Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of GO, MnO2, and GO-MnO2
2.3. Characterizations
2.4. Degradation Activity
2.4.1. Effect of pH
2.4.2. Effect of Ionic Strength
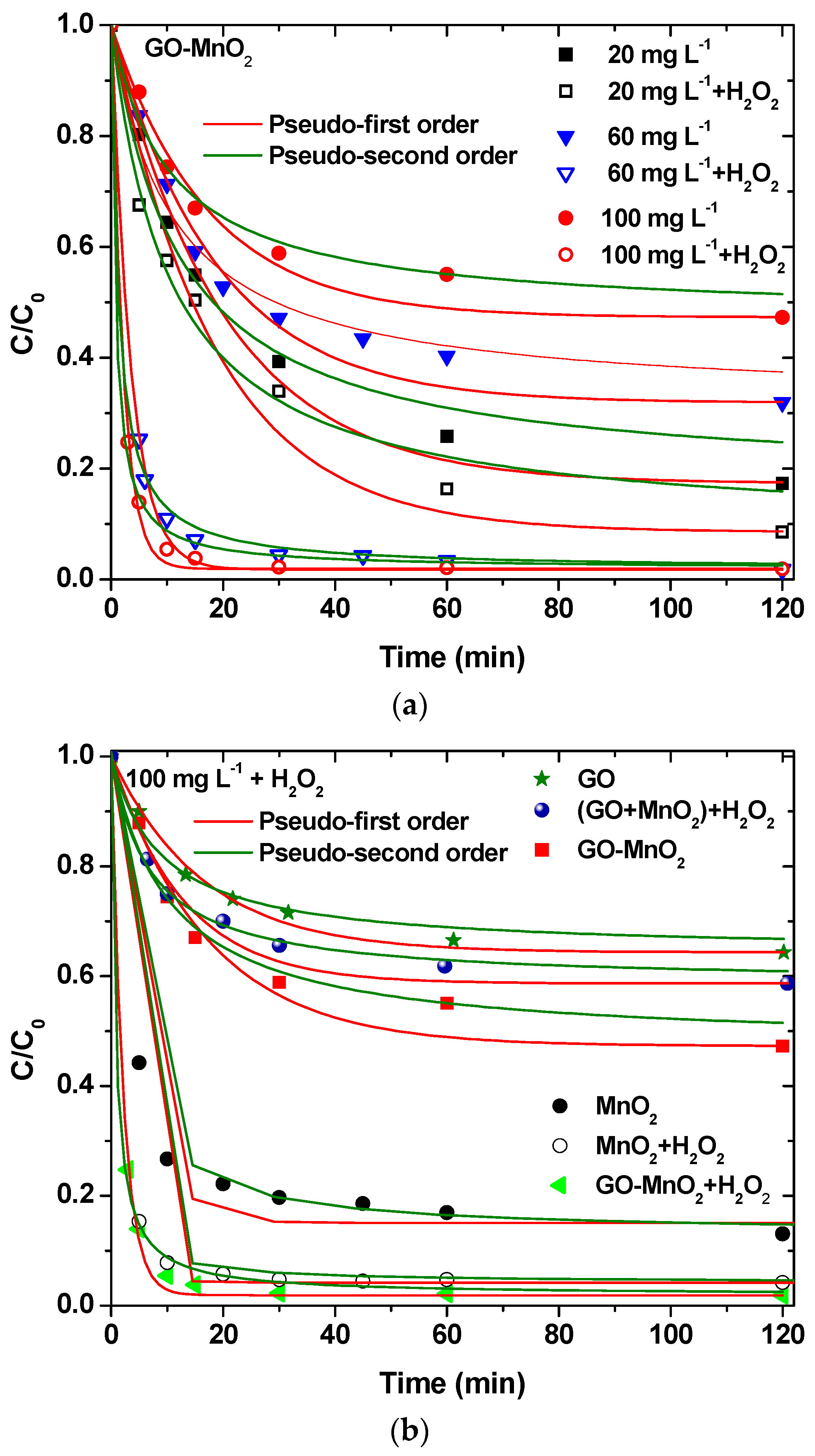
2.4.3. Kinetics
3. Results and Discussion
3.1. Characterizations
3.2. Dye Degradation
3.2.1. Effect of pH and Ionic Strength
3.2.2. Effect of Contact Time—Effect of H2O2
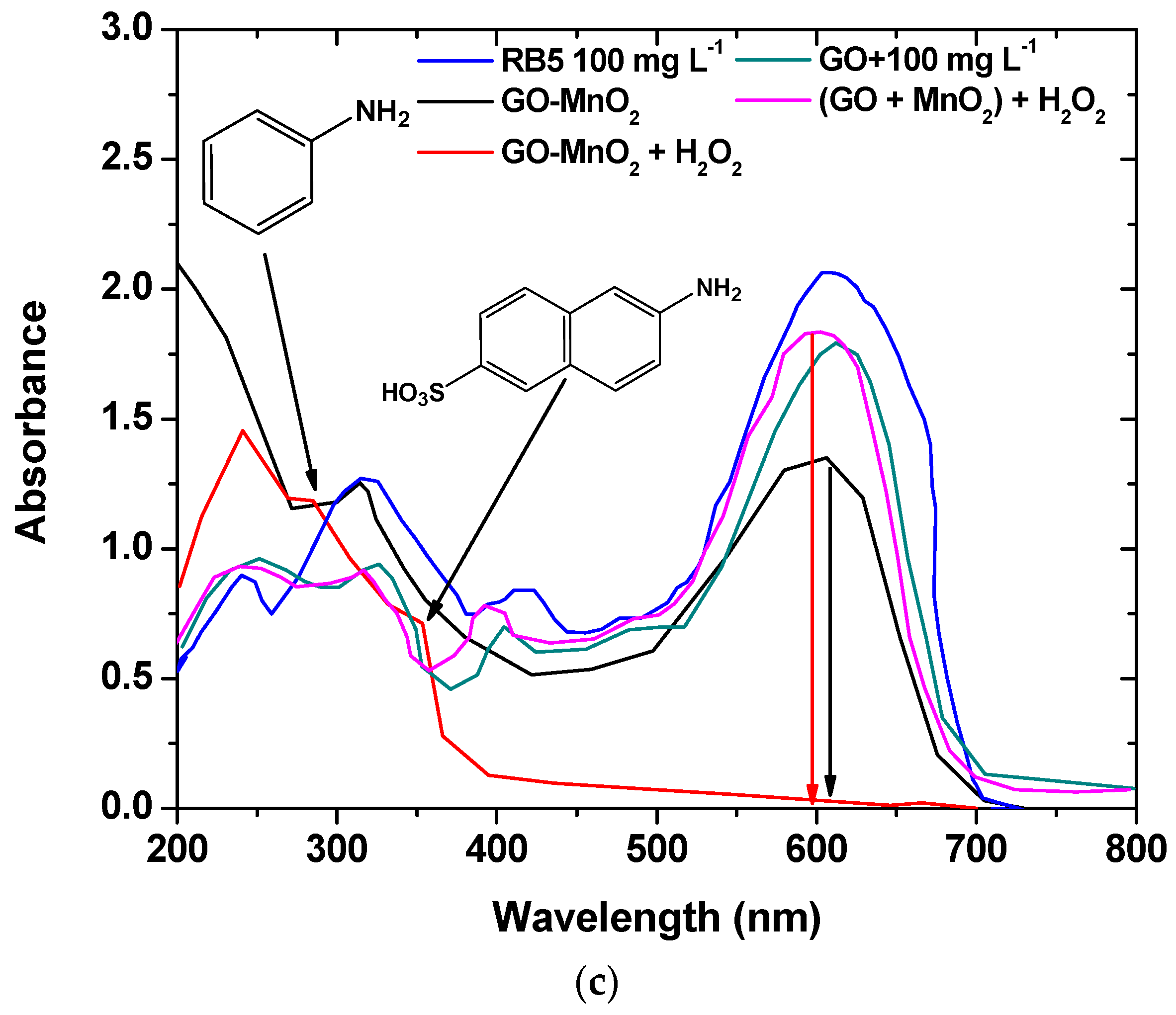
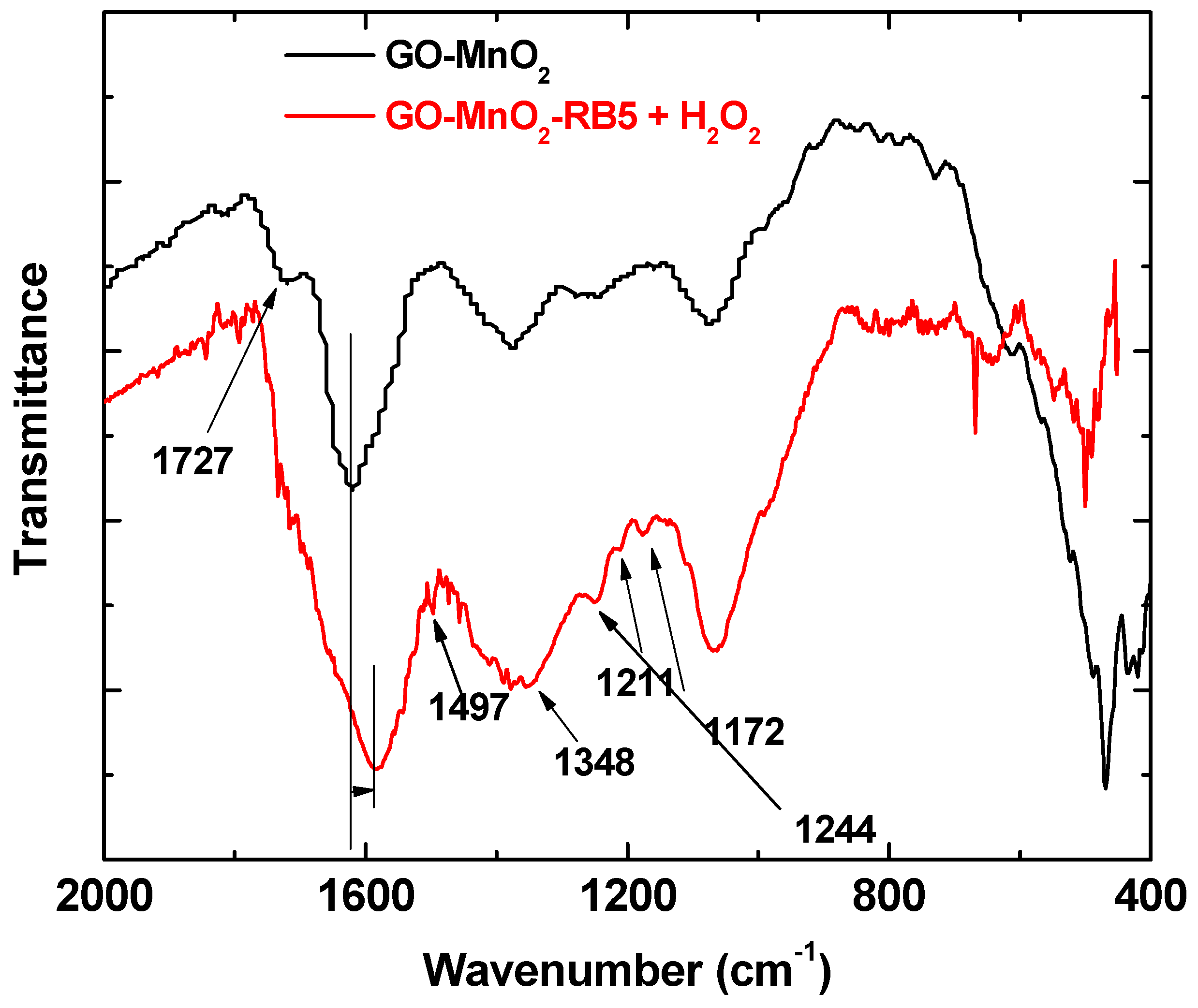
3.3. Analysis of Surface Features
3.4. Degradation Mechanism
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Przystas, W.; Zablocka-Godlewska, E.; Grabinska-Sota, E. Biological removal of azo and triphenylmethane dyes and toxicity of process by-products. Water Air Soil Pollut. 2012, 223, 1581–1592. [Google Scholar] [CrossRef] [PubMed]
- Chetima, A.; Wahabou, A.; Zomegni, G.; Rahman, A.N.; Nde, D.B. Bleaching of neutral cotton seed oil using organic activated carbon in a batch system: Kinetics and adsorption isotherms. Processes 2018, 6, 22. [Google Scholar] [CrossRef]
- Di Marcoberardino, G.; Vitali, D.; Spinelli, F.; Binotti, M.; Manzolini, G. Green hydrogen production from raw biogas: A techno-economic investigation of conventional processes using pressure swing adsorption unit. Processes 2018, 6, 19. [Google Scholar] [CrossRef]
- Yang, F.; Chen, S.; Shi, C.; Xue, F.; Zhang, X.; Ju, S.; Xing, W. A facile synthesis of hexagonal spinel λ-MnO2 ion-sieves for highly selective Li+ adsorption. Processes 2018, 6, 59. [Google Scholar] [CrossRef]
- You, Z.; Zhang, L.; Zhang, S.; Sun, Y.; Shah, K. Treatment of oil-contaminated water by modified polysilicate aluminum ferric sulfate. Processes 2018, 6, 95. [Google Scholar] [CrossRef]
- Bian, Y.; Xiong, N.; Zhu, G. Technology for the remediation of water pollution: A review on the fabrication of metal organic frameworks. Processes 2018, 6, 122. [Google Scholar] [CrossRef]
- Bafana, A.; Devi, S.S.; Chakrabarti, T. Azo dyes: Past, present and the future. Environ. Rev. 2011, 19, 350–370. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Nayak, A.; Agarwal, S.; Shrivastava, M. Removal of the hazardous dye-tartrazine by photodegradation on titanium dioxide surface. Mater. Sci. Eng. C 2011, 31, 1062–1067. [Google Scholar] [CrossRef]
- Vijayaraghavan, J.; Sardhar Basha, S.J.; Jegan, J. A review on efficacious methods to decolorize reactive azo dye. J. Urban Environ. Eng. 2013, 7, 30–47. [Google Scholar] [CrossRef]
- Han, F.; Kambala, V.S.R.; Srinivasan, M.; Rajarathnam, D.; Naidu, R. Tailored titanium dioxide photocatalysts for the degradation of organic dyes in wastewater treatment: A review. Appl. Catal. A Gen. 2009, 359, 25–40. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 photocatalysis: Design and applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Rauf, M.A.; Ashraf, S.S. Fundamental principles and application of heterogeneous photocatalytic degradation of dyes in solution. Chem. Eng. J. 2009, 151, 10–18. [Google Scholar] [CrossRef]
- Yin, B.; Zhang, S.; Jiao, Y.; Liu, Y.; Qu, F.; Wu, X. Facile synthesis of ultralong MnO2 nanowires as high performance supercapacitor electrodes and photocatalysts with enhanced photocatalytic activities. CrystEngComm 2014, 16, 9999–10005. [Google Scholar] [CrossRef]
- Caliskan, N.; Kul, A.R.; Alkan, S.; Sogut, E.G.; Alacabey, İ. Adsorption of Zinc(II) on diatomite and manganese-oxide-modified diatomite: A kinetic and equilibrium study. J. Hazard. Mater. 2011, 193, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Zhang, L.; Niu, Y. Fabrication of the magnetic manganese dioxide/graphene nanocomposite and its application in dye removal from the aqueous solution at room temperature. Mater. Res. Bull. 2015, 70, 82–86. [Google Scholar] [CrossRef]
- Tang, J.; Mu, B.; Wang, W.; Zheng, M.; Wang, A. Fabrication of manganese dioxide/carbon/attapulgite composites derived from spent bleaching earth for adsorption of Pb(II) and brilliant green. RSC Adv. 2016, 6, 36534–36543. [Google Scholar] [CrossRef]
- Debnath, B.; Roy, A.S.; Kapri, S.; Bhattacharyya, S. Efficient dye degradation catalyzed by manganese oxide nanoparticles and the role of cation valence. ChemistrySelect 2016, 1, 4265–4273. [Google Scholar] [CrossRef]
- Chacón-Patiño, M.L.; Blanco-Tirado, C.; Hinestroza, J.P.; Combariza, M.Y. Biocomposite of nanostructured MnO2 and fique fibers for efficient dye degradation. Green Chem. 2013, 15, 2920–2928. [Google Scholar] [CrossRef]
- Das, S.; Samanta, A.; Jana, S. Light-assisted synthesis of hierarchical flower-like MnO2 nanocomposites with solar light induced enhanced photocatalytic activity. ACS Sustain. Chem. Eng. 2017, 5, 9086–9094. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Guo, X.L.; Huang, M.; Hao, X.D.; Yuan, Y.; Hua, C. Engineering birnessite-type MnO2 nanosheets on fiberglass for pH-dependent degradation of methylene blue. J. Phys. Chem. Solids 2015, 83, 40–46. [Google Scholar] [CrossRef]
- Vandenbroucke, A.M.; Mora, M.; Jiménez-Sanchidrián, C.; Romero-Salguero, F.J.; De Geyter, N.; Leys, C.; Morent, R. Tce abatement with a plasma-catalytic combined system using MnO2 as catalyst. Appl. Catal. B Environ. 2014, 156–157, 94–100. [Google Scholar] [CrossRef]
- Liang, H.; Sun, H.; Patel, A.; Shukla, P.; Zhu, Z.H.; Wang, S. Excellent performance of mesoporous Co3O4/MnO2 nanoparticles in heterogeneous activation of peroxymonosulfate for phenol degradation in aqueous solutions. Appl. Catal. B Environ. 2012, 127, 330–335. [Google Scholar] [CrossRef]
- Adán-Más, A.; Wei, D. Photoelectrochemical properties of graphene and its derivatives. Nanomaterials 2013, 3, 325–356. [Google Scholar] [CrossRef] [PubMed]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Krishnamoorthy, K.; Veerapandian, M.; Yun, K.; Kim, S.J. The chemical and structural analysis of graphene oxide with different degrees of oxidation. Carbon 2013, 53, 38–49. [Google Scholar] [CrossRef]
- Yu, J.; Huang, H.; Gan, Y.; Xia, Y.; Liang, C.; Zhang, J.; Tao, X.; Zhang, W. A new strategy for the construction of 3D TiO2 nanowires/reduced graphene oxide for high-performance lithium/sodium batteries. J. Mater. Chem. A 2018, 6, 24256–24266. [Google Scholar] [CrossRef]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently cross-linked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef]
- Cseri, L.; Baugh, J.; Alabi, A.; AlHajaj, A.; Zou, L.; Dryfe, R.A.W.; Budd, P.M.; Szekely, G. Graphene oxide–polybenzimidazolium nanocomposite anion exchange membranes for electrodialysis. J. Mater. Chem. A 2018, 6, 24728–24739. [Google Scholar] [CrossRef]
- Chen, W.; Li, D.; Tian, L.; Xiang, W.; Wang, T.; Hu, W.; Hu, Y.; Chen, S.; Chen, J.; Dai, Z. Synthesis of graphene quantum dots from natural polymer starch for cell imaging. Green Chem. 2018, 20, 4438–4442. [Google Scholar] [CrossRef]
- He, Y.; Chen, W.; Li, X.; Zhang, Z.; Fu, J.; Zhao, C.; Xie, E. Freestanding three-dimensional graphene/MnO2 composite networks as ultralight and flexible supercapacitor electrodes. ACS Nano 2013, 7, 174–182. [Google Scholar] [CrossRef]
- Hu, M.; Hui, K.S.; Hui, K.N. Role of graphene in MnO2/graphene composite for catalytic ozonation of gaseous toluene. Chem. Eng. J. 2014, 254, 237–244. [Google Scholar] [CrossRef]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Ma, S.-B.; Ahn, K.-Y.; Lee, E.-S.; Oh, K.-H.; Kim, K.-B. Synthesis and characterization of manganese dioxide spontaneously coated on carbon nanotubes. Carbon 2007, 45, 375–382. [Google Scholar] [CrossRef]
- Ma, L.; Shen, X.; Ji, Z.; Zhu, G.; Zhou, H. Ag nanoparticles decorated MnO2/reduced graphene oxide as advanced electrode materials for supercapacitors. Chem. Eng. J. 2014, 252, 95–103. [Google Scholar] [CrossRef]
- Vieira, R.S.; Beppu, M.M. Interaction of natural and crosslinked chitosan membranes with Hg(II) ions. Colloid Surf. A 2006, 279, 196–207. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Handlingar 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; Ng, J.C.Y.; McKay, G. Kinetics of pollutant sorption by biosorbents: Review. Sep. Purif. Methods 2000, 29, 189–232. [Google Scholar] [CrossRef]
- Kim, I.-T.; Kouda, N.; Yoshimoto, N.; Morita, M. Preparation and electrochemical analysis of electrodeposited MnO2/C composite for advanced capacitor electrode. J. Power Sources 2015, 298, 123–129. [Google Scholar] [CrossRef]
- Liu, S.; Li, H.; Yan, L. Synthesis and photocatalytic activity of three-dimensional ZnS/CdS composites. Mater. Res. Bull. 2013, 48, 3328–3334. [Google Scholar] [CrossRef]
- Zhu, H.T.; Luo, J.; Yang, H.X.; Liang, J.K.; Rao, G.H.; Li, J.B.; Du, Z.M. Birnessite-type MnO2 nanowalls and their magnetic properties. J. Phys. Chem. C 2008, 112, 17089–17094. [Google Scholar] [CrossRef]
- Wu, Z.-S.; Ren, W.; Wang, D.-W.; Li, F.; Liu, B.; Cheng, H.-M. High-energy MnO2 nanowire/graphene and graphene asymmetric electrochemical capacitors. ACS Nano 2010, 4, 5835–5842. [Google Scholar] [CrossRef]
- Qu, J.; Shi, L.; He, C.; Gao, F.; Li, B.; Zhou, Q.; Hu, H.; Shao, G.; Wang, X.; Qiu, J. Highly efficient synthesis of graphene/MnO2 hybrids and their application for ultrafast oxidative decomposition of methylene blue. Carbon 2014, 66, 485–492. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Evaluation of GO/MnO2 composites as supercapacitors in neutral electrolytes: Role of graphite oxide oxidation level. J. Mater. Chem. 2012, 22, 23525–23533. [Google Scholar] [CrossRef]
- Tian, H.; He, J.; Liu, L.; Wang, D.; Hao, Z.; Ma, C. Highly active manganese oxide catalysts for low-temperature oxidation of formaldehyde. Micropor. Mesopor. Mater. 2012, 151, 397–402. [Google Scholar] [CrossRef] [Green Version]
- Jeong, Y.U.; Manthiram, A. Nanocrystalline manganese oxides for electrochemical capacitors with neutral electrolytes. J. Electrochem. Soc. India 2002, 149, A1419–A1422. [Google Scholar] [CrossRef]
- Seredych, M.; Bandosz, T.J. Manganese oxide and graphite oxide/MnO2 composites as reactive adsorbents of ammonia at ambient conditions. Micropor. Mesopor. Mater. 2012, 150, 55–63. [Google Scholar] [CrossRef]
- Feng, Q.; Kanoh, H.; Miyai, Y.; Ooi, K. Metal ion extraction/insertion reactions with todorokite-type manganese oxide in the aqueous phase. Chem. Mater. 1995, 7, 1722–1727. [Google Scholar] [CrossRef]
- Oba, M.; Oaki, Y.; Imai, H. A microbial-mineralization-inspired approach for synthesis of manganese oxide nanostructures with controlled oxidation states and morphologies. Adv. Funct. Mater. 2010, 20, 4279–4286. [Google Scholar] [CrossRef]
- Lin, S.-S.; Gurol, M.D. Catalytic decomposition of hydrogen peroxide on iron oxide: Kinetics, mechanism, and implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Xu, L.; Xu, C.; Zhao, M.; Qiu, Y.; Sheng, G.D. Oxidative removal of aqueous steroid estrogens by manganese oxides. Water Res. 2008, 42, 5038–5044. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.; Boroski, M.; da Silva, A.; Oliviera, J.; Nozaki, J.; Barreto, W. Solar and Trends in Solar Energy Research; Hough, T.P., Ed.; Nova Science Publishers: New York, NY, USA, 2006; pp. 107–132. [Google Scholar]
- Karadag, D.; Turan, M.; Akgul, E.; Tok, S.; Faki, A. Adsorption equilibrium and kinetics of Reactive Black 5 and Reactive Red 239 in aqueous solution onto surfactant-modified zeolite. J. Chem. Eng. Data 2007, 52, 1615–1620. [Google Scholar] [CrossRef]
- Bautista-Toledo, M.I.; Rivera-Utrilla, J.; Ocampo-Pérez, R.; Carrasco-Marín, F.; Sánchez-Polo, M. Cooperative adsorption of bisphenol-A and chromium(III) ions from water on activated carbons prepared from olive-mill waste. Carbon 2014, 73, 338–350. [Google Scholar] [CrossRef]
- Wolfenden, B.S.; Willson, R.L. Radical-cations as reference chromogens in kinetic studies of ono-electron transfer reactions: Pulse radiolysis studies of 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate). J. Chem. Soc. Perkin Trans. 1982, 2, 805–812. [Google Scholar] [CrossRef]
- Cao, J.; Wei, L.; Huang, Q.; Wang, L.; Han, S. Reducing degradation of azo dye by zero-valent iron in aqueous solution. Chemosphere 1999, 38, 565–571. [Google Scholar] [CrossRef]
- Nam, S.; Tratnyek, P.G. Reduction of azo dyes with zero-valent iron. Water Res. 2000, 34, 1837–1845. [Google Scholar] [CrossRef]
- Feng, W.; Nansheng, D.; Helin, H. Degradation mechanism of azo dye C.I. Reactive red 2 by iron powder reduction and photooxidation in aqueous solutions. Chemosphere 2000, 41, 1233–1238. [Google Scholar] [CrossRef]
- Stylidi, M.; Kondarides, D.I.; Verykios, X.E. Pathways of solar light-induced photocatalytic degradation of azo dyes in aqueous TiO2 suspensions. Appl. Catal. B Environ. 2003, 40, 271–286. [Google Scholar] [CrossRef]
- Bradu, C.; Frunza, L.; Mihalche, N.; Avramescu, S.-M.; Neaţă, M.; Udrea, I. Removal of Reactive Black 5 azo dye from aqueous solutions by catalytic oxidation using CuO/Al2O3 and NiO/Al2O3. Appl. Catal. B Environ. 2010, 96, 548–556. [Google Scholar] [CrossRef]
- Méndez-Martínez, A.J.; Dávila-Jiménez, M.M.; Ornelas-Dávila, O.; Elizalde-González, M.P.; Arroyo-Abad, U.; Sirés, I.; Brillas, E. Electrochemical reduction and oxidation pathways for Reactive Black 5 dye using nickel electrodes in divided and undivided cells. Electrochim. Acta 2012, 59, 140–149. [Google Scholar] [CrossRef]
- Zhang, M.; Lei, D.; Du, Z.; Yin, X.; Chen, L.; Li, Q.; Wang, Y.; Wang, T. Fast synthesis of SnO2/graphene composites by reducing graphene oxide with stannous ions. J. Mater. Chem. 2011, 21, 1673–1676. [Google Scholar] [CrossRef]
- Ben Mbarek, W.; Azabou, M.; Pineda, E.; Fiol, N.; Escoda, L.; Suñol, J.J.; Khitouni, M. Rapid degradation of azo-dye using Mn-Al powders produced by ball-milling. RSC Adv. 2017, 7, 12620–12628. [Google Scholar] [CrossRef]
- Liang, S.; Teng, F.; Bulgan, G.; Zong, R.; Zhu, Y. Effect of phase structure of MnO2 nanorod catalyst on the activity for CO oxidation. J. Phys. Chem. C 2008, 112, 5307–5315. [Google Scholar] [CrossRef]
- Vázquez-Olmos, A.; Redón, R.; Rodríguez-Gattorno, G.; Esther Mata-Zamora, M.; Morales-Leal, F.; Fernández-Osorio, A.L.; Saniger, J.M. One-step synthesis of Mn3O4 nanoparticles: Structural and magnetic study. J. Colloid Interface Sci. 2005, 291, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Bele, S.; Samanidou, V.; Deliyanni, E. Effect of the reduction degree of graphene oxide on the adsorption of bisphenol A. Chem. Eng. Res. Des. 2016, 109, 573–585. [Google Scholar] [CrossRef]
- Li, Y.; Qu, J.; Gao, F.; Lv, S.; Shi, L.; He, C.; Sun, J. In situ fabrication of Mn3O4 decorated graphene oxide as a synergistic catalyst for degradation of methylene blue. Appl. Catal. B Environ. 2015, 162, 268–274. [Google Scholar] [CrossRef]
- Travlou, N.A.; Kyzas, G.Z.; Lazaridis, N.K.; Deliyanni, E.A. Graphite oxide/chitosan composite for reactive dye removal. Chem. Eng. J. 2013, 217, 256–265. [Google Scholar] [CrossRef]
- Ramesh, M.; Nagaraja, H.S.; Rao, M.P.; Anandan, S.; Huang, N.M. Fabrication, characterization and catalytic activity of α-MnO2 nanowires for dye degradation of Reactive Black 5. Mater. Lett. 2016, 172, 85–89. [Google Scholar] [CrossRef]
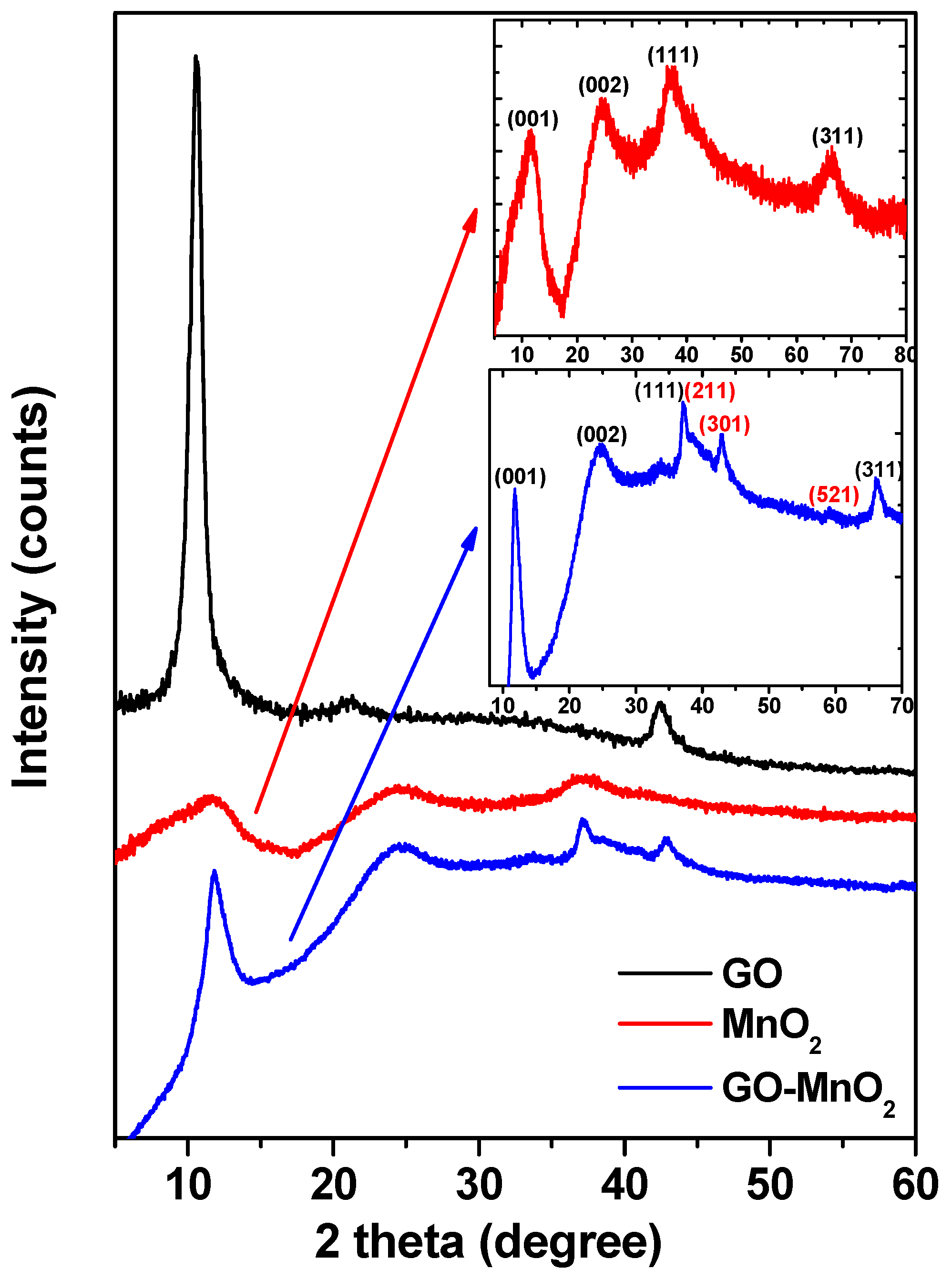

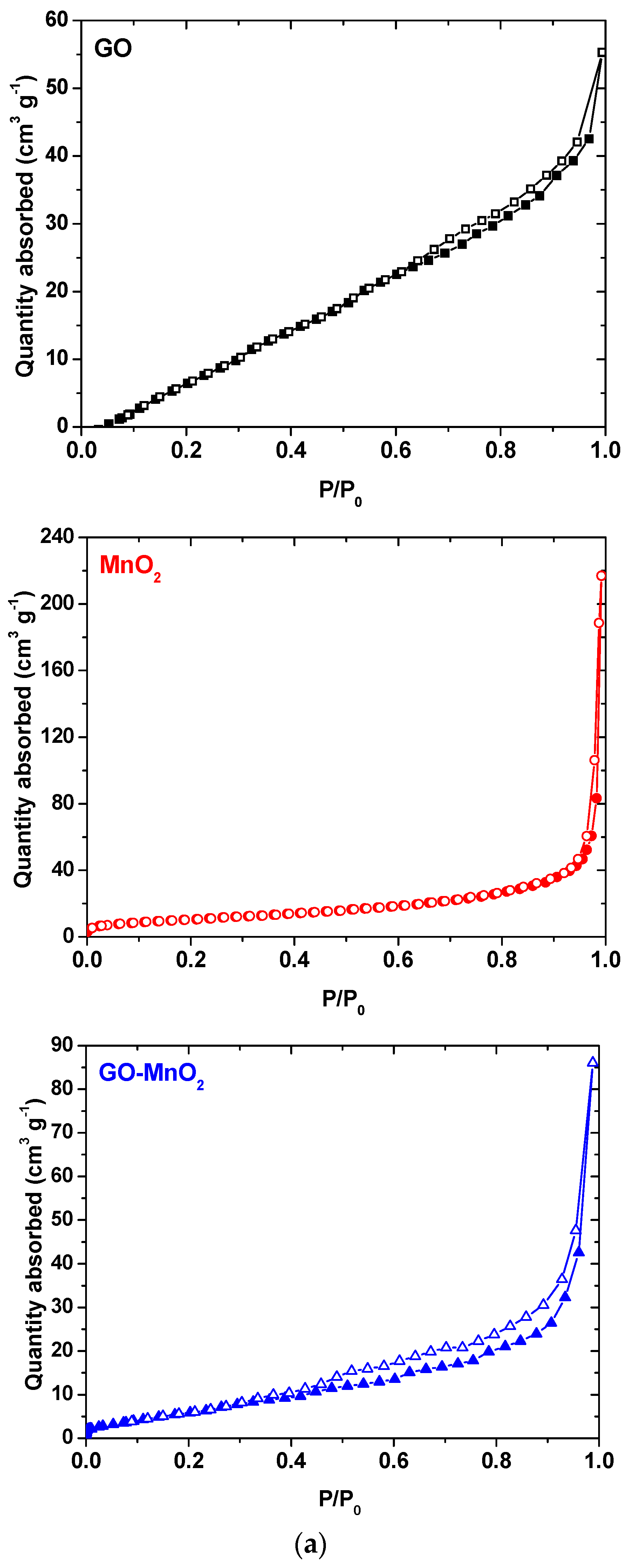
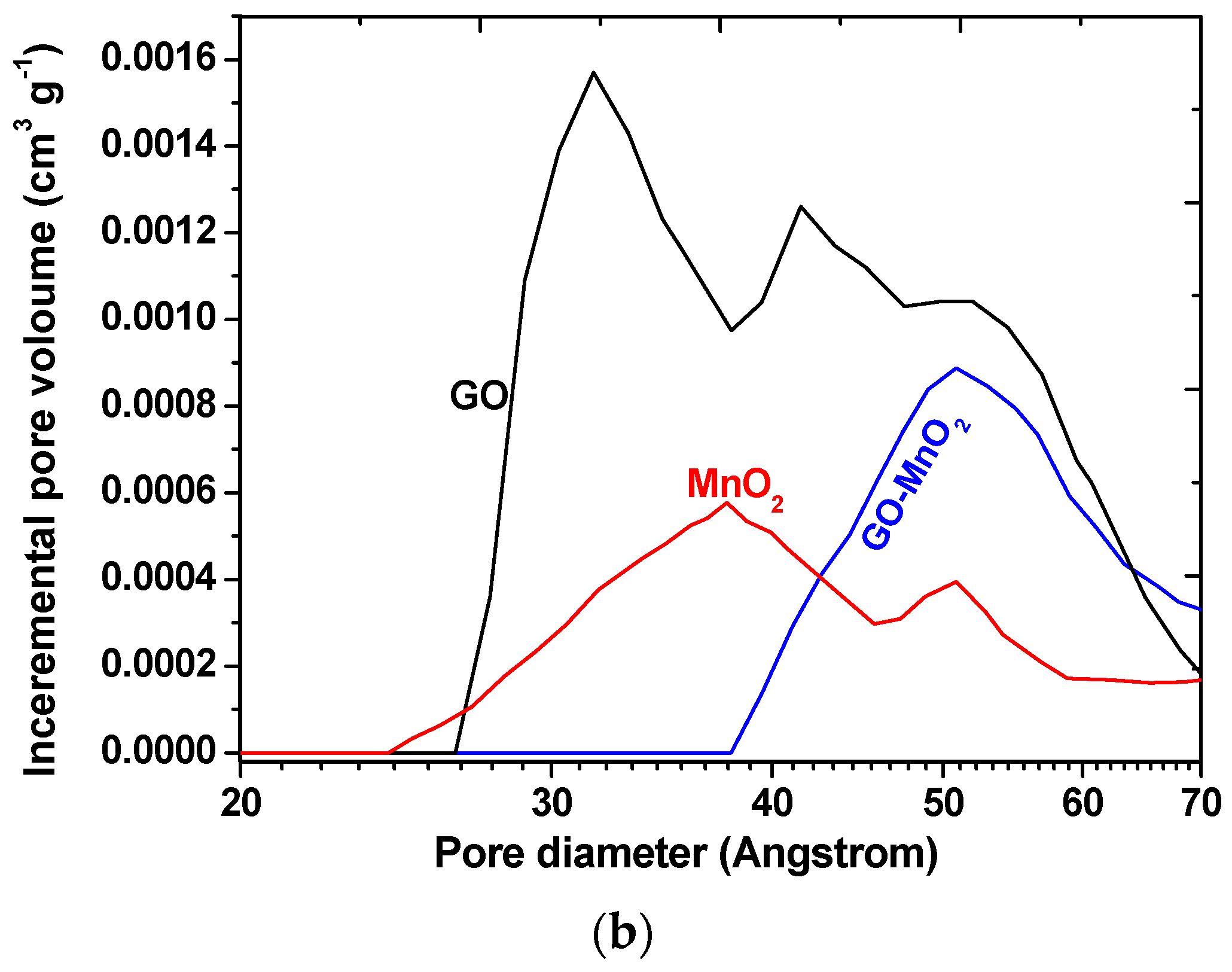
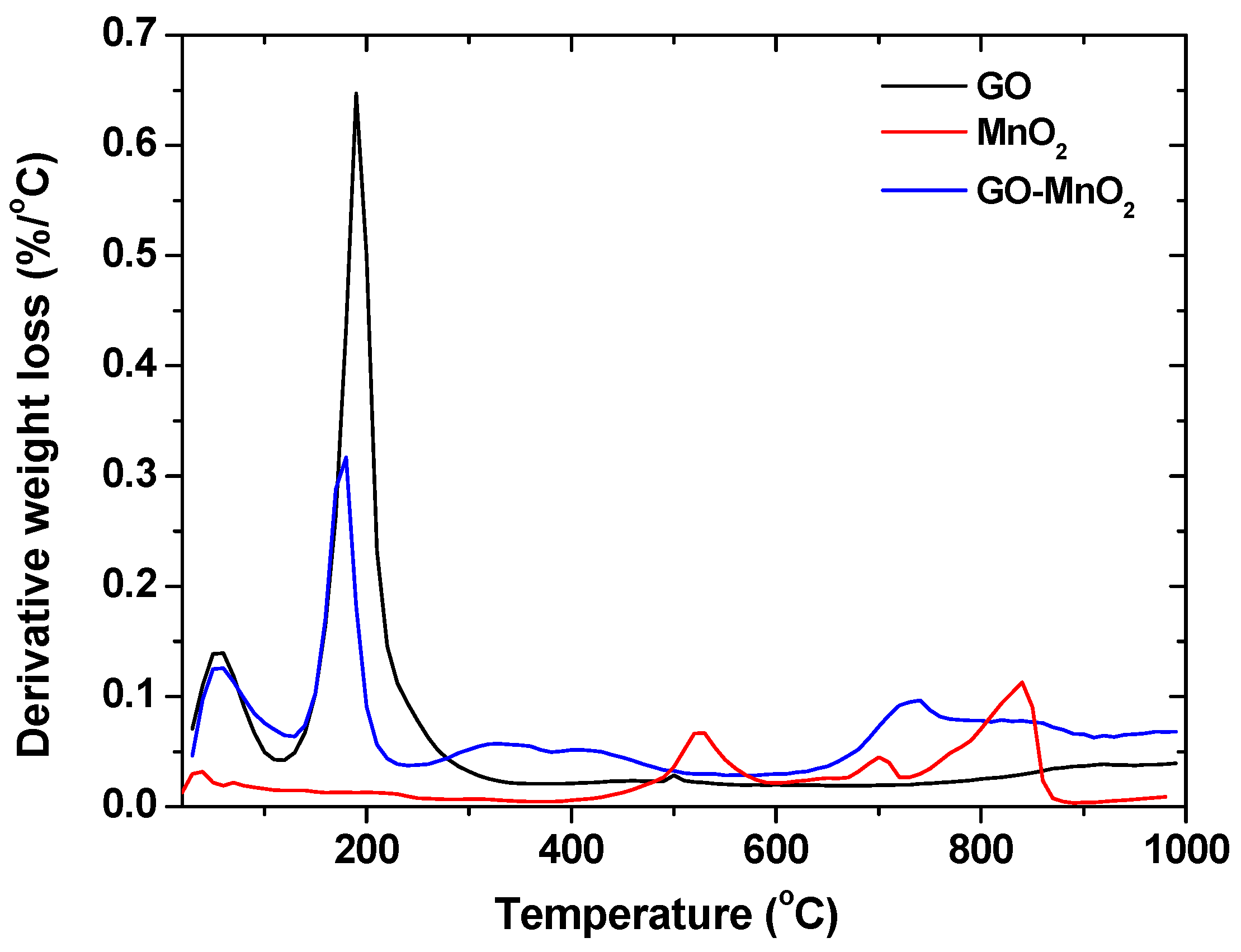



| Sample | SBET (m2 g−1) | Vtotal (cm3 g−1) | Vmeso (cm3 g−1) | Vmicro (cm3 g−1) |
|---|---|---|---|---|
| GO | 20.93 | 0.088 | 0.024 | 0.065 |
| MnO2 | 38.50 | 0.336 | 0.336 | 0 |
| GO-MnO2 | 25.85 | 0.133 | 0.133 | 0 |
| Pseudo-First Order | Pseudo-Second Order | ||||
|---|---|---|---|---|---|
| k1 | R2 | k2 | R2 | ||
| Curve | Figure | min−1 | - | min−1 | - |
| 100 mg L−1 RB5 (+H2O2) | 7a | 0.45993 | 0.998 | 1.34084 | 0.995 |
| 100 mg L−1 RB5 | - | 0.05804 | 0.974 | 0.09595 | 0.976 |
| 60 mg L−1 RB5 (+H2O2) | - | 0.28312 | 0.994 | 0.78528 | 0.997 |
| 60 mg L−1 RB5 | - | 0.05333 | 0.979 | 0.09390 | 0.975 |
| 20 mg L−1 RB5 (+H2O2) | - | 0.05450 | 0.965 | 0.09543 | 0.979 |
| 20 mg L−1 RB5 | - | 0.04981 | 0.991 | 0.08536 | 0.979 |
| MnO2 | 7b | 0.20301 | 0.983 | 0.40697 | 0.994 |
| MnO2 + H2O2 | - | 0.42056 | 0.999 | 1.81215 | 0.998 |
| GO | - | 0.06076 | 0.991 | 0.11268 | 0.980 |
| GO-MnO2 | - | 0.05802 | 0.974 | 0.09595 | 0.976 |
| GO-MnO2 (+H2O2) | - | 0.45993 | 0.997 | 1.34084 | 0.995 |
| GO + MnO2 (+H2O2) | - | 0.07789 | 0.970 | 0.14492 | 0.992 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saroyan, H.; Kyzas, G.Z.; Deliyanni, E.A. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Processes 2019, 7, 40. https://doi.org/10.3390/pr7010040
Saroyan H, Kyzas GZ, Deliyanni EA. Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide. Processes. 2019; 7(1):40. https://doi.org/10.3390/pr7010040
Chicago/Turabian StyleSaroyan, Hayarpi, George Z. Kyzas, and Eleni A. Deliyanni. 2019. "Effective Dye Degradation by Graphene Oxide Supported Manganese Oxide" Processes 7, no. 1: 40. https://doi.org/10.3390/pr7010040







