Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Waste Material
2.2. Analytical Methods
2.3. Extraction Procedure
2.4. Statistical Analysis
3. Results
3.1. Extraction of Phenolic Compounds
3.2. Analysis of HSPs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A. HSP Theory
References
- FAOSTAT. Food and Agricultural Commodities Production. Final 2013 Data. Available online: http://faostat3.fao.org/ (accessed on 17 December 2018).
- Eaton, B. An Overview of Brewing. In Handbook of Brewing, 3rd ed.; Stewart, G.G., Russell, I., Anstruther, A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 53–66. [Google Scholar]
- Xiros, C.; Christakopoulos, P. Biotechnological potential of brewers spent grain and its recent applications. Waste Biomass Valorization 2012, 3, 213–232. [Google Scholar] [CrossRef]
- Mussatto, S.I. Brewer’s spent grain: A valuable feedstock for industrial applications. J. Sci. Food Agric. 2014, 94, 1264–1275. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Dragone, G.; Roberto, I.C. Brewers’ spent grain: Generation, characteristics and potential applications. J. Cereal Sci. 2006, 43, 1–14. [Google Scholar] [CrossRef]
- Lynch, K.M.; Steffen, E.J.; Arendt, E.K. Brewers’ spent grain: A review with an emphasis on food and health. J. Inst. Brew. 2016, 122, 553–568. [Google Scholar] [CrossRef]
- Wannenmacher, J.; Gastl, M.; Becker, T. Phenolic substances in beer: Structural diversity, reactive potential and relevance for brewing process and beer quality. Compr. Rev. Food. Sci. Food Saf. 2018, 17, 953–988. [Google Scholar] [CrossRef]
- McCarthy, A.L.; O’Callaghan, Y.C.; Piggott, C.O.; FitzGerald, R.J.; O’Brien, N.M. Brewers’ spent grain; bioactivity of phenolic component, its role in animal nutrition and potential for incorporation in functional foods: A review. Proc. Nutr. Soc. 2013, 72, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Fărcaş, A.C.; Socaci, S.A.; Dulf, F.V.; Tofană, M.; Mudura, E.; Diaconeasa, Z. Volatile profile, fatty acids composition and total phenolics content of brewers’ spent grain by-product with potential use in the development of new functional foods. J. Cereal Sci. 2015, 64, 34–42. [Google Scholar] [CrossRef]
- Ameer, K.; Shahbaz, H.M.; Kwon, J.-H. Green extraction methods for polyphenols from plant matrices and their byproducts: A review. Compr. Rev. Food. Sci. Food Saf. 2017, 16, 295–315. [Google Scholar] [CrossRef]
- Chaturvedi, A.K. Extraction of nutraceuticals from plants by microwave assisted extraction. Syst. Rev. Pharm. 2018, 9, 31–35. [Google Scholar] [CrossRef]
- Zuorro, A.; Lavecchia, R.; Medici, F.; Piga, L. Use of cell wall degrading enzymes for the production of high-quality functional products from tomato processing waste. Chem. Eng. Trans. 2014, 38, 355–360. [Google Scholar]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Optimization of enzyme-assisted lipid extraction from Nannochloropsis microalgae. J. Taiwan Inst. Chem. Eng. 2016, 67, 106–114. [Google Scholar] [CrossRef]
- Kammerer, D.R.; Kammerer, J.; Valet, R.; Carle, R. Recovery of polyphenols from the by-products of plant food processing and application as valuable food ingredients. Food Res. Int. 2014, 65, 2–12. [Google Scholar] [CrossRef]
- Zuorro, A. Optimization of polyphenol recovery from espresso coffee residues using factorial design and response surface methodology. Sep. Purif. Technol. 2015, 152, 64–69. [Google Scholar] [CrossRef]
- Xu, C.C.; Wang, B.; Pu, Y.Q.; Tao, J.S.; Zhang, T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef]
- Meneses, N.G.T.; Martins, S.; Teixeira, J.A.; Mussatto, S.L. Influence of extraction solvents on the recovery of antioxidant phenolic compounds from brewer’s spent grains. Sep. Purif. Technol. 2013, 108, 152–158. [Google Scholar] [CrossRef] [Green Version]
- Socaci, S.A.; Fărcaş, A.C.; Diaconeasa, Z.M.; Vodnar, D.C.; Rusu, B.; Tofană, M. Influence of the extraction solvent on phenolic content, antioxidant, antimicrobial and antimutagenic activities of brewers’ spent grain. J. Cereal Sci. 2018, 80, 180–187. [Google Scholar] [CrossRef]
- Hansen, C.M. The three dimensional solubility parameter—Key to paint component affinities. I. Solvents, plasticizers polymers, and resins. J. Paint Technol. 1967, 39, 104–117. [Google Scholar]
- Mohammad, M.A.; Alhalaweh, A.; Velaga, S.P. Hansen solubility parameter as a tool to predict cocrystal formation. Int. J. Pharm. 2011, 407, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Lavecchia, R.; Zuorro, A. Evaluation of olive pomace as a source of phenolic antioxidants for the production of functional cosmetics. Int. J. Appl. Eng. Res. 2015, 14, 34405–34409. [Google Scholar]
- Zuorro, A.; Lavecchia, R. Influence of extraction conditions on the recovery of phenolic antioxidants from spent coffee grounds. Am. J. Appl. Sci. 2013, 10, 478–486. [Google Scholar] [CrossRef]
- Maietta, M.; Colombo, R.; Lavecchia, R.; Sorrenti, M.; Zuorro, A.; Papetti, A. Artichoke (Cynara cardunculus L. var. scolymus) waste as a natural source of carbonyl trapping and antiglycative agents. Food Res. Int. 2017, 100, 180–190. [Google Scholar]
- Hoftyzer, P.J.; Van Krevelen, D.W. Properties of Polymers, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 1976; pp. 152–155. [Google Scholar]
- Fedors, R. A method for estimating both the solubility parameters and molar volumes of liquids. Polym. Eng. Sci. 1974, 14, 147–154. [Google Scholar] [CrossRef]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 27–43. [Google Scholar]
- Szwajgier, D.; Waśko, A.; Targoński, Z.; Niedźwiadek, M.; Bancarzewska, M. The use of a novel ferulic acid esterase from Lactobacillus acidophilus K1 for the release of phenolic acids from brewer’s spent grain. J. Inst. Brew. 2010, 116, 293–303. [Google Scholar] [CrossRef]
- Kowalska, H.; Czajkowska, K.; Cichowska, J.; Lenart, A. What’s new in biopotential of fruit and vegetable by-products applied in the food processing industry. Trends Food Sci. Technol. 2017, 67, 150–159. [Google Scholar] [CrossRef]
- Kumar, K.; Yadav, A.N.; Kumar, V.; Vyas, P.; Dhaliwal, H.S. Food waste: A potential bioresource for extraction of nutraceuticals and bioactive compounds. Bioresour. Bioprocess. 2017, 4, 18. [Google Scholar] [CrossRef]
- Panusa, A.; Zuorro, A.; Lavecchia, R.; Marrosu, G.; Petrucci, R. Recovery of natural antioxidants from spent coffee grounds. J. Agric. Food Chem. 2015, 61, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Zuorro, A.; Maffei, G.; Lavecchia, R. Effect of solvent type and extraction conditions on the recovery of phenolic compounds from artichoke waste. Chem. Eng. Trans. 2014, 39, 463–468. [Google Scholar]
- Dorta, E.; Lobo, M.G.; Gonzalez, M. Reutilization of mango byproducts: Study of the effect of extraction solvent and temperature on their antioxidant properties. J. Food Sci. 2012, 77, C80–C88. [Google Scholar] [CrossRef] [PubMed]
- Guiné, R.P.F.; Gonçalves, F.; Lerat, C.; El Idrissi, T.; Rodrigo, E.; Correia, P.M.R.; Gonçalves, J.C. Extraction of phenolic compounds with antioxidant activity from beetroot (Beta vulgaris L.). Curr. Nutr. Food Sci. 2018, 14, 350–357. [Google Scholar] [CrossRef]
- Liu, F.F.; Ang, C.Y.W.; Springer, D. Optimization of extraction conditions for active components in Hypericum perforatum using response surface methodology. J. Agric. Food Chem. 2000, 48, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Hernanz, D.; Nuñez, V.; Sancho, A.I.; Faulds, C.B.; Williamson, G.; Bartolomé, B.; Gómez-Cordovés, C. Hydroxycinnamic acids and ferulic acid dehydrodimers in barley and processed barley. J. Agric. Food Chem. 2001, 49, 4884–4888. [Google Scholar] [CrossRef] [PubMed]
- Dykes, L.; Rooney, L.W. Phenolic compounds in cereal grains and their health benefits. Cereal Foods World 2007, 52, 105–111. [Google Scholar] [CrossRef]
- Albersheim, P.; Darvill, A.; Roberts, K.; Sederoff, R.; Staehelin, A. Plant Cell Walls: From Chemistry to Biology; Garland Science: New York, NY, USA, 2010. [Google Scholar]
- Lavecchia, R.; Zuorro, A. Cellulase Applications in Pigment and Bioactive Compound Extraction. In New and Future Developments in Microbial Biotechnology and Bioengineering: Microbial Cellulase System Properties and Applications; Gupta, V.K., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–222. [Google Scholar]
- El Seoud, O.A. Understanding solvation. Pure Appl. Chem. 2009, 81, 697–707. [Google Scholar] [CrossRef] [Green Version]
- Fidale, L.C.; Ruiz, N.; Heinze, T.; El Seoud, O.A. Cellulose swelling by aprotic and protic solvents: What are the similarities and differences? Macromol. Chem. Phys. 2008, 209, 1240–1254. [Google Scholar] [CrossRef]
- Idehen, E.; Tang, Y.; Sang, S. Bioactive phytochemicals in barley. J. Food Drug Anal. 2017, 25, 148–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, R.C.; Sun, X.F.; Wang, S.Q.; Zhu, W.; Wang, X.Y. Ester and ether linkages between hydroxycinnamic acids and lignin from wheat, rice rye, and barley straws, maize stems, and fast-growing poplar wood. Ind. Crop Prod. 2002, 15, 179–188. [Google Scholar] [CrossRef]
- Damoderan, S. Protein: Danaturation. In Handbook of Food Science, Technology and Engineering; Hui, Y.K., Ed.; CRC Press: Boca Raton, FL, USA, 2005; pp. 6-1–6-14. [Google Scholar]
- Hildebrand, J.H.; Scott, R.L. Solutions of nonelectrolytes. Annu. Rev. Phys. Chem. 1950, 1, 75–92. [Google Scholar] [CrossRef]
- Güner, A. The algorithmic calculations of solubility parameter for the determination of interactions in dextran/certain polar solvent systems. Eur. Polym. J. 2004, 40, 1587–1594. [Google Scholar] [CrossRef]
- Hansen, C.M.; Skaarup, K. The three dimensional solubility parameter—Key to paint component affinities III. J. Paint Technol. 1967, 39, 511–514. [Google Scholar]




| Solvent | v/v (%) | c (mg GAE/L) | AA (mg TE/L) |
|---|---|---|---|
| Water | 100 | 0.013 ± 0.002 | 0.011 ± 0.001 |
| Acetone | 20 | 0.073 ± 0.003 | 0.034 ± 0.003 |
| 40 | 0.101 ± 0.006 | 0.051 ± 0.003 | |
| 60 | 0.160 ± 0.005 | 0.064 ± 0.005 | |
| 80 | 0.091 ± 0.004 | 0.048 ± 0.004 | |
| 100 | 0.022 ± 0.001 | 0.017 ± 0.002 | |
| Ethanol | 20 | 0.034 ± 0.002 | 0.019 ± 0.003 |
| 40 | 0.083 ± 0.004 | 0.029 ± 0.003 | |
| 60 | 0.106 ± 0.005 | 0.043 ± 0.005 | |
| 80 | 0.067 ± 0.004 | 0.033 ± 0.004 | |
| 100 | 0.023 ± 0.002 | 0.019 ± 0.003 |
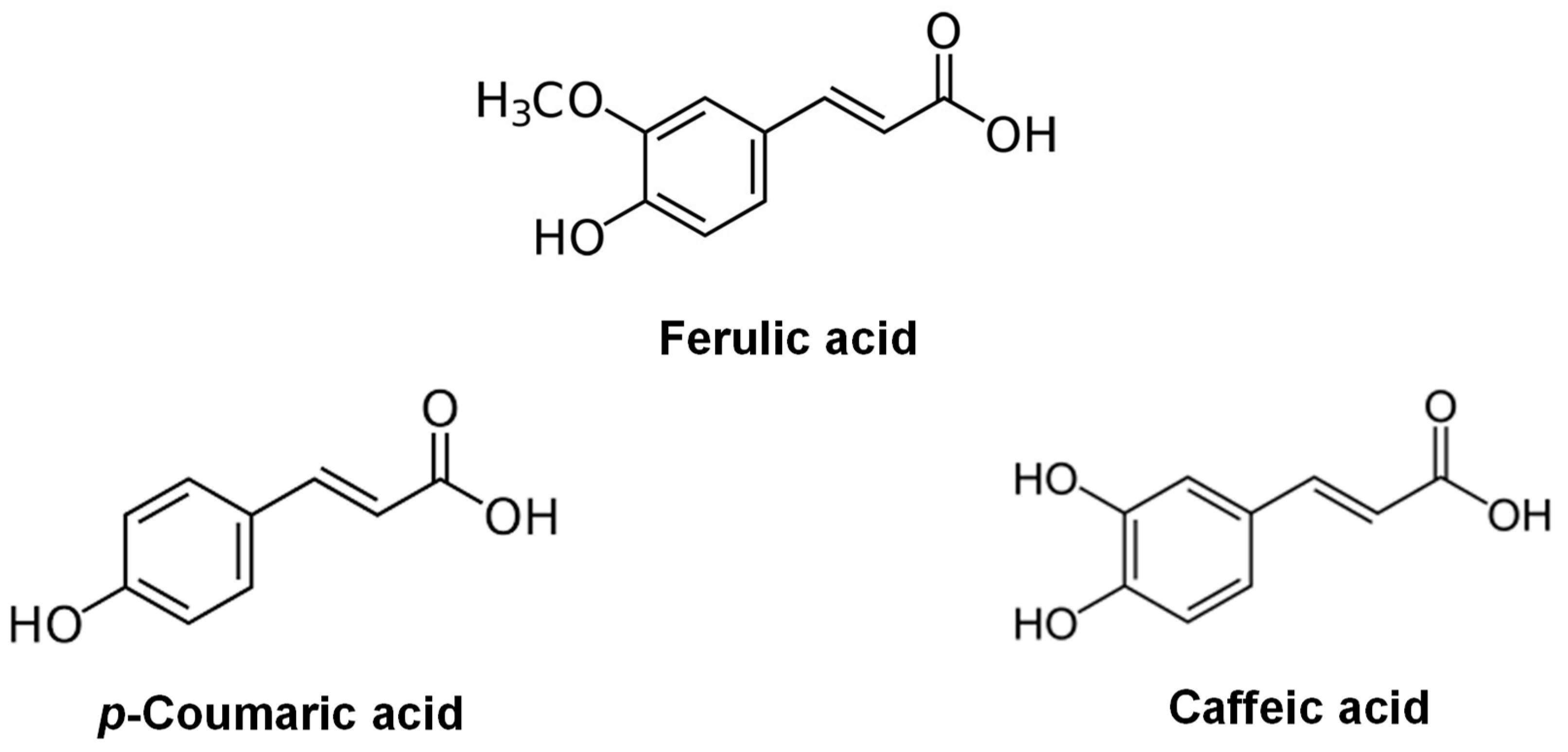
| Compound | MW (Da) | MV (cm3/mol) | δD (MPa0.5) | δP (MPa0.5) | δH (MPa0.5) | δT (MPa0.5) |
|---|---|---|---|---|---|---|
| Water | 18.01 | 18.02 | 15.5 | 16 | 42.3 | 47.8 |
| Acetone | 58.08 | 73.40 | 15.5 | 10.4 | 7.0 | 19.9 |
| Ethanol | 46.07 | 58.68 | 15.8 | 8.8 | 19.4 | 26.5 |
| Ferulic acid | 194.19 | 136.2 | 22.7 | 5.7 | 15.6 | 28.1 |
| Caffeic acid | 180.16 | 108.9 | 25.5 | 10.0 | 21.4 | 34.8 |
| p-Coumaric acid | 164.16 | 117.9 | 20.4 | 5.6 | 16.0 | 26.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuorro, A.; Iannone, A.; Lavecchia, R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes 2019, 7, 126. https://doi.org/10.3390/pr7030126
Zuorro A, Iannone A, Lavecchia R. Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes. 2019; 7(3):126. https://doi.org/10.3390/pr7030126
Chicago/Turabian StyleZuorro, Antonio, Annalaura Iannone, and Roberto Lavecchia. 2019. "Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain" Processes 7, no. 3: 126. https://doi.org/10.3390/pr7030126
APA StyleZuorro, A., Iannone, A., & Lavecchia, R. (2019). Water–Organic Solvent Extraction of Phenolic Antioxidants from Brewers’ Spent Grain. Processes, 7(3), 126. https://doi.org/10.3390/pr7030126






