Comparison between Conventional Ageing Process in Barrels and a New Rapid Aging Process Based on RSLDE: Analysis of Bioactive Compounds in Spirit Drinks
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Reagents, and Instrumentation
2.2. Materials and Preparation of Samples
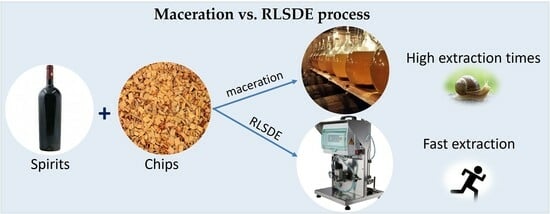
2.3. Maceration vs. RSLDE Techniques
2.4. Analysis of the Extracted Samples
2.4.1. Determination of Dry Residue
2.4.2. Determination of Total Polyphenols Using the Folin–Ciocalteu Method
2.5. HPLC Analysis
2.6. Analysis of a Young Commercial Grappa
2.7. Statistical Analyses
3. Results and Discussion
3.1. Extraction of Model Solutions
3.1.1. Extraction of Nobile® Fresh Chips
3.1.2. Extraction of Nobile® Sweet Chips
3.2. HPLC Analysis
3.3. Aging Tests on Commercial Grappa
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Śliwińska, M.; Wiśniewska, P.; Dymerski, T.; Wardencki, W.; Namieśnik, J. The flavour of fruit spirits and fruit liqueurs: A review. Flavour Fragr. J. 2015, 30, 197–207. [Google Scholar] [CrossRef]
- Tonutti, I.; Liddle, P. Aromatic plants in alcoholic beverages. A review. Flavour Fragr. J. 2010, 25, 341–350. [Google Scholar] [CrossRef]
- Wang, L.; Chen, S.; Xu, Y. Distilled beverage aging: A review on aroma characteristics, maturation mechanisms, and artificial aging techniques. Compr. Rev. Food Sci. Food Saf. 2023, 22, 502–534. [Google Scholar] [CrossRef]
- Christoph, N.; Bauer-Christoph, C. Flavour of spirit drinks: Raw materials, fermentation, distillation, and ageing. In Flavours and Fragrances: Chemistry, Bioprocessing and Sustainability; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–239. [Google Scholar]
- Marianski, S.; Marianski, A. Home Production of Vodkas, Infusions & Liqueurs; Bookmagic LLC: Seminole, FL, USA, 2012. [Google Scholar]
- Conner, J. Maturation. In Whisky and Other Spirits; Academic Press: Cambridge, MA, USA; Elsevier Ltd.: Amsterdam, The Netherlands, 2022; pp. 291–311. [Google Scholar]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine aging technology: Fundamental role of wood barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- Bortoletto, A.M.; Silvello, G.C.; Alcarde, A.R. Aromatic profiling of flavor active compounds in sugarcane spirits aged in tropical wooden barrels. Braz. J. Food Technol. 2021, 24, e2019071. [Google Scholar] [CrossRef]
- Krstić, J.D.; Kostić, S.M.M.; Veljović, S.P. Traditional and innovative aging technologies of distilled beverages: The influence on the quality and consumer preferences of aged spirit drinks. J. Agric. Sci. Belgrade 2021, 66, 209–230. [Google Scholar] [CrossRef]
- Petrozziello, M.; Nardi, T.; Asproudi, A.; Cravero, M.C.; Bonello, F. Chemistry and technology of wine aging with oak chips. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; BoD–Books on Demand: Norderstedt, Germany, 2021; Volume 9. [Google Scholar]
- Rubio-Bretón, P.; Lorenzo, C.; Salinas, M.R.; Martínez, J.; Garde-Cerdán, T. Influence of Oak Barrel Aging on the Quality of Red Wines. In Oak: Ecology, Types and Management; Chuteira, C.A., Grao, A.B., Eds.; Nova Science Publishers: New York, NY, USA, 2013; Volume 2, pp. 59–86. ISBN 978-1-61942-493-7. [Google Scholar]
- Rubio-Bretón, P.; Garde-Cerdán, T.; Martínez, J. Use of oak fragments during the aging of red wines. Effect on the phenolic, aromatic, and sensory composition of wines as a function of the contact time with the wood. Beverages 2018, 4, 102. [Google Scholar] [CrossRef]
- Li, Q.; Xu, H.; Yu, Y.; Zheng, Q. Why does distilled liquor has a soft and harmonious flavor after long-time ageing? A thermodynamic analysis. J. Food Compos. Anal. 2023, 123, 105609. [Google Scholar] [CrossRef]
- Arapitsas, P.; Antonopoulos, A.; Stefanou, E.; Dourtoglou, V.G. Artificial aging of wines using oak chips. Food Chem. 2004, 86, 563–570. [Google Scholar] [CrossRef]
- Caldeira, I.; Anjos, O.; Portal, V.; Belchior, A.P.; Canas, S. Sensory and chemical modifications of wine-brandy aged with chestnut and oak wood fragments in comparison to wooden barrels. Anal. Chim. Acta 2010, 660, 43–52. [Google Scholar] [CrossRef]
- Madrera, R.R.; Valles, B.S.; García, Y.D.; del Valle Argüelles, P.; Lobo, A.P. Alternative woods for aging distillates-an insight into their phenolic profiles and antioxidant activities. Food Sci. Biotechnol. 2010, 19, 1129–1134. [Google Scholar] [CrossRef]
- Jordão, A.M.; Correia, A.C.; DelCampo, R.; González SanJosé, M.L. Antioxidant capacity, scavenger activity, and ellagitannins content from commercial oak pieces used in winemaking. Eur. Food Res. Technol. 2012, 235, 817–825. [Google Scholar] [CrossRef]
- Madrera, R.R.; Hevia, A.G.; Valles, B.S. Comparative study of two aging systems for cider brandy making. Changes in chemical composition. LWT-Food Sci. Technol. 2013, 54, 513–520. [Google Scholar] [CrossRef]
- Taloumi, T.; Makris, D.P. Accelerated aging of the traditional greek distillate tsipouro using wooden chips. part i: Effect of static maceration vs. ultrasonication on the polyphenol extraction and antioxidant activity. Beverages 2017, 3, 5. [Google Scholar] [CrossRef]
- Alañón, M.E.; Marchante, L.; Alarcón, M.; Díaz-Maroto, I.J.; Pérez-Coello, S.; Díaz-Maroto, M.C. Fingerprints of acacia aging treatments by barrels or chips based on volatile profile, sensorial properties, and multivariate analysis. J. Sci. Food Agric. 2018, 98, 5795–5806. [Google Scholar] [CrossRef] [PubMed]
- Dumitriu, G.D.; Teodosiu, C.; Gabur, I.; Cotea, V.V.; Peinado, R.A.; López de Lerma, N. Evaluation of aroma compounds in the process of wine ageing with oak chips. Foods 2019, 8, 662. [Google Scholar] [CrossRef]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2019, 99, 436–448. [Google Scholar] [CrossRef]
- Cerdán, T.G.; Mozaz, S.R.; Azpilicueta, C.A. Volatile composition of aged wine in used barrels of French oak and of American oak. Food Res. Int. 2022, 35, 603–610. [Google Scholar] [CrossRef]
- Krüger, R.T.; Alberti, A.; Nogueira, A. Current technologies to accelerate the aging process of alcoholic beverages: A review. Beverages 2022, 8, 65. [Google Scholar] [CrossRef]
- Solar, S.; Castro, R.; Guerrero, E.D. New accelerating techniques applied to the ageing of oenological products. Food Rev. Int. 2023, 39, 1526–1546. [Google Scholar] [CrossRef]
- Naviglio, D. Naviglio’s principle and presentation of an innovative solid–liquid extraction technology: Extractor Naviglio®. Anal. Lett. 2003, 36, 1647–1659. [Google Scholar] [CrossRef]
- Formato, A.; Gallo, M.; Ianniello, D.; Montesano, D.; Naviglio, D. Supercritical fluid extraction of α-and β-acids from hops compared to cyclically pressurized solid–liquid extraction. J. Supercrit. Fluids 2013, 84, 113–120. [Google Scholar] [CrossRef]
- Naviglio, D.; Formato, A.; Vitulano, M.; Cozzolino, I.; Ferrara, L.; Zanoelo, E.F.; Gallo, M. Comparison between the kinetics of conventional maceration and a cyclic pressurization extraction process for the production of lemon liqueur using a numerical model. J. Food Process Eng. 2017, 40, e12350. [Google Scholar] [CrossRef]
- Naviglio, D.; Scarano, P.; Ciaravolo, M.; Gallo, M. Rapid Solid-Liquid Dynamic Extraction (RSLDE): A powerful and greener alternative to the latest solid-liquid extraction techniques. Foods 2019, 8, 245. [Google Scholar] [CrossRef] [PubMed]
- Ouerghemmi, I.; Rebey, I.B.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Tounsi, M.S. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown Tunisian Ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, M.J.; Dias, C.B.; Freitas, A.M.C. Phenolic acids, phenolic aldehudes and furanic derivatives in oak composition of white wine. S. Afr. J. Enol. Vitic. 2011, 32, 204–210. [Google Scholar]
- Vivas, N.; Bourden-Nonier, M.F.; de Gaulejac, N.V.; Mouche, C.; Rossy, C. Origin and characterisation of the extractable colour of oak heartwood used for ageing spirits. J. Wood Sci. 2020, 66, 21. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Belchior, A.P. Extraction/oxidation kinetics of low molecular weight compounds in wine brandy resulting from different ageing technologies. Food Chem. 2013, 138, 2460–2467. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Rubio, H.; Díaz-Maroto, M.C.; Pérez-Coello, M.S. Monosaccharide anhydrides, new markers of toasted oak wood used for ageing wines and distillates. Food Chem. 2010, 119, 505–512. [Google Scholar] [CrossRef]
- Zamora, F. Barrel aging; types of wood. In Red Wine Technology; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 125–147. [Google Scholar]
- Comuzzo, P.; Battistutta, F. Acidification and pH control in red wines. In Red Wine Technology; Academic Press: Cambridge, MA, USA; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 17–34. [Google Scholar]
- Nunes, I.; Correia, A.C.; Jordão, A.M.; MRicardo-da-Silva, J. Use of oak and cherry wood chips during alcoholic fermentation and the maturation process of rosé wines: Impact on phenolic composition and sensory profile. Molecules 2020, 25, 1236. [Google Scholar] [CrossRef]
- Canas, S.; Belchior, A.P.; Spranger, M.I.; Bruno-de-Sousa, R. High-performance liquid chromatography method for analysis of phenolic acids, phenolic aldehydes, and furanic derivatives in brandies. Development and validation. J. Sep. Sci. 2003, 26, 496–502. [Google Scholar] [CrossRef]
- De Rosso, M.; Cancian, D.; Panighel, A.; Dalla Vedova, A.; Flamini, R. Chemical compounds released from five different woods used to make barrels for aging wines and spirits: Volatile compounds and polyphenols. Wood Sci. Technol. 2009, 43, 375–385. [Google Scholar] [CrossRef]
- Iacumin, L.; Manzano, M.; Cecchini, F.; Orlic, S.; Zironi, R.; Comi, G. Influence of specific fermentation conditions on natural microflora of pomace in “Grappa” production. World J. Microbiol. Biotechnol. 2012, 28, 1747–1759. [Google Scholar] [CrossRef] [PubMed]







| Time, min | Reservoir of Water-Formic Acid (98:2, v/v) | Reservoir of Methanol-Water-Formic Acid (70:28:2 v/v) |
|---|---|---|
| 0 | 90% | 10% |
| 3 | 90% | 10% |
| 25 | 40% | 60% |
| 43 | 40% | 60% |
| 55 | 0% | 100% |
| 65 | 0% | 100% |
| Time, Days | Maceration | RSLDE 49 Cycles (196 min) | RSLDE 268 Cycles (1072 min) | RSLDE 360 Cycles (1440 min) | ||||
|---|---|---|---|---|---|---|---|---|
| Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | |
| 0.005 | --- | --- | 0.62 ± 0.03 | 3.5 | --- | --- | --- | --- |
| 0.734 | --- | --- | --- | --- | 0.75 ± 0.02 | 4.5 | --- | --- |
| 1 | --- | --- | --- | --- | --- | --- | 1.51 ± 0.06 | 8.6 |
| 6 | 0.63 ± 0.07 | --- | --- | --- | 0.85 ± 0.03 | 5.5 | 1.75 ± 0.05 | 10.5 |
| 15 | 1.37 ± 0.02 | 8.2 | 0.61 ± 0.02 | 3.7 | 0.83 ± 0.02 | 5 | 1.77 ± 0.03 | 11.1 |
| 80 | 1.45 ± 0.02 | 8.7 | 0.56 ± 0.04 | 3.4 | 0.98 ± 0.03 | 5.9 | 1.81 ± 0.04 | 12.5 |
| 210 | 1.71 ± 0.03 | 10.3 | 0.68 ± 0.5 | 4.2 | --- | --- | --- | --- |
| Time, Days | Maceration | RSLDE 49 Cycles (196 min) | RSLDE 268 Cycles (1072 min) | RSLDE 360 Cycles (1440 min) |
|---|---|---|---|---|
| 0.005 | --- | 170 ± 5 | --- | --- |
| 0.734 | --- | --- | 368 ± 5 | --- |
| 1 | --- | --- | --- | 440 ± 6 |
| 6 | 257 ± 9 | --- | 380 ± 6 | 480 ± 2 |
| 15 | 510 ± 12 | 176 ± 7 | 402 ± 3 | 510 ± 9 |
| 80 | 426 ± 8 | 168 ± 6 | 446 ± 2 | 553 ± 4 |
| 210 | 593 ± 8 | 187 ± 2 | --- | --- |
| Solutions | pH |
|---|---|
| Hydroalcoholic solution | 6.39 |
| Maceration after 210 days | 3.66 |
| RSLDE 49 cycles after 210 days | 4.44 |
| RSLDE 268 cycles after 80 days | 4.22 |
| Time, Days | Maceration | RSLDE 49 Cycles (196 min) | RSLDE 268 Cycles (1072 min) | RSLDE 360 Cycles (1440 min) | ||||
|---|---|---|---|---|---|---|---|---|
| Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | Dry residue (g/L) | Non-volatile amount extracted (%) | |
| 0.005 | --- | --- | 0.30 ± 0.03 | 1.6 | --- | --- | --- | --- |
| 0.734 | --- | --- | --- | --- | 0.34 ± 0.02 | 2 | --- | --- |
| 1 | --- | --- | --- | --- | --- | --- | 1.2 ± 0.05 | 6.5 |
| 6 | --- | --- | --- | --- | 0.34 ± 0.03 | 2 | 1.3 ± 0.07 | 7.8 |
| 15 | 1.10 ± 0.02 | 6.6 | 0.29 ± 0.03 | 1.7 | 0.58 ± 0.03 | 1.7 | 1.45 ± 0.10 | 8.5 |
| 80 | 1.31 ± 0.02 | 7.9 | 0.40 ± 0.02 | 2.4 | 0.75 ± 0.05 | 4.5 | 1.51 ± 0.09 | 8.9 |
| 210 | 1.38 ± 0.02 | 8.1 | 1.11 ± 0.07 | 6.3 | --- | --- | --- | --- |
| Time, Days | Maceration | RSLDE 49 Cycles (196 min) | RSLDE 268 Cycles (1072 min) | RSLDE 360 Cycles (1440 min) |
|---|---|---|---|---|
| 0.005 | --- | 110 ± 5 | --- | --- |
| 0.734 | --- | --- | 198 ± 3 | --- |
| 1 | --- | --- | 228 ± 4 | 403 ± 8 |
| 6 | --- | --- | 220 ± 5 | 410 ± 5 |
| 15 | 330 ± 10 | 116 ± 6 | 212 ± 4 | 440 ± 4 |
| 80 | 304 ± 6 | 132 ± 8 | ± | 445 ± 7 |
| 210 | 379 ± 8 | 149 ± 3 | --- | --- |
| Solutions | pH |
|---|---|
| Hydroalcoholic solution | 6.39 |
| Maceration after 210 days | 3.63 |
| RSLDE 49 cycles after 210 days | 4.58 |
| RSLDE 268 cycles after 80 days | 4.30 |
| Compounds | Retention Time | Wavelengths | Wavelengths |
|---|---|---|---|
| Furfural | 13.40 ± 1.11 | 233 | 276 |
| Vanillin | 26.14 ± 0.49 | 238 | 280–309 |
| Syringaldehyde | 28.55 ± 0.45 | 238 | 309 |
| Coniferaldehyde | 33.69 ± 0.51 | 243 | 306–342 |
| Sinapaldehyde | 34.89 ± 0.33 | 246 | 347 |
| Ellagic acid | 42.40 ± 2.48 | 252 | 365 |
| Compounds | RSLDE 49 Cycles, % | RSLDE 268 Cycles, % |
|---|---|---|
| Furfural | 36 | 48 |
| Vanillin | 46 | 58 |
| Syringaldehyde | 36 | 54 |
| Coniferaldehyde | 30 | 47 |
| Sinapaldehyde | 48 | 81 |
| Ellagic acid | 30 | 36 |
| Sample | Dry Residue g/L | Polyphenols GAE/L |
|---|---|---|
| Young commercial grappa | 1.69 ± 0.02 | 16 ± 2 |
| RSLDE 1030 cycles with Nobile® Sweet chips | 2.84 ± 0.05 | 483 ± 7 |
| Aged commercial grappa | 6.99 ± 0.07 | 32 ± 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naviglio, D.; Trucillo, P.; Perrone, A.; Montesano, D.; Gallo, M. Comparison between Conventional Ageing Process in Barrels and a New Rapid Aging Process Based on RSLDE: Analysis of Bioactive Compounds in Spirit Drinks. Processes 2024, 12, 829. https://doi.org/10.3390/pr12040829
Naviglio D, Trucillo P, Perrone A, Montesano D, Gallo M. Comparison between Conventional Ageing Process in Barrels and a New Rapid Aging Process Based on RSLDE: Analysis of Bioactive Compounds in Spirit Drinks. Processes. 2024; 12(4):829. https://doi.org/10.3390/pr12040829
Chicago/Turabian StyleNaviglio, Daniele, Paolo Trucillo, Angela Perrone, Domenico Montesano, and Monica Gallo. 2024. "Comparison between Conventional Ageing Process in Barrels and a New Rapid Aging Process Based on RSLDE: Analysis of Bioactive Compounds in Spirit Drinks" Processes 12, no. 4: 829. https://doi.org/10.3390/pr12040829