A Data-Driven Reaction Network for the Fluid Catalytic Cracking of Waste Feeds
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Properties
2.2. Feed Properties
2.3. Reaction Conditions and Product Properties
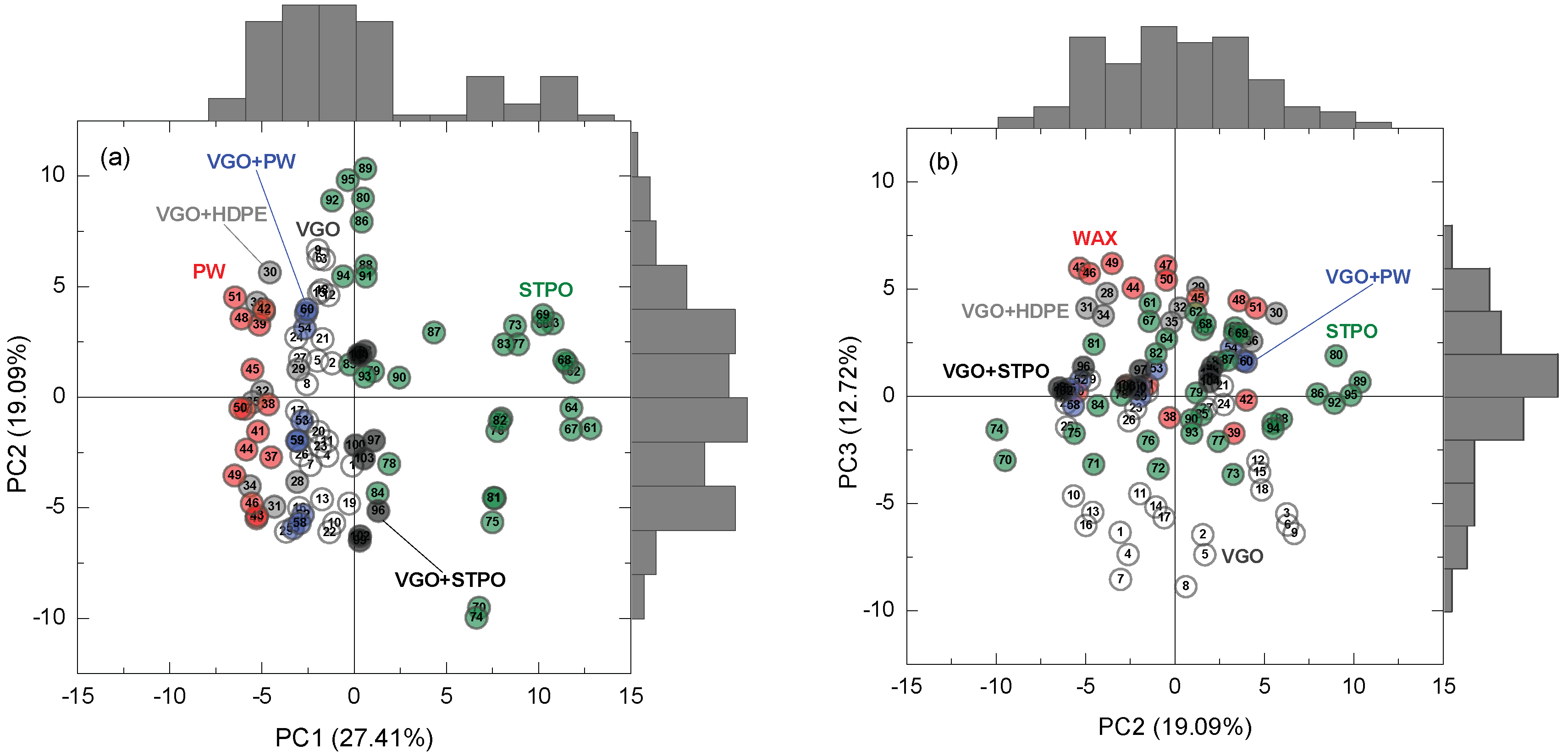
2.4. Principal Component Analysis (PCA)
3. Results
3.1. Properties of the Catalysts and Feeds
3.2. Multivariate Analysis
- Among the feed properties, the lump fraction with more than 20 carbons is the one affecting the most on the yields of cycle oils, whereas the fraction with less than 20 carbons is correlated with the amount and aromatic content of gasoline. The chemical composition of the feed is of lesser relevance than its boiling point distribution.
- Among the process conditions, the key variables are predominantly time and temperature, which are strongly correlated with the amount and olefin content of the lighter fractions (dry and liquified petroleum gases, with attention to propylene).
- Among the catalyst properties, two sets of variables have been obtained: those positively affecting the FCC performance, such as acidity and micropore area, and detrimental variables, such as the content of P, Na, V or Ni.
3.3. Reaction Network
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charpentier, J.C. In the frame of globalization and sustainability, process intensification, a path to the future of chemical and process engineering (molecules into money). Chem. Eng. J. 2007, 134, 84–92. [Google Scholar] [CrossRef]
- Gao, X.; Shang, C.; Jiang, Y.; Huang, D.; Chen, T. Refinery scheduling with varying crude: A deep belief network classification and multimodel approach. AIChE J. 2014, 60, 2525–2532. [Google Scholar] [CrossRef]
- Yang, S.; Wang, N. A P systems based hybrid optimization algorithm for parameter estimation of FCCU reactor-regenerator model. Chem. Eng. J. 2012, 211–212, 508–518. [Google Scholar] [CrossRef]
- Letzsch, W. Fluid Catalytic Cracking (FCC) in Petroleum Refining. In Handbook of Petroleum Processing; Treese, A.S., Pujadó, R.P., Jones, J.D.S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 261–316. ISBN 978-3-319-14529-7. [Google Scholar]
- Chen, Y.M. Recent advances in FCC technology. Powder Technol. 2006, 163, 2–8. [Google Scholar] [CrossRef]
- Fogassy, G.; Thegarid, N.; Schuurman, Y.; Mirodatos, C. The fate of bio-carbon in FCC co-processing products. Green Chem. 2012, 14, 1367–1371. [Google Scholar] [CrossRef]
- Zhang, Y.; Brown, T.R.; Hu, G.; Brown, R.C. Techno-economic analysis of two bio-oil upgrading pathways. Chem. Eng. J. 2013, 225, 895–904. [Google Scholar] [CrossRef]
- Corma, A.; Corresa, E.; Mathieu, Y.; Sauvanaud, L.; Al-Bogami, S.; Al-Ghrami, M.S.; Bourane, A. Crude oil to chemicals: Light olefins from crude oil. Catal. Sci. Technol. 2017, 7, 12–46. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. Fluid catalytic cracking: Recent developments on the grand old lady of zeolite catalysis. Chem. Soc. Rev. 2015, 44, 7342–7370. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Sauvanaud, L. FCC testing at bench scale: New units, new processes, new feeds. Catal. Today 2013, 218–219, 107–114. [Google Scholar] [CrossRef]
- Arandes, J.M.; Torre, I.; Azkoiti, M.J.; Ereña, J.; Olazar, M.; Bilbao, J. HZSM-5 zeolite as catalyst additive for residue cracking under FCC conditions. Energy Fuels 2009, 23, 4215–4223. [Google Scholar] [CrossRef]
- Arandes, J.M.; Torre, I.; Azkoiti, M.J.; Ereña, J.; Bilbao, J. Effect of atmospheric residue incorporation in the fluidized catalytic cracking (FCC) feed on product stream yields and composition. Energy Fuels 2008, 22, 2149–2156. [Google Scholar] [CrossRef]
- García, J.R.; Falco, M.; Sedran, U. Intracrystalline mesoporosity over Y zeolites. Processing of VGO and resid-VGO mixtures in FCC. Catal. Today 2017, 296, 247–253. [Google Scholar] [CrossRef]
- Passamonti, F.J.; Sedran, U. Recycling of waste plastics into fuels. LDPE conversion in FCC. Appl. Catal. B Environ. 2012, 125, 499–506. [Google Scholar] [CrossRef]
- Odjo, A.O.; García, A.N.; Marcilla, A. Conversion of low density polyethylene into fuel through co-processing with vacuum gas oil in a fluid catalytic cracking riser reactor. Fuel Process. Technol. 2013, 113, 130–140. [Google Scholar] [CrossRef]
- Torre, I.; Arandes, J.M.; Castaño, P.; Azkoiti, M.J.; Bilbao, J.; de Lasa, H.I. Catalytic Cracking of Plastic Pyrolysis Waxes with Vacuum Gasoil: Effect of HZSM-5 Zeolite in the FCC Catalyst. Int. J. Chem. React. Eng. 2006, 4. [Google Scholar] [CrossRef]
- Arandes, J.M.; Azkoiti, M.J.; Torre, I.; Olazar, M.; Castaño, P. Effect of HZSM-5 catalyst addition on the cracking of polyolefin pyrolysis waxes under FCC conditions. Chem. Eng. J. 2007, 132, 17–26. [Google Scholar] [CrossRef]
- Arandes, J.M.M.; Torre, I.; Azkoiti, M.J.J.; Castaño, P.; Bilbao, J.; de Lasa, H. Effect of catalyst properties on the cracking of polypropylene pyrolysis waxes under FCC conditions. Catal. Today 2008, 133, 413–419. [Google Scholar] [CrossRef]
- Arandes, J.M.; Torre, I.; Castaño, P.; Olazar, M.; Bilbao, J. Catalytic cracking of waxes produced by the fast pyrolysis of polyolefins. Energy Fuels 2007, 21, 561–569. [Google Scholar] [CrossRef]
- Lovás, P.; Hudec, P.; Jambor, B.; Hájeková, E.; Horňáček, M. Catalytic cracking of heavy fractions from the pyrolysis of waste HDPE and PP. Fuel 2017, 203, 244–252. [Google Scholar] [CrossRef]
- Hita, I.; Rodríguez, E.; Olazar, M.; Bilbao, J.; Arandes, J.M.M.; Castaño, P. Prospects for Obtaining High Quality Fuels from the Hydrocracking of a Hydrotreated Scrap Tires Pyrolysis Oil. Energy Fuels 2015, 29, 5458–5466. [Google Scholar] [CrossRef]
- Hita, I.; Gutiérrez, A.; Olazar, M.; Bilbao, J.; Arandes, J.M.; Castaño, P. Upgrading model compounds and Scrap Tires Pyrolysis Oil (STPO) on hydrotreating NiMo catalysts with tailored supports. Fuel 2015, 145, 158–169. [Google Scholar] [CrossRef]
- Cordero-Lanzac, T.; Hita, I.; Veloso, A.; Arandes, J.M.; Rodríguez-Mirasol, J.; Bilbao, J.; Cordero, T.; Castaño, P. Characterization and controlled combustion of carbonaceous deactivating species deposited on an activated carbon-based catalyst. Chem. Eng. J. 2017, 327, 454–464. [Google Scholar] [CrossRef]
- Errekatxo, A.; Ibarra, A.; Gutierrez, A.; Bilbao, J.; Arandes, J.M.; Castaño, P. Catalytic deactivation pathways during the cracking of glycerol and glycerol/VGO blends under FCC unit conditions. Chem. Eng. J. 2017, 307, 955–965. [Google Scholar] [CrossRef]
- De Pinho, A.R.; de Almeida, M.B.B.; Mendes, F.L.; Ximenes, V.L.; Casavechia, L.C. Co-processing raw bio-oil and gasoil in an FCC Unit. Fuel Process. Technol. 2015, 131, 159–166. [Google Scholar] [CrossRef]
- Ma, W.; Liu, B.; Zhang, R.; Gu, T.; Ji, X.; Zhong, L.; Chen, G.; Ma, L.; Cheng, Z.; Li, X. Co-upgrading of raw bio-oil with kitchen waste oil through fluid catalytic cracking (FCC). Appl. Energy 2018, 217, 233–240. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Li, J. Preparation of biofuels with waste cooking oil by fluid catalytic cracking: The effect of catalyst performance on the products. Renew. Energy 2018, 124, 34–39. [Google Scholar] [CrossRef]
- Ibarra, Á.; Rodríguez, E.; Sedran, U.; Arandes, J.M.; Bilbao, J. Synergy in the Cracking of a Blend of Bio-oil and Vacuum Gasoil under Fluid Catalytic Cracking Conditions. Ind. Eng. Chem. Res. 2016, 55, 1872–1880. [Google Scholar] [CrossRef]
- Bertero, M.; La Puente, G.D.; Sedran, U. Products and coke from the conversion of bio-oil acids, esters, aldehydes and ketones over equilibrium FCC catalysts. Renew. Energy 2013, 60, 349–354. [Google Scholar] [CrossRef]
- Ibarra, A.; Veloso, A.; Bilbao, J.; Arandes, J.M.; Castaño, P. Dual coke deactivation pathways during the catalytic cracking of raw bio-oil and vacuum gasoil in FCC conditions. Appl. Catal. B Environ. 2016, 182, 336–346. [Google Scholar] [CrossRef]
- Bezergianni, P.S.; Dimitriadis, A.; Kikhtyanin, O.; Kubi Cka, D. Refinery co-processing of renewable feeds. Prog. Energy Combust. Sci. 2018, 68, 29–64. [Google Scholar] [CrossRef]
- Feital, T.; Kruger, U.; Dutra, J.; Pinto, J.C.; Lima, E.L. Modeling and performance monitoring of multivariate multimodal processes. AIChE J. 2013, 59, 1557–1569. [Google Scholar] [CrossRef]
- Rato, T.; Reis, M.; Schmitt, E.; Hubert, M.; De Ketelaere, B. A systematic comparison of PCA-based Statistical Process Monitoring methods for high-dimensional, time-dependent Processes. AIChE J. 2016, 62, 1478–1493. [Google Scholar] [CrossRef]
- Tomita, R.K.; Park, S.W.; Sotomayor, O.A.Z. Analysis of activated sludge process using multivariate statistical tools—A PCA approach. Chem. Eng. J. 2002, 90, 283–290. [Google Scholar] [CrossRef]
- Zhang, J.; Martin, E.B.; Morris, A.J. Process monitoring using non-linear statistical techniques. Chem. Eng. J. 1997, 67, 181–189. [Google Scholar] [CrossRef]
- Gregersen, L.; Jørgensen, S.B. Supervision of fed-batch fermentations. Chem. Eng. J. 1999, 75, 69–76. [Google Scholar] [CrossRef]
- Kashani, M.N.; Shahhosseini, S. A methodology for modeling batch reactors using generalized dynamic neural networks. Chem. Eng. J. 2010, 159, 195–202. [Google Scholar] [CrossRef]
- Sjögren, M.; Li, H.; Rannug, U.; Westerholm, R. A multivariate statistical analysis of chemical composition and physical characteristics of ten diesel fuels. Fuel 1995, 74, 983–989. [Google Scholar] [CrossRef]
- Pasadakis, N.; Yiokari, C.; Varotsis, N.; Vayenas, C. Characterization of hydrotreating catalysts using the principal component analysis. Appl. Catal. A Gen. 2001, 207, 333–341. [Google Scholar] [CrossRef]
- Mostad, H.B.; Riis, T.U.; Ellestad, O.H. Use of principal component analysis in catalyst characterization. Catalytic cracking of decalin over Y-zeolites. Appl. Catal. 1990, 64, 119–141. [Google Scholar] [CrossRef]
- Héberger, K.; Németh, A.; Cotarca, L.; Delogu, P. Principal component analysis of data on the catalytic oxidation of toluene. Appl. Catal. A Gen. 1994, 119, L7–L12. [Google Scholar] [CrossRef]
- Aguado, R.; Elordi, G.; Arrizabalaga, A.; Artetxe, M.; Bilbao, J.; Olazar, M. Principal component analysis for kinetic scheme proposal in the thermal pyrolysis of waste HDPE plastics. Chem. Eng. J. 2014, 254, 357–364. [Google Scholar] [CrossRef]
- Aguado, R.; Arrizabalaga, A.; Arabiourrutia, M.; Lopez, G.; Bilbao, J.; Olazar, M. Principal component analysis for kinetic scheme proposal in the thermal and catalytic pyrolysis of waste tyres. Chem. Eng. Sci. 2014, 106, 9–17. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Z.; Liu, Z. Application of Artificial Neural Networks for Catalysis: A Review. Catalysts 2017, 7, 306. [Google Scholar] [CrossRef]
- Cheng, Z.; Yang, B.; Chen, Q.; Gao, X.; Tan, Y.; Ma, Y.; Shen, Z. A Quantitative-Structure-Activity-Relationship (QSAR) model for the reaction rate constants of organic compounds during the ozonation process at different temperatures. Chem. Eng. J. 2018, 353, 288–296. [Google Scholar] [CrossRef]
- Zhang, Y.; Teng, Y. Process data modeling using modified kernel partial least squares. Chem. Eng. Sci. 2010, 65, 6353–6361. [Google Scholar] [CrossRef]
- Tonetto, G.M.; Ferreira, M.L.; Atias, J.A.; De Lasa, H.I. Effect of steaming treatment in the structure and reactivity of FCC catalysts. AIChE J. 2006, 52, 754–768. [Google Scholar] [CrossRef]
- Johnson, M. Estimation of the zeolite content of a catalyst from nitrogen adsorption isotherms. J. Catal. 1978, 52, 425–431. [Google Scholar] [CrossRef]
- Elordi, G.; Olazar, M.; Lopez, G.; Artetxe, M.; Bilbao, J. Product Yields and Compositions in the Continuous Pyrolysis of High-Density Polyethylene in a Conical Spouted Bed Reactor. Ind. Eng. Chem. Res 2011, 50, 6650–6659. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Lopez, G.; Elordi, G.; Olazar, M.; Aguado, R.; Bilbao, J. Product distribution obtained in the pyrolysis of tyres in a conical spouted bed reactor. Chem. Eng. Sci. 2007, 62, 5271–5275. [Google Scholar] [CrossRef]
- Al-Sabawi, M.; De Lasa, H. Kinetic modeling of catalytic conversion of methylcyclohexane over USY zeolites: Adsorption and reaction phenomena. AIChE J. 2009, 55, 1538–1558. [Google Scholar] [CrossRef]
- Anderson, J.E.; Kramer, U.; Mueller, S.A.; Wallington, T.J. Octane Numbers of Ethanol- and Methanol-Gasoline Blends Estimated from Molar Concentrations. Energy Fuels 2010, 24, 6576–6585. [Google Scholar] [CrossRef]
- Arabiourrutia, M.; Olazar, M.; Aguado, R.; López, G.; Barona, A.; Bilbao, J. HZSM-5 and HY Zeolite Catalyst Performance in the Pyrolysis of Tires in a Conical Spouted Bed Reactor. Ind. Eng. Chem. Res. 2008, 47, 7600–7609. [Google Scholar] [CrossRef]
- Olazar, M.; Aguado, R.; Arabiourrutia, M.; Lopez, G.; Barona, A.; Bilbao, J. Catalyst Effect on the Composition of Tire Pyrolysis Products. Energy Fuels 2008, 22, 2909–2916. [Google Scholar] [CrossRef]
- Torre, I.; Arandes, J.M.; Azkoiti, M.J.; Olazar, M.; Bilbao, J. Cracking of coker naphtha with gas-oil. Effect of HZSM-5 zeolite addition to the catalyst. Energy Fuels 2007, 21, 11–18. [Google Scholar] [CrossRef]
- Li, J.; Li, T.; Ma, H.; Sun, Q.; Li, C.; Ying, W.; Fang, D. Kinetics of coupling cracking of butene and pentene on modified HZSM-5 catalyst. Chem. Eng. J. 2018, 346, 397–405. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Wang, B.; Dai, X.; Sun, C.; Bai, Z.; Guo, Q.; Seshan, K. Metals modification of the paraffin dehydrogenation catalyst regenerated by coke combustion. Appl. Catal. A Gen. 2016, 513, 82–88. [Google Scholar] [CrossRef]
- Bhasin, M.M.; McCain, J.H.; Vora, B.V.; Imai, T.; Pujadó, P.R. Dehydrogenation and oxydehydrogenation of paraffins to olefins. Appl. Catal. A Gen. 2001, 221, 397–419. [Google Scholar] [CrossRef]
- Van Borm, R.; Reyniers, M.-F.; Marin, G.B. Catalytic cracking of alkanes on FAU: Single-event microkinetic modeling including acidity descriptors. AIChE J. 2012, 58, 2202–2215. [Google Scholar] [CrossRef]
- Gutiérrez, A.; Arandes, J.M.; Castaño, P.; Olazar, M.; Bilbao, J. Preliminary studies on fuel production through LCO hydrocracking on noble-metal supported catalysts. Fuel 2012, 94, 504–515. [Google Scholar] [CrossRef]
- Epelde, E.; Ibañez, M.; Aguayo, A.T.; Gayubo, A.G.; Bilbao, J.; Castaño, P. Differences among the deactivation pathway of HZSM-5 zeolite and SAPO-34 in the transformation of ethylene or 1-butene to propylene. Microporous Mesoporous Mater. 2014, 195, 284–293. [Google Scholar] [CrossRef]




| Var. | Name | Units | Var. | Name | Units |
|---|---|---|---|---|---|
| Catalysts properties | LPG | Yield of LPGb (C3–C4) | wt % | ||
| ST | BETa surface area | m2 g−1 | GL | Yield of gasoline (C5–C12) | wt % |
| Sm | Micropore area | m2 g−1 | iGL | Gasoline identified | wt % |
| SM | Matrix or mesopore area | m2 g−1 | LCO | Yield of light cycle oil (C13–C20) | wt % |
| Z | Zeolite percentage | wt % | HCO | Yield of heavy cycle oil (C20+) | wt % |
| Vm | Micropore volume | cm3 g−1 | Ck | Coke content on the catalyst | wt % |
| mV | Mean pore volume | cm3 g−1 | RON | Octane number (GL) | |
| Dc | Zeolite cell unit size | Å | A6 | Benzene | wt % |
| Cu | Content of Cu | ppm | A7 | Toluene | wt % |
| Ni | Content of Ni | ppm | A8 | Xylenes | wt % |
| V | Content of V | ppm | A9 | Aromatics with 9 carbons | wt % |
| Fe | Content of Fe | wt % | A10+ | Aromatics with 10 carbons | wt % |
| Na | Content of Na | wt % | AT | Total aromatics | wt % |
| Al | Content of Al2O3 | wt % | NT | Total naphthenes | wt % |
| Re | Content of rare earths Re2O3 | wt % | P3 | Propane | wt % |
| P | Content of P2O5 | wt % | P4 | Butane | wt % |
| Ac | Total acidity | mmol g−1 | PT | Total paraffins | wt % |
| BL | Brönsted-Lewis acid site ratio | mol mol−1 | O3 | Propylene | wt % |
| Feed properties | O4 | Butenes | wt % | ||
| Pf | Paraffins | wt % | O5 | Pentenes | wt % |
| Of | Olefins | wt % | OT | Total olefins | wt % |
| Nf | Naphthenes | wt % | IT | Total isoparaffins | wt % |
| Af | Aromatics | wt % | C3 | C3 hydrocarbons | wt % |
| Sf | Sulfur | wt % | C4 | C4 hydrocarbons | wt % |
| Gf | Gasoline (C5–C12) | wt % | C5 | C5 hydrocarbons | wt % |
| Lf | Light cycle oil (C13–C20) | wt % | C6 | C6 hydrocarbons | wt % |
| Hf | Heavy cycle oil (C20+) | wt % | C7 | C7 hydrocarbons | wt % |
| Reaction conditions | C8 | C8 hydrocarbons | wt % | ||
| CO | Catalyst-to-oil ratio | g g−1 | C9 | C9 hydrocarbons | wt % |
| t | Contact time | s | C10 | C10+ hydrocarbons | wt % |
| T | Temperature | °C | CT | Total hydrocarbons | wt % |
| Product properties | iOT | Total isoolefins | wt % | ||
| X | Conversion | wt % | nOT | Total linear olefins | wt % |
| LG | Yield of light gases (C1–C2) | wt % | cOT | Total cycloolefins | wt % |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvira, J.I.; Hita, I.; Rodríguez, E.; Arandes, J.M.; Castaño, P. A Data-Driven Reaction Network for the Fluid Catalytic Cracking of Waste Feeds. Processes 2018, 6, 243. https://doi.org/10.3390/pr6120243
Alvira JI, Hita I, Rodríguez E, Arandes JM, Castaño P. A Data-Driven Reaction Network for the Fluid Catalytic Cracking of Waste Feeds. Processes. 2018; 6(12):243. https://doi.org/10.3390/pr6120243
Chicago/Turabian StyleAlvira, José Ignacio, Idoia Hita, Elena Rodríguez, José M. Arandes, and Pedro Castaño. 2018. "A Data-Driven Reaction Network for the Fluid Catalytic Cracking of Waste Feeds" Processes 6, no. 12: 243. https://doi.org/10.3390/pr6120243
APA StyleAlvira, J. I., Hita, I., Rodríguez, E., Arandes, J. M., & Castaño, P. (2018). A Data-Driven Reaction Network for the Fluid Catalytic Cracking of Waste Feeds. Processes, 6(12), 243. https://doi.org/10.3390/pr6120243








