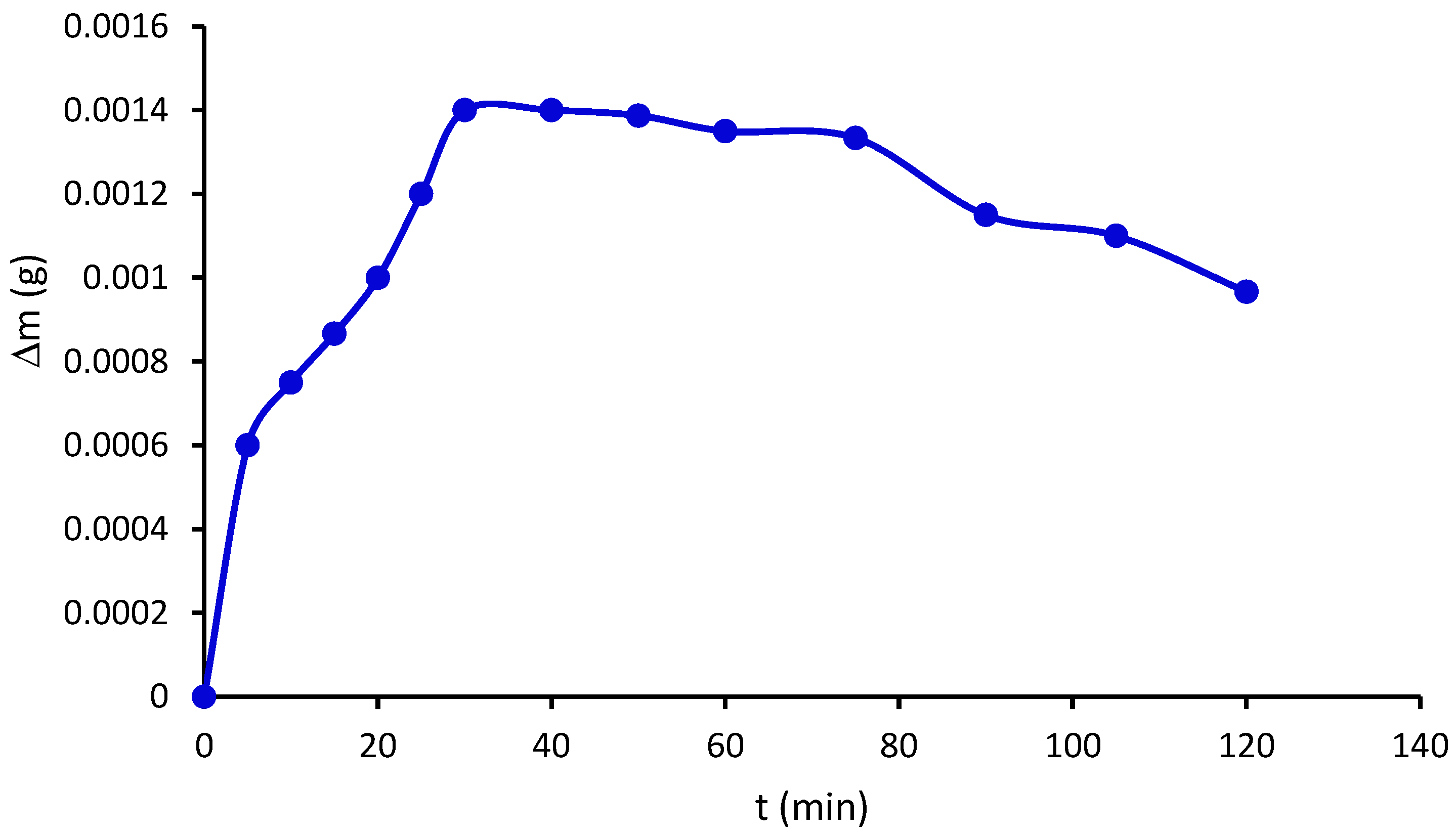
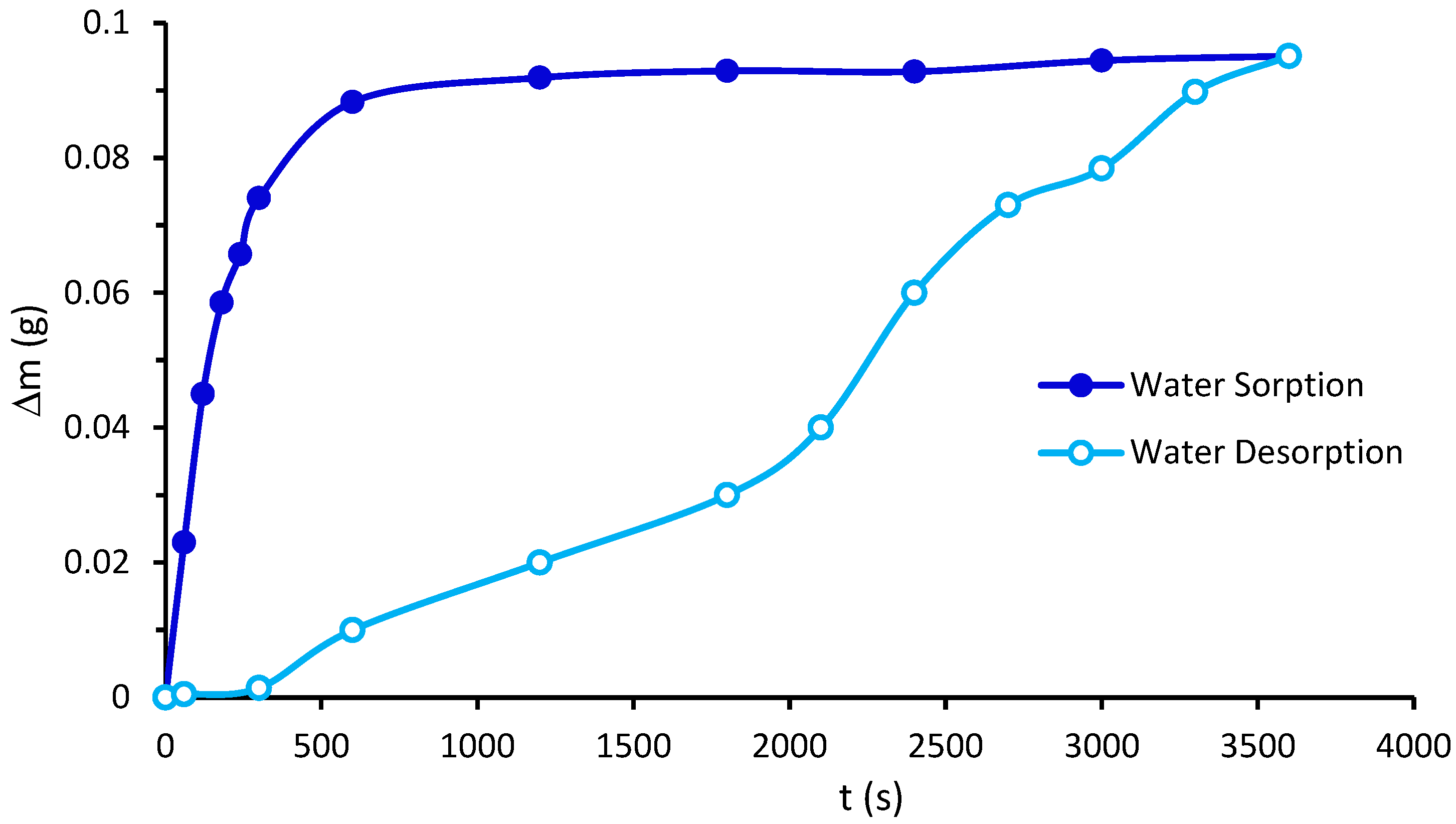
2.9. Water Vapor Permeability and Water Vapor Sorption
2.10. Swelling Index
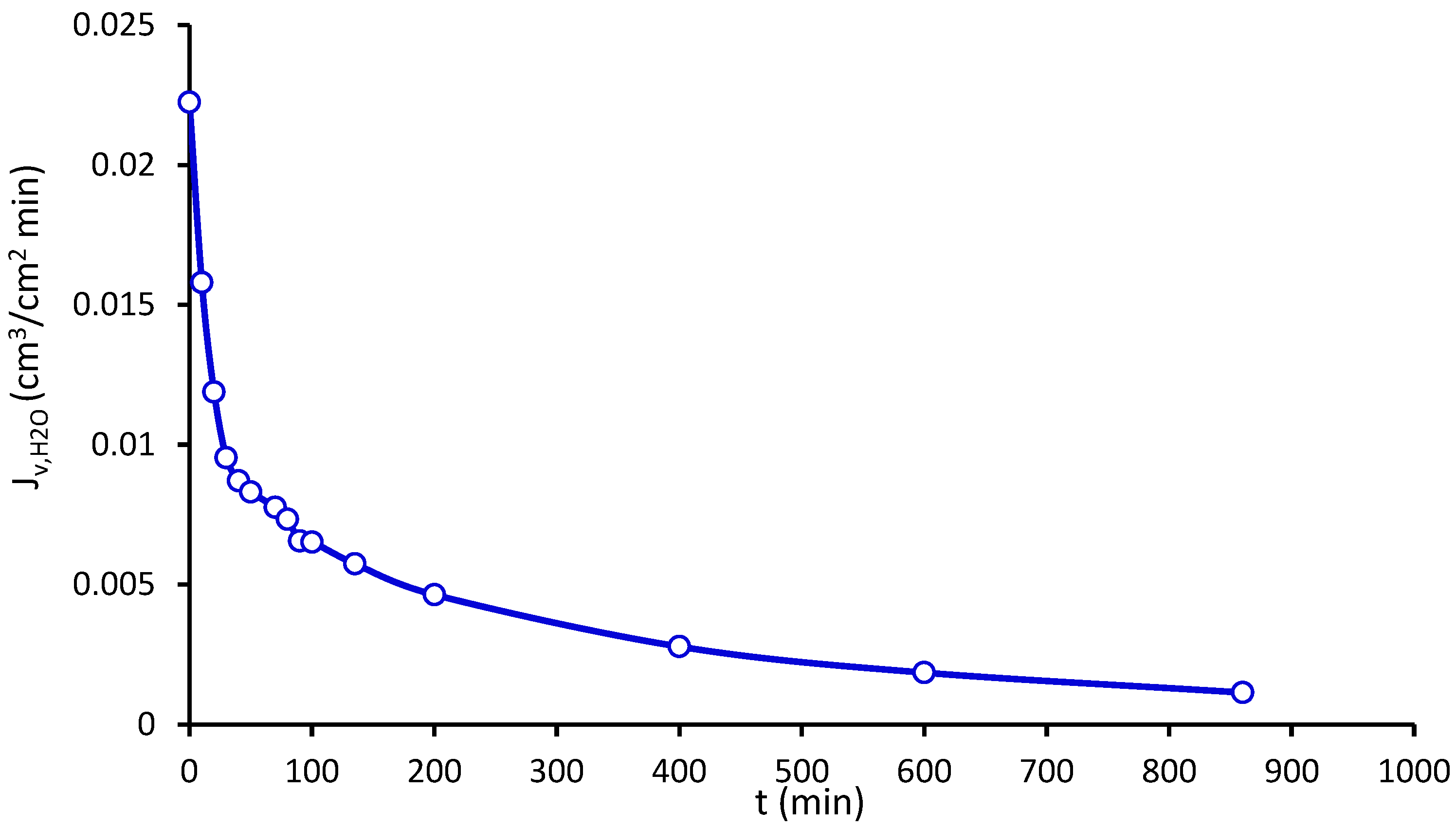
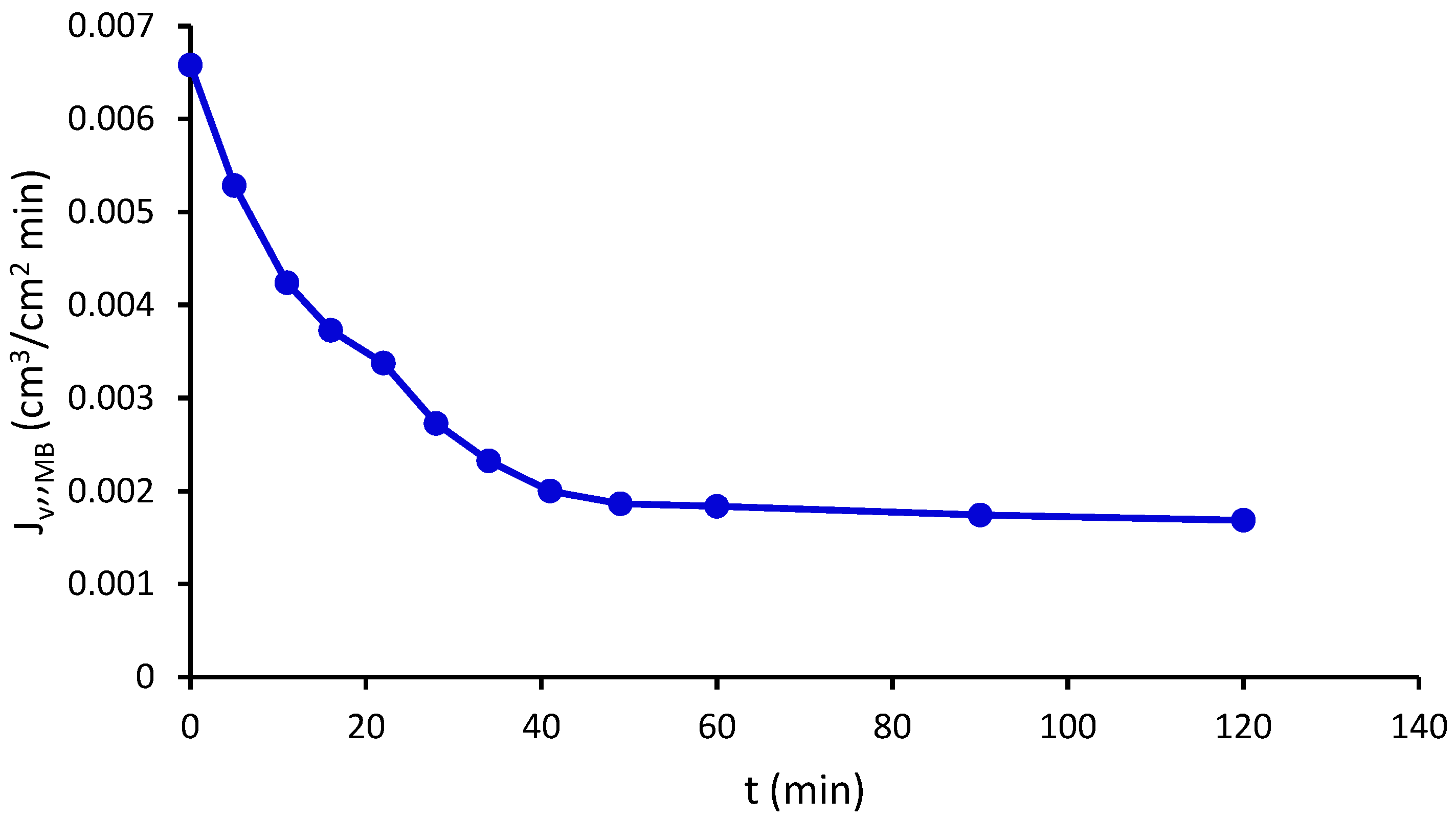
2.11. Pure Water Flux and Methylene Blue Flux
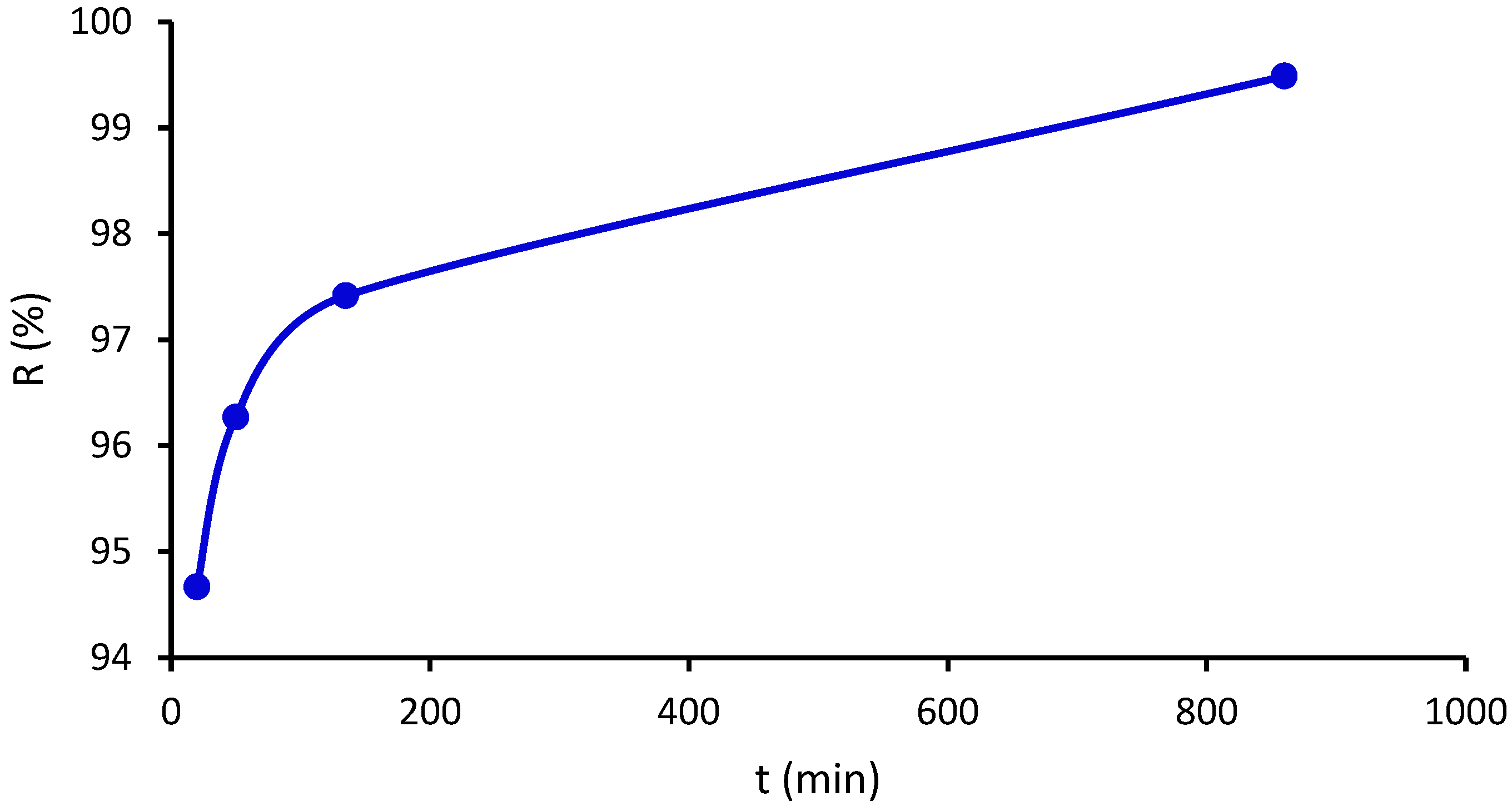
2.12. Biodegradability
3. Materials and Methods
3.1. Raw Material
3.2. Degree of Acetylation
3.3. Solubility
3.4. Fourier Transform Infrared Spectroscopy
3.5. X-ray Diffraction (XRD)
3.6. Differential Scanning Calorimetric Analysis (DSC)
3.7. Thermogravimetric Analysis—Differential Thermogravimetric Analysis
3.8. Scanning Electron Microscopy–Electron Dispersive X-ray Spectroscopy (SEM-EDX)
3.9. Colorimetry
3.10. Mechanical Tests
3.11. Contact Angle
3.12. Water Vapor Permeability
3.13. Water Vapor Sorption
3.14. Swelling Index
3.15. Pure Water Flux and Methylene Blue Flux
3.16. Biodegradability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cortes, C.N.; Narosky, T. Cien Caracoles Argentinos; Ed. Albatros: Buenos Aires, Argentina, 1997. [Google Scholar]
- Lasta, M.L.; Ciocco, N.F.; Bremec, C.; Roux, A. Moluscos bivalvos y gasterópodos. Mar Argent. Sus Recur. Pesq. 1998, 2, 115–142. [Google Scholar]
- Carranza, A.; Scarabino, F.; Ortega, L. Distribution of large benthic gastropods in the Uruguayan continental shelf and Río de la Plata estuary. J. Coast. Res. 2008, 24, 161–168. [Google Scholar] [CrossRef]
- Tsai, W.T.; Yang, J.M.; Lai, C.W.; Cheng, Y.H.; Lin, C.C.; Yeh, C.W. Characterization and adsorption properties of eggshells and eggshell membrane. Bioresour. Technol. 2006, 97, 488–493. [Google Scholar] [CrossRef]
- Yasothai, R.; Kavithaa, N.V. Chemical characterization of egg shell meal. Int. J. Sci. Environ. Technol. 2014, 3, 1436–1439. [Google Scholar]
- Zaman, T.; Mostari, M.; Mahmood, M.A.A.; Rahman, M.S. Evolution and characterization of eggshell as a potential candidate of raw material. Cerâmica 2018, 64, 236–241. [Google Scholar] [CrossRef]
- Ajala, E.O.; Eletta, O.A.A.; Ajala, M.A.; Oyeniyi, S.K. Characterization and evaluation of chicken eggshell for use as a bio-resource. Arid Zone J. Eng. Technol. Environ. 2018, 14, 26–40. [Google Scholar]
- Yi, F.; Guo, Z.X.; Zhang, L.-X.; Yu, J.; Li, Q. Soluble eggshell membrane protein: Preparation, characterization and biocompatibility. Biomaterials 2004, 25, 4591–4599. [Google Scholar] [CrossRef]
- Baláž, M. Eggshell membrane biomaterial as a platform for applications in materials science. Acta Biomater. 2014, 10, 3827–3843. [Google Scholar] [CrossRef]
- Dinculescu, D.; Gîjiu, C.L.; Apetroaei, M.R.; Isopescu, R.; Rău, I.; Schröder, V. Optimization of chitosan extraction process from Rapanavenosa egg capsules waste using experimental design. Materials 2023, 16, 525. [Google Scholar] [CrossRef]
- Schröder, V.; Rau, I.; Dobrin, N.; Stefanov, C.; Mihali, C.-V.; Paduretu, C.-C.; Apetroaei, M.R. Micromorphological details and identification of chitinous wall structures in Rapanavenosa (Gastropoda, Mollusca) egg capsules. Sci. Rep. 2020, 10, 14550. [Google Scholar] [CrossRef] [PubMed]
- Oyekunle, D.T.; Omoleye, J.A. New process for synthesizing chitosan from snail shells. In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2019; Volume 1299, p. 012089. [Google Scholar]
- Shi, Y.; Zhou, K.; Li, D.; Guyonnet, V.; Hincke, M.T.; Mine, Y. Avian eggshell membrane as a novel biomaterial: A review. Foods 2021, 10, 2178. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Muralisankar, T.; Jayakumar, R.; Rajeevgandhi, C. A study on structural comparisons of α-chitin extracted from marine crustacean shell waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Dahmane, E.M.; Taourirte, M.; Eladlani, N.; Rhazi, M. Extraction and characterization of chitin and chitosan from Parapenaeus longirostris from Moroccan local sources. Int. J. Polym. Anal. Charact. 2014, 19, 342–351. [Google Scholar] [CrossRef]
- Guru, P.S.; Dash, S. Sorption on eggshell waste—A review on ultrastructure, biomineralization and other applications. Adv. Colloid Interface Sci. 2014, 209, 49–67. [Google Scholar] [CrossRef] [PubMed]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Cardenas, G.; Miranda, S.P. FTIR and TGA studies of chitosan composite films. J. Chil. Chem. Soc. 2004, 49, 291–295. [Google Scholar] [CrossRef]
- Beppu, M.M.; Vieira, R.S.; Aimoli, C.G.; Santana, C.C. Crosslinking of chitosan membranes using glutaraldehyde: Effect on ion permeability and water absorption. J. Membr. Sci. 2007, 301, 126–130. [Google Scholar] [CrossRef]
- Al Sagheer, F.A.; Al-Sughayer, M.A.; Muslim, S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf. Carbohydr. Polym. 2009, 77, 410–419. [Google Scholar] [CrossRef]
- Barbosa, H.F.; Francisco, D.S.; Ferreira, A.P.; Cavalheiro, É.T. A new look towards the thermal decomposition of chitins and chitosans with different degrees of deacetylation by coupled TG-FTIR. Carbohydr. Polym. 2019, 225, 115232. [Google Scholar] [CrossRef] [PubMed]
- González-Campos, J.B.; Prokhorov, E.; Luna-Bárcenas, G.; Galván, A.M.; Sanchez, I.C.; Donlucas, S.M.N.; Garcia-Gaitan, B.; Kovalenko, Y. Relaxations in chitin: Evidence for a glass transition. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 932–943. [Google Scholar] [CrossRef]
- Zheng, Y.; Li, X.; Huang, Y.; Li, H.; Chen, L.; Liu, X. Two colorimetric films based on chitin whiskers and sodium alginate/gelatin incorporated with anthocyanins for monitoring food freshness. Food Hydrocoll. 2022, 127, 107517. [Google Scholar] [CrossRef]
- Kaewmanee, T.; Benjakul, S.; Visessanguan, W. Changes in chemical composition, physical properties and microstructure of duck egg as influenced by salting. Food Chem. 2009, 112, 560–569. [Google Scholar] [CrossRef]
- Yu, H.; Tang, Q.; Wu, J.; Lin, Y.; Fan, L.; Huang, M.; Lin, J.; Li, Y.; Yu, F. Using eggshell membrane as a separator in supercapacitor. J. Power Sources 2012, 206, 463–468. [Google Scholar] [CrossRef]
- Park, S.; Choi, K.S.; Lee, D.; Kim, D.; Lim, K.T.; Lee, K.-H.; Seonwoo, H.; Kim, J. Eggshell membrane: Review and impact on engineering. Biosyst. Eng. 2016, 151, 446–463. [Google Scholar] [CrossRef]
- Jayaraj, K.; Gopi, S.; Rajeswari, A.; Christy, E.J.S.; Pius, A. Microscopic studies on chitin and chitosan-based interpenetrating polymer networks, gels, blends, composites, and nano-composites. In Handbook of Chitin and Chitosan; Elsevier: Amsterdam, The Netherlands, 2020; pp. 95–138. [Google Scholar]
- Illanes, C.O.; Takara, E.A.; Masuelli, M.A.; Ochoa, N.A. pH-responsive gum tragacanth hydrogels for high methylene blue adsorption. J. Chem. Technol. Biotechnol. 2024, 99, 31–39. [Google Scholar] [CrossRef]
- Battampara, P.; Sathish, T.N.; Reddy, R.; Guna, V.; Nagananda, G.; Reddy, N.; Ramesha, B.; Maharaddi, V.; Rao, A.P.; Ravikumar, H.; et al. Properties of chitin and chitosan extracted from silkworm pupae and egg shells. Int. J. Biol. Macromol. 2020, 161, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- de Moraes, M.A.; Cocenza, D.S.; da Cruz Vasconcellos, F.; Fraceto, L.F.; Beppu, M.M. Chitosan and alginate biopolymer membranes for remediation of contaminated water with herbicides. J. Environ. Manag. 2013, 131, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Hou, Y.; Liu, Q.; Hu, Z.; Xie, Q.; Shan, Y.; Li, G.; Ding, S. Physicochemical and antimicrobial properties of chitosan composite films incorporated with glycerol monolaurate and nano-TiO2. Food Hydrocoll. 2021, 119, 106846. [Google Scholar] [CrossRef]
- Rupiasih, N.N.; Sumadiyasa, M.; Putra, I.K. The effect of variations in the ratio of matrix/solvent on the physical and mechanical properties of chitosan biopolymer membranes. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 196, p. 012039. [Google Scholar]
- Baig, U.; Faizan, M.; Waheed, A. A review on super-wettable porous membranes and materials based on bio-polymeric chitosan for oil-water separation. Adv. Colloid Interface Sci. 2022, 303, 102635. [Google Scholar] [CrossRef] [PubMed]
- Mamba, F.B.; Mbuli, B.S.; Ramontja, J. Recent advances in biopolymeric membranes towards the removal of emerging organic pollutants from water. Membranes 2021, 11, 798. [Google Scholar] [CrossRef] [PubMed]
- Salehi, E.; Daraei, P.; Shamsabadi, A.A. A review on chitosan-based adsorptive membranes. Carbohydr. Polym. 2016, 152, 419–432. [Google Scholar] [CrossRef] [PubMed]
- Hardian, R.; Alammar, A.; Holtzl, T.; Szekely, G. Fabrication of sustainable organic solvent nanofiltration membranes using cellulose–chitosan biopolymer blends. J. Membr. Sci. 2022, 658, 120743. [Google Scholar] [CrossRef]
- Honarkar, H.; Barikani, M. Applications of biopolymers I: Chitosan. Monatshefte Chem. -Chem. Mon. 2009, 140, 1403–1420. [Google Scholar] [CrossRef]
- Zango, Z.U.; Dennis, J.O.; Aljameel, A.I.; Usman, F.; Ali, M.K.M.; Abdulkadir, B.A.; Algessair, S.; Aldaghri, O.A.; Ibnaouf, K.H. Effective removal of methylene blue from simulated wastewater using ZnO-chitosan nano-composites: Optimization, kinetics, and isotherm studies. Molecules 2022, 27, 4746. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Rong, Z.; Zhu, K.; Wu, Y. Chitosan-based dual network composite hydrogel for efficient adsorption of methylene blue dye. Int. J. Biol. Macromol. 2022, 222, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Shi, W.; Li, K.; Cai, J.; Xu, C.; Gao, L.; Lu, J.; Ding, F. Chitosan-based hollow nanofiber membranes with polyvinylpyrrolidone and polyvinyl alcohol for efficient removal and filtration of organic dyes and heavy metals. Int. J. Biol. Macromol. 2023, 239, 124264. [Google Scholar] [CrossRef] [PubMed]
- Khoerunnisa, F.; Amanda, P.C.; Nurhayati, M.; Hendrawan, H.; Lestari, W.W.; Sanjaya, E.H.; Handayani, M.; Oh, W.-D.; Lim, J. Promotional effect of ammonium chloride functionalization on the performance of polyethersulfone/chitosan composite-based ultrafiltration membrane. Chem. Eng. Res. Des. 2023, 190, 366–378. [Google Scholar] [CrossRef]
- Heredia-Guerrero, J.A.; Benítez, J.J.; Porras-Vázquez, J.M.; Tedeschi, G.; Morales, Y.; Fernández-Ortuño, D.; Athanassiou, A.; Guzman-Puyol, S. Plasticized, greaseproof chitin bioplastics with high transparency and biodegradability. Food Hydrocoll. 2023, 145, 109072. [Google Scholar] [CrossRef]
- De Carli, C.; Aylanc, V.; Mouffok, K.M.; Santamaria-Echart, A.; Barreiro, F.; Tomás, A.; Pereira, C.; Rodrigues, P.; Vilas-Boas, M.; Falcão, S.I. Production of chitosan-based biodegradable active films using bio-waste enriched with polyphenol propolis extract envisaging food packaging applications. Int. J. Biol. Macromol. 2022, 213, 486–497. [Google Scholar] [CrossRef] [PubMed]
- Yanat, M.; Schroën, K. Advances in chitin-based nanoparticle use in biodegradable polymers: A review. Carbohydr. Polym. 2023, 312, 120789. [Google Scholar] [CrossRef] [PubMed]
- Domard, A.; Rinaudo, M. Preparation and characterization of fully deacetylated chitosan. Int. J. Biol. Macromol. 1983, 5, 49–52. [Google Scholar] [CrossRef]
- Brine, C.J.; Austin, P.R. Chitin variability with species and method of preparation. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 69, 283–286. [Google Scholar] [CrossRef]
- Mohan, K.; Ganesan, A.R.; Ezhilarasi, P.N.; Kondamareddy, K.K.; Rajan, D.K.; Sathishkumar, P.; Rajarajeswaran, J.; Conterno, L. Green and eco-friendly approaches for the extraction of chitin and chitosan: A review. Carbohydr. Polym. 2022, 287, 119349. [Google Scholar] [CrossRef] [PubMed]
- Zanon, M.; Masuelli, M. Alcayota Gum Films: Experimental Reviews. J. Mater. Sci. Chem. Eng. 2018, 6, 11. [Google Scholar] [CrossRef]
- Lazo, L.; Melo, G.M.; Auad, M.L.; Filippa, M.; Masuelli, M.A. Synthesis and characterization of Chañar gum films. Colloids Interfaces 2022, 6, 10. [Google Scholar] [CrossRef]
- Saheed, I.O.; Da Oh, W.; Suah, F.B.M. Chitosan modifications for adsorption of pollutants–A review. J. Hazard. Mater. 2021, 408, 124889. [Google Scholar] [CrossRef] [PubMed]
- da Silva Alves, D.C.; Healy, B.; Pinto, L.A.D.A.; Cadaval Jr, T.R.S.A.; Breslin, C.B. Recent developments in chitosan-based adsorbents for the removal of pollutants from aqueous environments. Molecules 2021, 26, 594. [Google Scholar] [CrossRef] [PubMed]
- Scudeler, C.G.D.S.; Costa, T.D.L.; Cortez-Vega, W.R.; Prentice, C.; Fonseca, G.G. Development and characterization of Nile tilapia (Oreochromis niloticus) protein isolate-based biopolymer films incorporated with essential oils and nanoclay. Food Packag. Shelf Life 2020, 25, 100542. [Google Scholar] [CrossRef]
- Ruano, P.; Delgado, L.L.; Picco, S.; Villegas, L.; Tonelli, F.; Merlo, M.E.A.; Rigau, J.; Diaz, D.; Masuelli, M. Extraction and characterization of pectins from peels of Criolla Oranges (Citrus sinensis): Experimental reviews. In Pectins-Extraction, Purification, Characterization and Applications; INTECH Publishing: Rijeka, Croatia, 2020; pp. 1–45. [Google Scholar]
- Bielska, M.; Szymanowski, J. Removal of methylene blue from waste water using micellar enhanced ultrafiltration. Water Res. 2006, 40, 1027–1033. [Google Scholar] [CrossRef]
- Masuelli, M.A. Ultrafiltration of oil/water emulsions using PVDF/PC blend membranes. Desalination Water Treat. 2015, 53, 569–578. [Google Scholar] [CrossRef]
- Masuelli, M.A. Synthesis polysulfone-acetylethanol ultrafiltration membranes. Application to oily wastewater treatment. J. Mater. Phys. Chem. 2013, 1, 37–44. [Google Scholar]




| 2θ | dspacing (Å) |
|---|---|
| 10.88 | 8.09 |
| 21.26 | 4.17 |
| L* | a* | b* | Online Color | Real Color | |
|---|---|---|---|---|---|
| PBn Dry | 59.09 | 0.91 | 23.70 |  |  |
| PBn Wet | 67.23 | 4.45 | 13.33 |  |  |
| RH (%) | ε (%) | σ (MPa) | E (MPa) |
|---|---|---|---|
| 10 | 13.75 | 11.5 | 14.8 |
| 30 | 20.31 | 11.4 | 9.06 |
| 50 | 29.65 | 0.93 | 5.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masuelli, M.A.; Lazo, L.; Becerra, F.; Torres, F.; Illanes, C.O.; Takara, A.; Auad, M.L.; Bercea, M. Physical and Chemical Properties of Pachycymbiola brasiliana Eggshells—From Application to Separative Processes. Processes 2024, 12, 814. https://doi.org/10.3390/pr12040814
Masuelli MA, Lazo L, Becerra F, Torres F, Illanes CO, Takara A, Auad ML, Bercea M. Physical and Chemical Properties of Pachycymbiola brasiliana Eggshells—From Application to Separative Processes. Processes. 2024; 12(4):814. https://doi.org/10.3390/pr12040814
Chicago/Turabian StyleMasuelli, Martin A., Lismet Lazo, Federico Becerra, Fernanda Torres, Cristian O. Illanes, Andres Takara, Maria Lujan Auad, and Maria Bercea. 2024. "Physical and Chemical Properties of Pachycymbiola brasiliana Eggshells—From Application to Separative Processes" Processes 12, no. 4: 814. https://doi.org/10.3390/pr12040814





