Biopolymer Solution Evaluation Methodology: Thermal and Mechanical Assessment for Enhanced Oil Recovery with High Salinity Brines
Abstract
:1. Introduction
2. Experimental
2.1. Materials
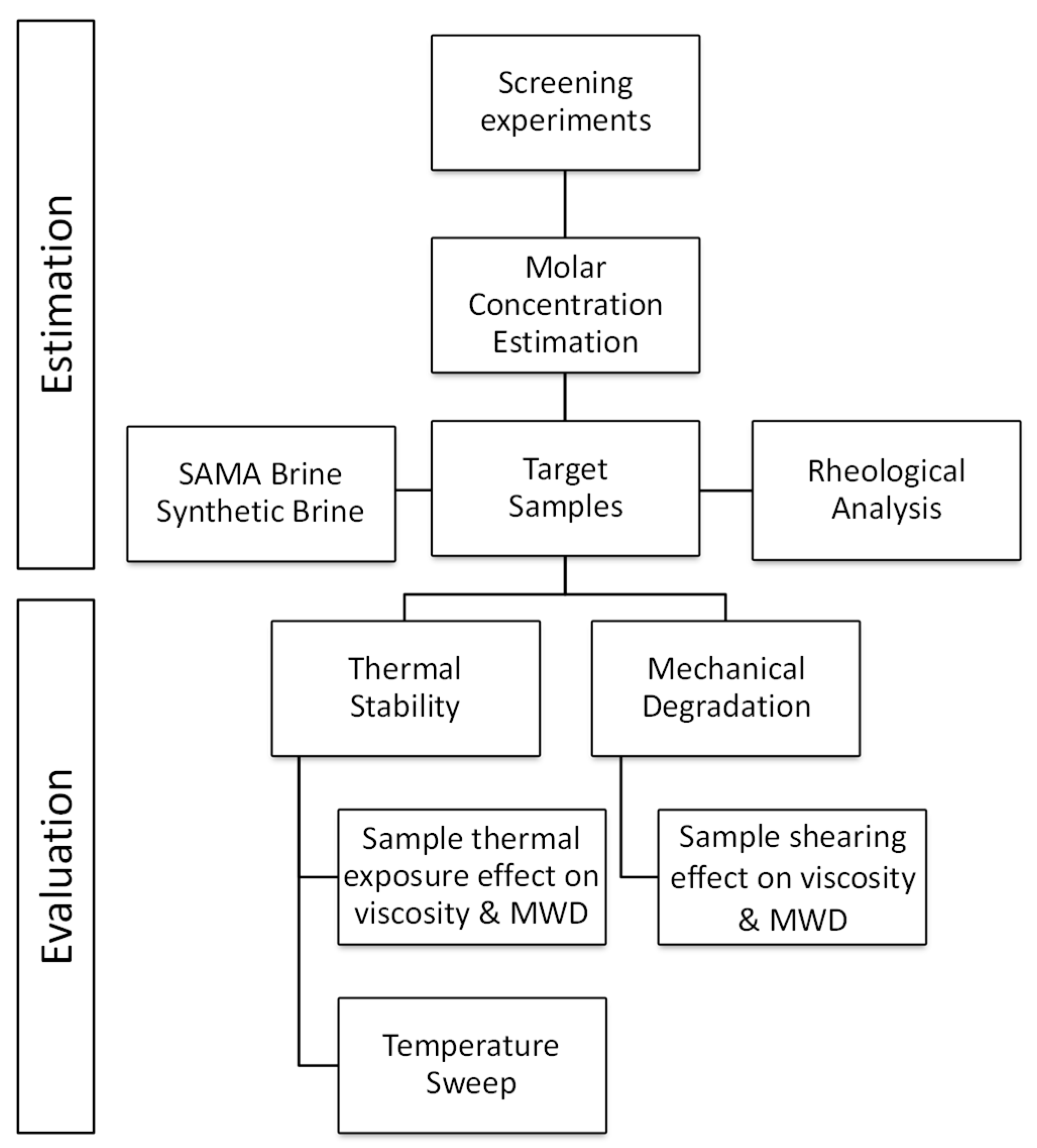
2.2. Methodology
2.3. Reservoir Selection
2.4. Brine Preparation
2.5. Screening Region Boundaries of Samples
2.6. Characterization and Evaluation
3. Results, Discussion and Evaluation
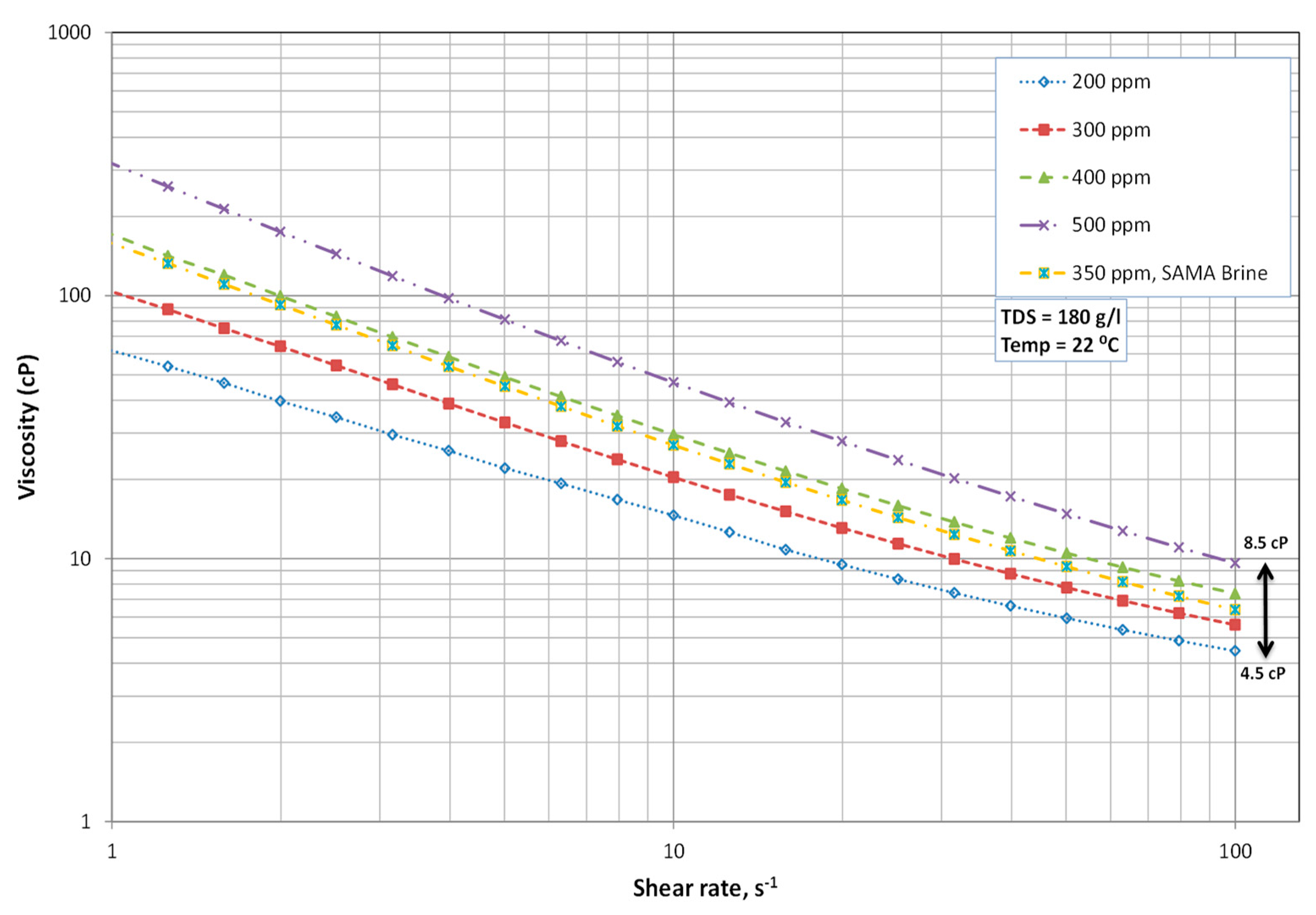
3.1. Polymer Molar Concentration Optimization
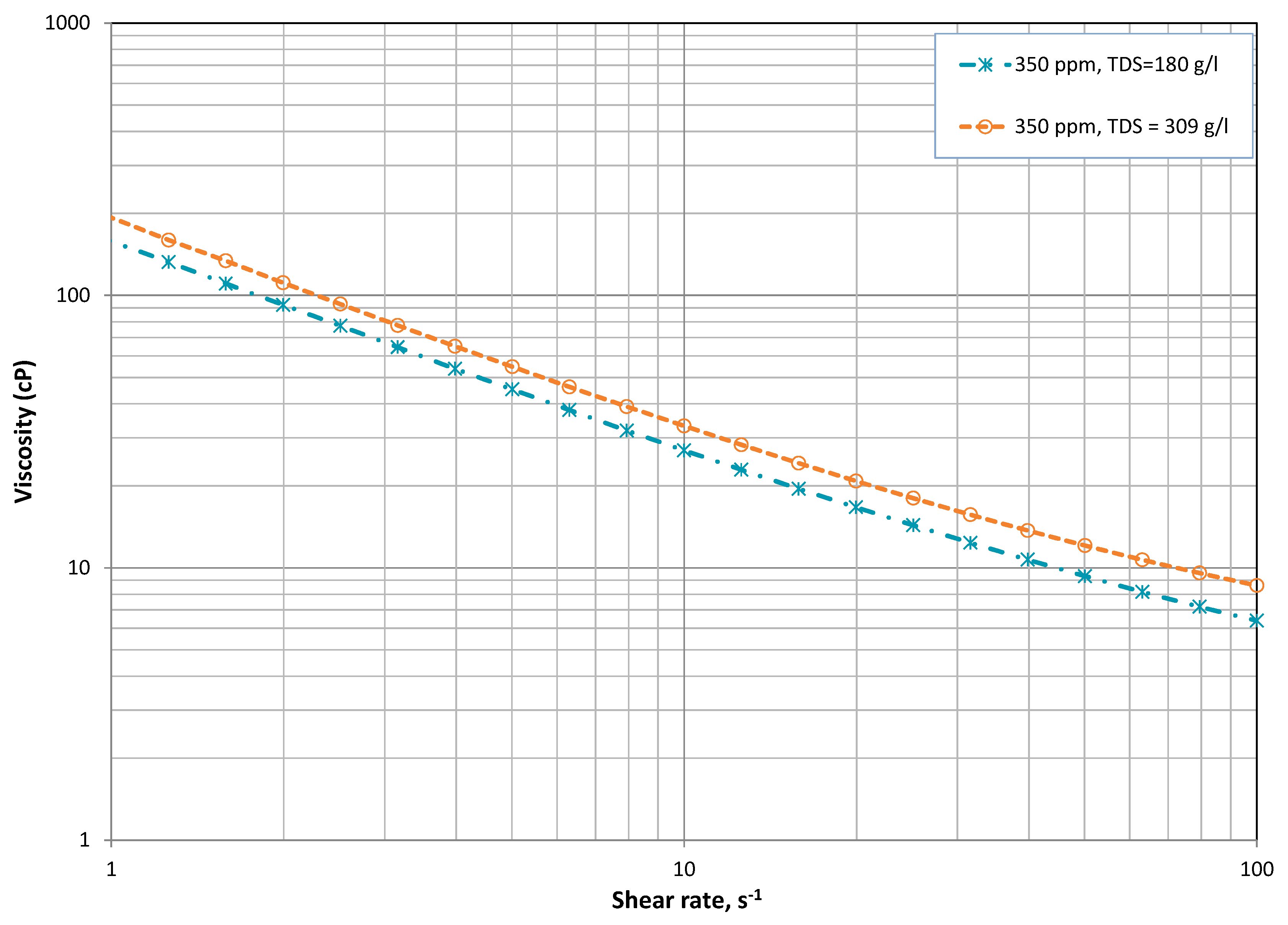
3.2. Salinity Effect
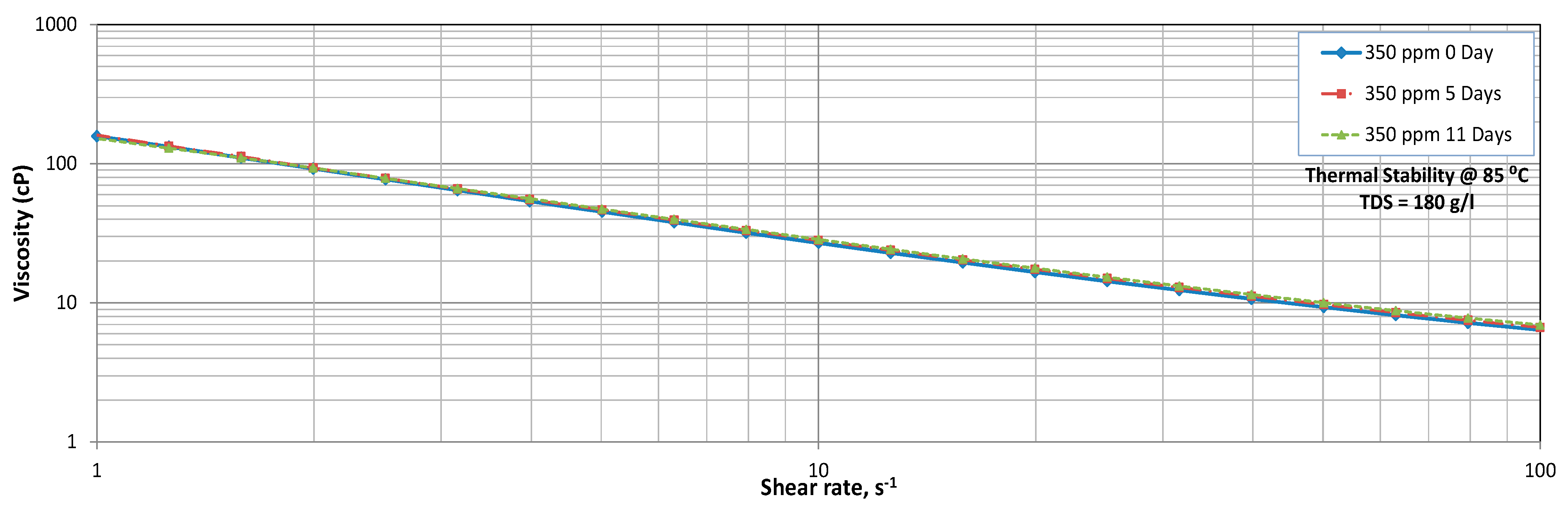
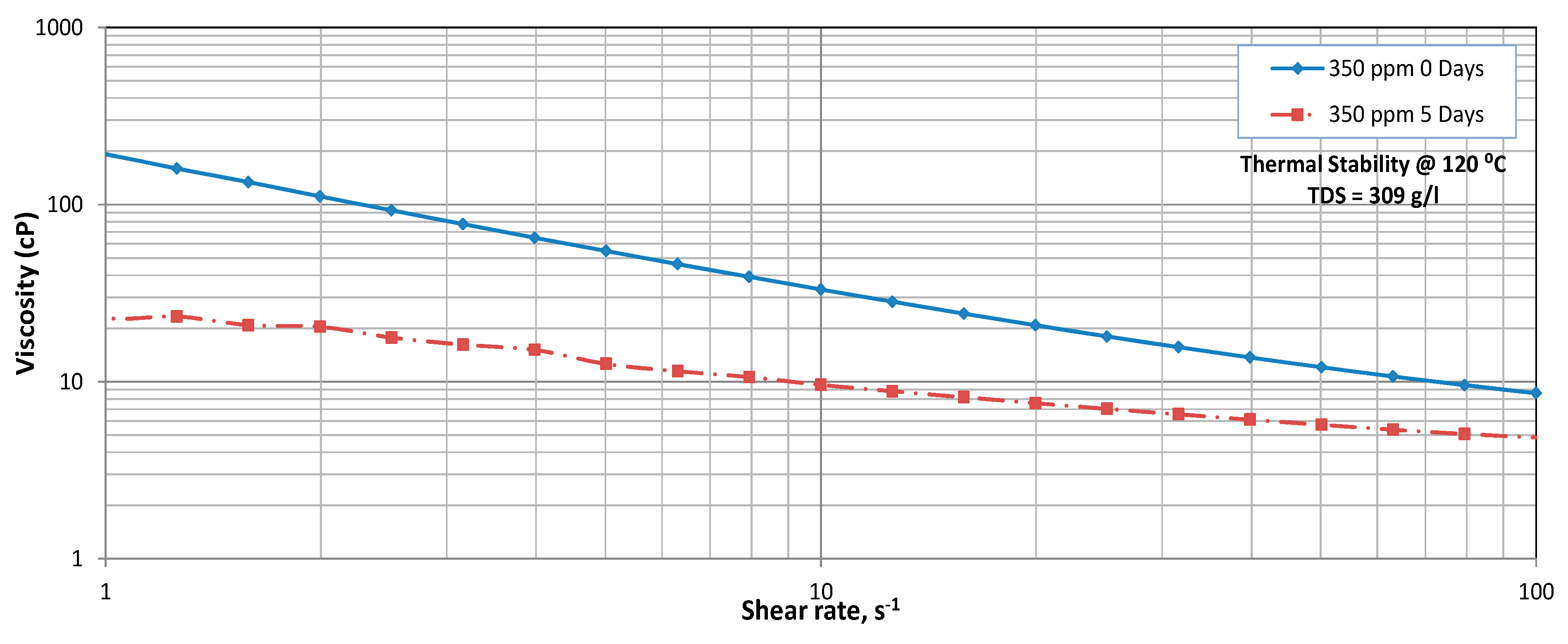
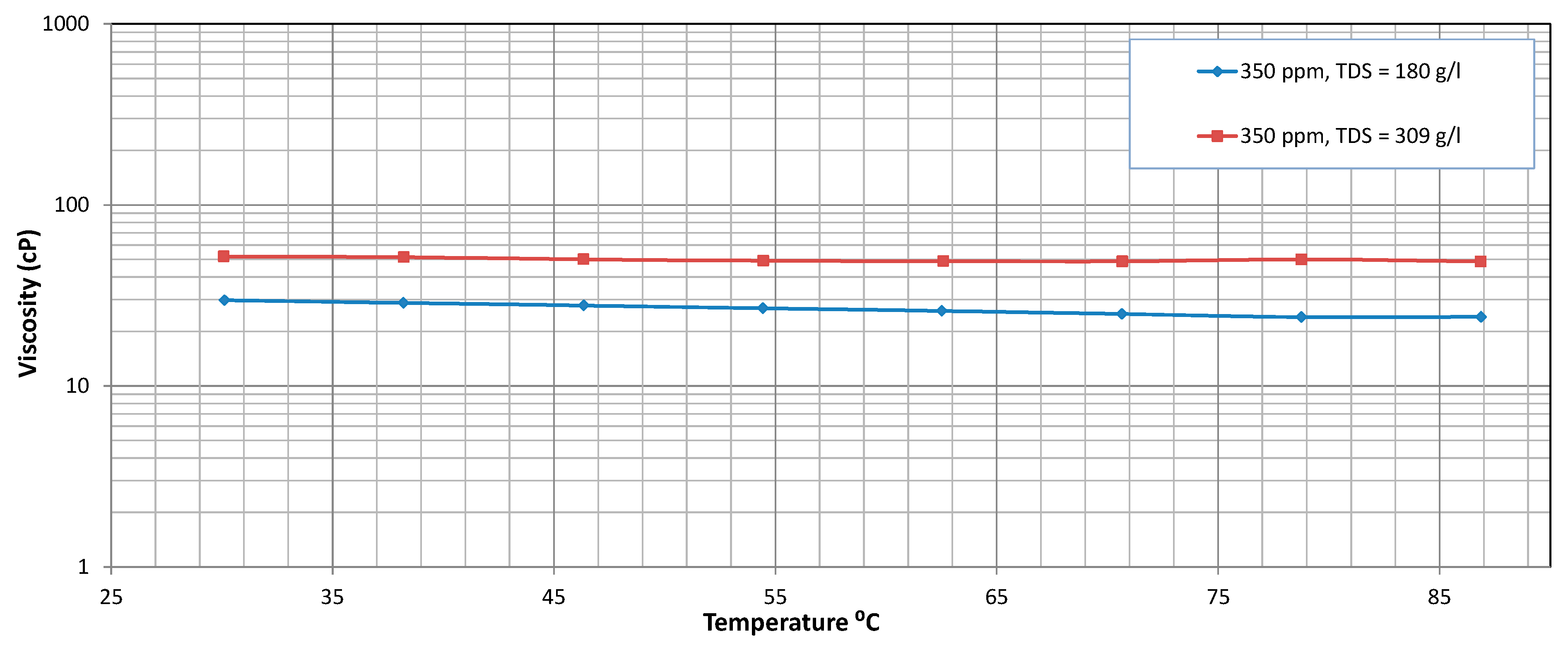
3.3. Thermal Stability
Temperature Sweep
3.4. Mechanical Degradation
3.5. Thermal and Mechanical Shear Impact on the Molecular Weight Distribution
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Thomas, S. Enhanced oil recovery—An overview. Oil Gas Sci. Technol. Rev. IFP 2008, 63, 9–19. [Google Scholar] [CrossRef]
- Vladimir, A.; Manrique, E. Enhanced oil recovery: An update review. Energies 2010, 3, 1529–1575. [Google Scholar]
- Sun, Y.; Saleh, L.; Bai, B. Measurement and impact factors of polymer rheology in porous media. Reology 2012, 8, 187–202. [Google Scholar]
- Wang, W.; Liu, Y.; Gu, Y. Application of a novel polymer system in chemical enhanced oil recovery (EOR). Colloid Polymer Sci. 2003, 281, 1046–1054. [Google Scholar] [CrossRef]
- DaSilva, I.; Lucas, E.; de Franca, F. Study of conditions for polyacrylamide use in petroleum reservoirs: Physical flow simulation in porous media. Chem. Chem. Technol. 2010, 4, 73–80. [Google Scholar]
- Leiting, S.; Zhongbin, Y.; Zhuo, Z.; Changjiang, Z.; Shanshan, Z.; Zhidong, G. Necessity and feasibility of improving the residual resistance factor of polymer flooding in heavy oil reservoirs. Pet. Sci. 2010, 7, 251–256. [Google Scholar] [Green Version]
- Du, Y. Field-scale polymer flooding: Lessons learnt and experiences gained during past 40 years. In Proceedings of the SPE International Petroleum Conference in Mexico, Society of Petroleum Engineers 91787, Puebla Pue, Mexico, 7–9 November 2004. [Google Scholar]
- Brouzet, C.; Mittal, N.; Lundell, F.; Söderberg, L.D. Characterizing the orientational and network dynamics of polydisperse nanofibers on the nanoscale. Macromolecules 2019, 52, 2286–2295. [Google Scholar] [CrossRef]
- Brouzet, C.; Mittal, N.; Söderberg, L.D.; Lundell, F. Size-dependent orientational dynamics of brownian nanorods. ACS Macro Lett. 2018, 7, 1022–1027. [Google Scholar] [CrossRef]
- Del Giudice, F.; Tassieri, M.; Oelschlaeger, C.; Shen, A.Q. When microrheology, bulk rheology, and microfluidics meet: Broadband rheology of hydroxyethyl cellulose water solutions. Macromolecules 2017, 50, 2951–2963. [Google Scholar] [CrossRef]
- Haward, S.J.; Sharma, V.; Butts, C.P.; McKinley, G.H.; Rahatekar, S.S. Shear and Extensional Rheology of Cellulose/Ionic Liquid Solutions. Biomacromolecules 2012, 13, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Zamani, N.; Bondino, I.; Kaufmann, R.; Skauge, A. Computation of polymer in-situ rheology using direct numerical simulation. J. Pet. Sci. Eng. 2017, 159, 92–102. [Google Scholar] [CrossRef]
- Saavedra, N.F.; Gaviria, W.; Davitt, J. Laboratory testing of polymer flood candidates: San Francisco Field. In Proceedings of the SPE/DOE Improved Oil Recovery Symposium, San Francisco, CA, USA, 13 April 2002; 75182. Society of Petroleum Engineers: Richardson, TX, USA, 2002. [Google Scholar]
- Martin, I.; Lozanski, W.R. Taber South-Canada’s first polymer flood. In Proceedings of the SPE California Regional Meeting, Santa Barbara, CA, USA, 28–30 October 1970; 3180. Society of Petroleum Engineers: Richardson, TX, USA. [Google Scholar]
- Ustick, R.E.; Hillhouse, J.D. Comparison of polymer flooding and waterflooding. In Proceedings of the Society of Petroleum Engineers, Huntington Beach, CA, USA, 19 February 1967; 1734. Society of Petroleum Engineers: Richardson, TX, USA, 1967. [Google Scholar]
- Luo, X.; Zhao, C. Practices and experiences of seven-year polymer flooding history matching in China offshore oil field: A case study. In Proceedings of the SPE Reservoir Characterisation and Simulation Conference and Exhibition, Abu Dhabi, UAE, 9–11 October 2011; 147807. Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Dong, H.Z. Review of practical experience and management by polymer flooding at Daqing. In Proceedings of the SPE Symposium on Improved Oil Recovery, Tulsa, OK, USA, 20–23 April 2008; 114342. Society of Petroleum Engineers: Richardson, TX, USA, 2008. [Google Scholar]
- He, L. An enhanced oil recovery technology continually after polymer-flooding. In Proceedings of the SPE Enhanced Oil Recovery Conference, Kuala Lumpur, Malaysia, 19–21 July 2011; 144250. Society of Petroleum Engineers: Richardson, TX, USA, 2011. [Google Scholar]
- Tabary, R.; Bazin, B. Advances in chemical flooding, improved oil recovery (IOR) techniques and their role in boosting the recovery factor. In Proceedings of the IFP-OAPEC Joint Seminar, Rueil-Malmaison, France, 26–28 June 2007. [Google Scholar]
- Gao, C. Application of a novel biopolymer to enhance oil recovery. J. Petrol. Explor. Prod. Technol. 2016, 6, 749–753. [Google Scholar] [CrossRef]
- Zhang, Y.; Kong, H.; Fang, Y.; Nishinari, K.; Phillips, G.O. Schizophyllan: A review on its structure, properties, bioactivities and recent developments. Bioactive Carbohydr. Diet. Fibre 2013, 1, 53–71. [Google Scholar] [CrossRef]
- Al-Matrouk, M.F.; Oskui, R.P.; Al-Qahtani, M.M. Assessment of Enhanced Oil Recovery (EOR) Techniques for Selected Fifteen Kuwait Reservoirs; Report No. KISR9550; Kuwait Institute for Scientific Research: Kuwait, Kuwait, 2009. [Google Scholar]
- Kwak, H.-T.; Zhang, G.; Chen, S. The effects of salt type and salinity on formation water viscosity and NMR responses. In Proceedings of the International Symposium of the Society of Core Analysts, Toronto, ON, Canada, 21–25 August 2005. [Google Scholar]






| Salt | Fresh Brine (g/L) | Synthetic Brine (g/L) |
|---|---|---|
| NaCl | 134.2 | 240.3 |
| CaCl2 | 31.8 | 52.2 |
| MgCl2 | 9.9 | 12.9 |
| KCl | 4.3 | 3.7 |
| TDS | 180.2 | 309.1 |
| Biopolymer, ppm | 500 | 400 | 300 | 200 |
|---|---|---|---|---|
| Biopolymer, wt | 17.85 | 14.28 | 10.71 | 7.14 |
| Brine, wt | 182.15 | 185.72 | 189.29 | 192.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saleh, M.A.; Yussuf, A.A.; Jumaa, M.A.; Hammoud, A.; Al-Shammari, T. Biopolymer Solution Evaluation Methodology: Thermal and Mechanical Assessment for Enhanced Oil Recovery with High Salinity Brines. Processes 2019, 7, 339. https://doi.org/10.3390/pr7060339
Al-Saleh MA, Yussuf AA, Jumaa MA, Hammoud A, Al-Shammari T. Biopolymer Solution Evaluation Methodology: Thermal and Mechanical Assessment for Enhanced Oil Recovery with High Salinity Brines. Processes. 2019; 7(6):339. https://doi.org/10.3390/pr7060339
Chicago/Turabian StyleAl-Saleh, Mohammad A., Abdirahman A. Yussuf, Mohammad A. Jumaa, Abbas Hammoud, and Tahani Al-Shammari. 2019. "Biopolymer Solution Evaluation Methodology: Thermal and Mechanical Assessment for Enhanced Oil Recovery with High Salinity Brines" Processes 7, no. 6: 339. https://doi.org/10.3390/pr7060339




