Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection Site and Treatment of Agricultural Residues Coffee Pulp (CP) and Plantain Pseudostem (PP)
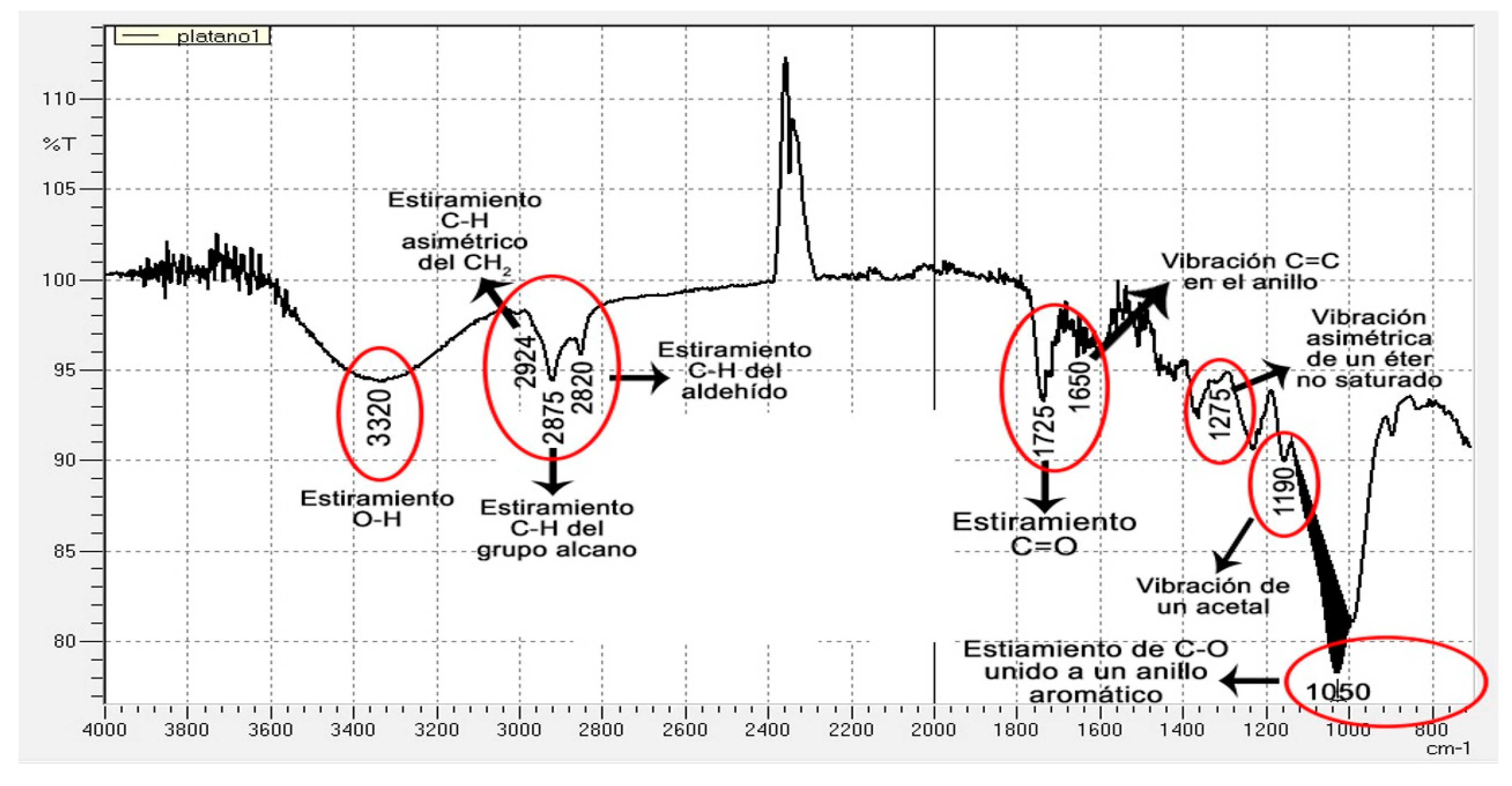
2.2. Bromatological Analysis, Lignocellulosic Content and Infrared Spectrum (IR)
2.3. Quantification of Cr (VI)
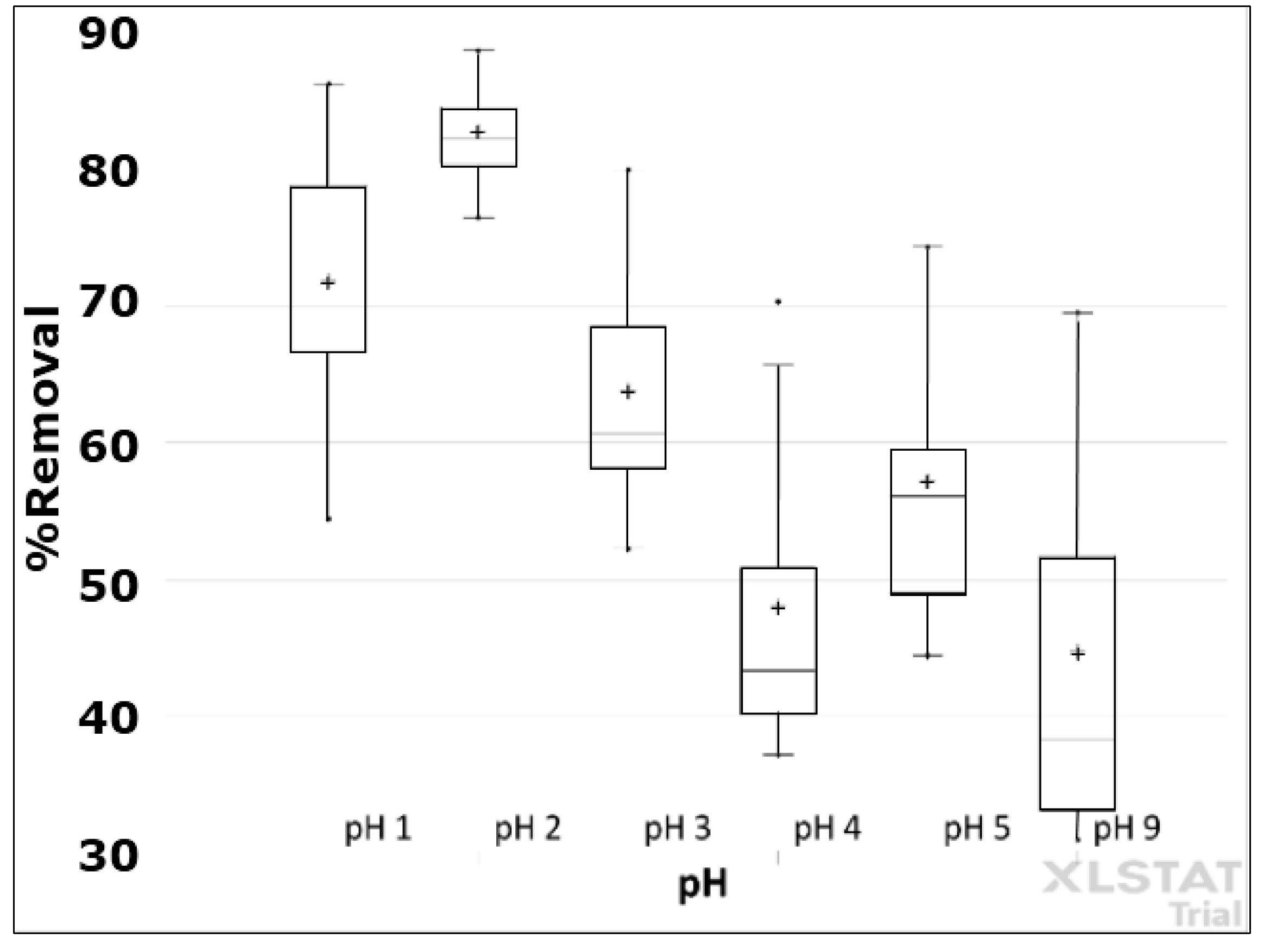
2.4. Determination of the Optimum pH of Adsorption with CP
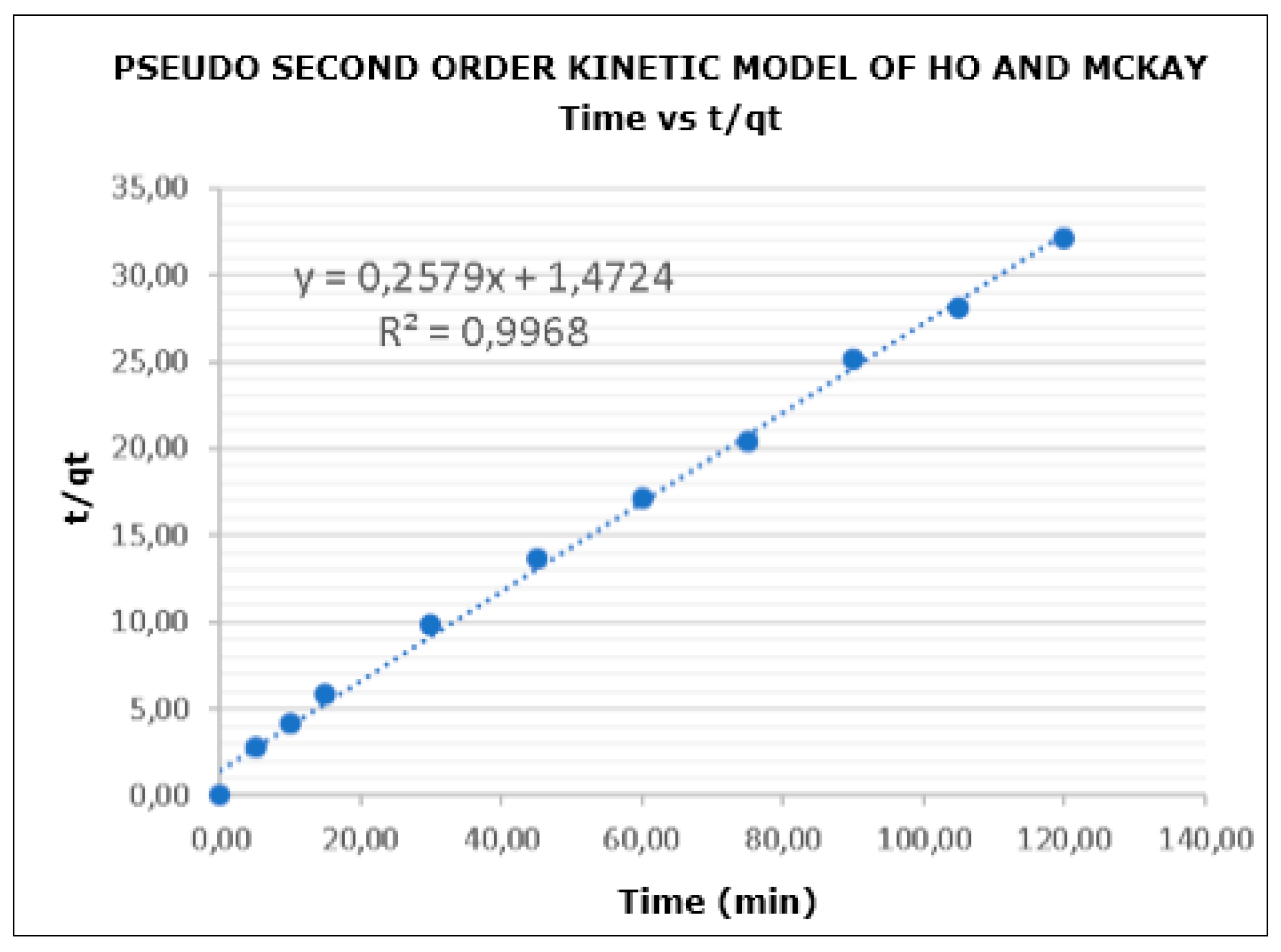
2.5. Kinetic Adsorption with CP
2.6. Isotherm Adsorption with CP
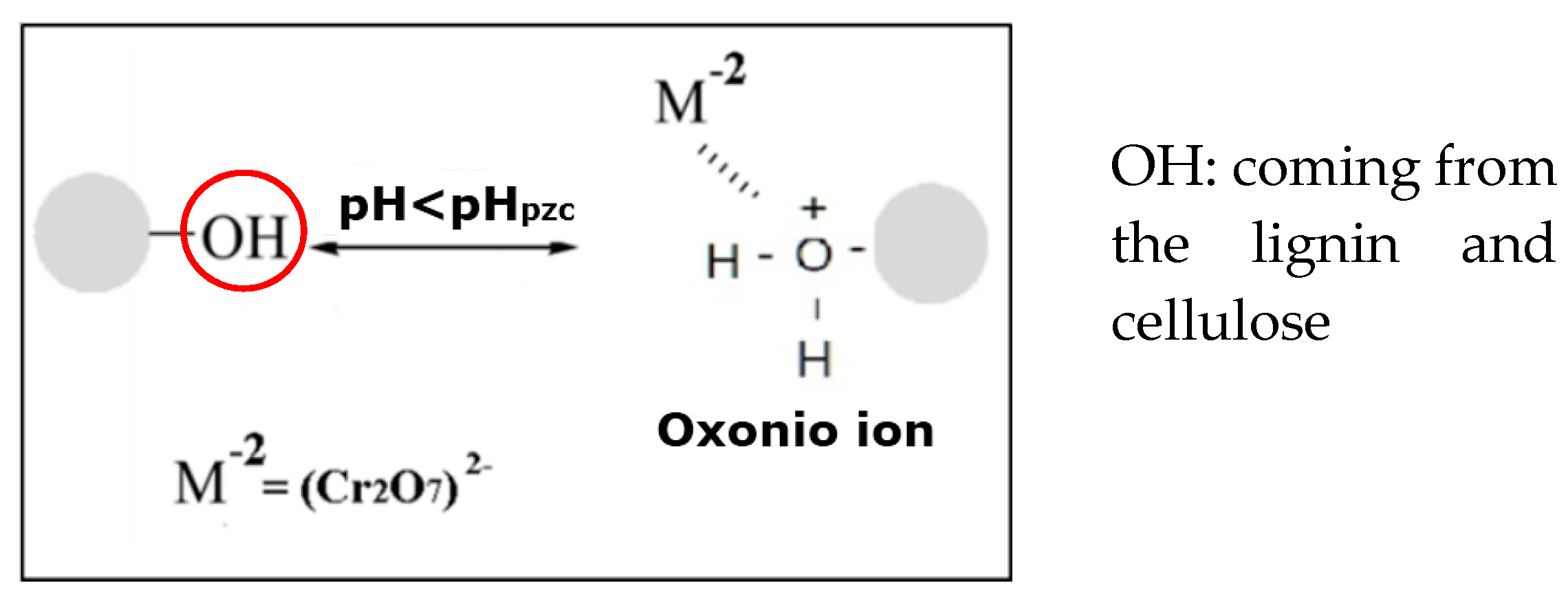
2.7. Determination of the Zero Charge Point (pHpzc) and Active Sites
2.8. Determination of the Removal Percentage with Mixtures of Lignocellulosic Residues (CP and PP)
3. Results and Discussion
3.1. Bromatological Analysis and Lignin and Cellulose Percentage
3.2. Infrared (IR) Spectrum of the CP and PP
3.3. Determination of Optimal pH of Adsorption in the CP
3.4. Determination pHpzc and Active Sites of the Surface of the CP
3.5. Adsorption Kinetics of CP
3.6. Adsorption Isotherms of the CP
3.7. Mixtures of more Efficient Bio Adsorbents for the Removal of Chromium (VI) in Synthetic Wastewater
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Srivastava, N.K.; Majumder, C.B. Novel biofiltration methods for the treatment of heavy metals from industrial wastewater. J. Hazard. Mater. 2008, 151, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, D. Absorption and Adsorption of Heavy Metals by Microalgae. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Richmond, A., Hu, Q., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA; Blackwell Publishing Ltd.: Israel, Middle East, 2013; pp. 602–611. [Google Scholar]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Wendling, Z.A.; Emerson, J.W.; Esty, D.C.; Levy, M.A.; de Sherbinin, A.; Spiegel, N.; Pinkerton, V.; Bouncher, L.; Ratté, S.; Mardel, S.; et al. 2018 Environmental Performance Index; Yale Center for Environmental Law & Policy: New Haven, CT, USA, 2018; Available online: https://epi.envirocenter.yale.edu/2018/report/category/hlt (accessed on 26 March 2019).
- Wang, J.; Chen, C. Biosorbents for heavy metals removal and their future. J. Biotechnol. Adv. 2009, 27, 195–226. [Google Scholar] [CrossRef] [PubMed]
- Organización Mundial de la Salud (OMS) (2011). Adverse health effects of heavy metals in children. Available online: https://www.who.int/ceh/capacity/heavy_metals.pdf (accessed on 26 March 2019).
- Coreño-Alonso, A.; Tomasini, A.; Reyna, G. Cromo: Lo bueno y lo malo, los inicios de una historia. R. Enl. Quím. 2010, 2, 14–54. [Google Scholar]
- Barthelmy, D. Mineralogy Database. 2014. Available online: http://webmineral.com/ (accessed on 26 March 2019).
- Álvarez, E.; Garcia, A.; Querol, X. Adsorption of Cr (VI) from Synthetic Solutions and Electroplating Wastewaters on Amorphous Aluminium Oxide. J. Haz. Mater. 2007, 142, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Artunduaga, F. Tratamiento para la remoción de Cr (VI) presente en aguas residuales. R. Nov. 2015, 1, 66–73. [Google Scholar]
- Vargas, Y.; Pérez, L. Use of Agro-Industrial Waste in Improving the Quality of the Environment. R. Fac. Cienc. Básic. 2018, 14, 1–14. [Google Scholar]
- Saval, S. Aprovechamiento de residuos agroindustriales: pasado, presente y futuro. R. Biotecn. 2012, 16, 14–16. [Google Scholar]
- Alonso, M.; Ramírez, C.; Rigal, L. Valorización de residuos agroindustriales del tequila para alimentación de rumiantes. R. Chap. Ser. Cien. Forest. Amb. 2012, 18, 449–457. [Google Scholar]
- Blandón, G.; Dávila, M.; Rodríguez, N. Caracterización microbiológica y físico-química de la pulpa de café sola y con mucílago, en proceso de lombricompostaje. R. Cenic. 1999, 50, 5–23. [Google Scholar]
- Rodríguez, V.; Gómez, C. Cultive Hongos Comestibles en Pulpa de Café. 2001. Available online: https://www.cenicafe.org/es/index.php/nuestras_publicaciones/avances_tecnicos/avance_tecnico_0285 (accessed on 26 March 2019).
- Rodríguez-Estupiñán, P.; Giraldo, L.; Moreno-Piraján, J.C. Adsorción simple y competitiva de níquel y cadmio sobre carbón activado granular: Efecto del pH. R. Afinid. 2010, LXVII, 449–454. [Google Scholar]
- Segovia-Sandoval, S.J.; Ocampo-Pérez, R.; Berber-Mendoza, M.S.; Leyva-Ramos, R.; Jacobo-Azuara, A.; Medellin-Castillo, N.A. Alnut shell treated with citric acid and its application as biosorbent in the removal of Zn (II). J. Wat. Proc. Engine. 2018, 25, 45–53. [Google Scholar] [CrossRef]
- Bernal, I. Análisis de Alimentos, 2nd ed.; Academia Colombiana de Ciencias Exacta físicas y Naturales: Bogotá, Colombia, 1998. [Google Scholar]
- ASTM International. ANSI/ASTM D1103-60: Method of Test for Alpha-Cellulose in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 1960. [Google Scholar]
- ASTM International. ANSI/ASTM D1106-56: Standard Test Method for Acid-Insoluble Lignin in Wood; ASTM International (ASTM): West Conshohocken, PA, USA, 2001. [Google Scholar]
- Calderón, C. Manual Para la Interpretación de Espectros Infrarrojos; Universidad Nacional de Colombia: Bogotá, Colombia, 1995. [Google Scholar]
- Rubio, S. Recuperación de celulosa en un residuo forestal mediante líquidos iónicos. In Tesis de Maestría en Ciencia y Tecnología Ambiental; Centro de Investigación en Materiales Avanzados: Chihuahua, México, 2016. [Google Scholar]
- Moreno, J. Importancia y Aplicaciones de la Adsorción en Fase Líquida. En Sólidos Porosos; Universidad de los Andes: Bogotá, Colombia, 2007; pp. 155–207. [Google Scholar]
- Acosta, I.; López, V.; Coronado, E.; Cárdenas, J.; Martínez, V. Remoción de Cromo (VI) en solución acuosa por la biomasa de la cáscara de tamarindo (Tamarindus indica). R. Biotecn. 2010, 14, 11–23. [Google Scholar]
- Ho, Y.S.; Mckay, G. Pseudo-second order model for sorption processes. J. Proc. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ayawei, N.; Ebelegi, A.N.; Wankasi, D. Modelling and Interpretation of Adsorption Isotherms. J. Chem. Hind. 2017, 2017. [Google Scholar] [CrossRef]
- Nascimento, R.F.; Lima, A.C.; Vidal, C.B.; Melo, D.Q.; Cabral Raulino, G.S. Adsorção: Aspectos Teóricos e Aplicações Ambientais; Universitária da Universidade Federal do Ceará (UFC): Imprensa Universitária, 2014. [Google Scholar]
- Alemayehu, H.; Burkute, A.; Ede, A. Adsorptive removal of Pb (II) and Cr (VI) from wastewater used acid untreated coffee husk, Interlink continental. J. Environ. Sci. Toxicol. 2014, 1, 9–16. [Google Scholar]
- Anastopoulos, I.; Karamesouti, M.; Mitropoulos, A.; Kyzas, G. A review for coffee adsorbents. J. Mol. Liq. 2017, 229, 555–565. [Google Scholar] [CrossRef]
- Ede, A. Adsorptive removal of Cr (VI) ion from wastewater by activated coffee husk and banana peel: Comparative study. World J. Pharm. Res. 2014, 4, 212–225. [Google Scholar]
- Ede, A.; Alemayehu, H.; Burkute, A. Adsorptive removal of Pb (II) and Cr (VI) from wastewater using acid untreated and treated coffee husks: Comparative study. Int. J. Res. Chem. Environ. 2014, 4, 26–31. [Google Scholar]

| PARAMETER | AGRICULTURAL RESIDUALS | ||
|---|---|---|---|
| COFFEE PULP (CP) | PLANTAIN PSEUDOSTEM (PP) | METODOLOGY USED | |
| % Humidity | 12.40 ± 0.00 | 13.40 ± 0.64 | AOAC 7.003/84, 930.15/90 Adapted [18] (p. 47) |
| % Ashes | 10.43 ± 0.18 | 10.61 ± 0.24 | AOAC 7.009/84, 942.05/90 Adapted [18] (p. 47) |
| % Fats and Oils (F and O) | 1.85 ± 0.08 | 1.00 ± 0.25 | AOAC 7.060/84, 920.39/90 Adapted [18] (p. 48) |
| %Protein | 10.53 ± 0.64 | 3.73 ± 0.18 | Kjeldahl Method-Gunning-Arnold Adapted-Griffin-1995 [19] (p. 51) |
| % Raw fiber | 16.29 ± 0.50 | 16.71 ± 0.11 | AOAC 7.066/84, 962.09/90 Adapted [18] (p. 48) |
| % Carbohydrates | 48.50 ± 0.00 | 54.55 ± 0.00 | Found between the difference of the parameters (humidity, ash, crude fiber, ether extract and total protein) |
| % Cellulose | 29.93 ± 0.21 | 49.20 ± 2.65 | ANSI/ASTM D1103-60 [19] |
| % Lignin | 19.25 ± 0.16 | 5.49 ± 0.25 | ANSI/ASTM D1106-56 [20]. |
| Total Active Sites | pHpzc | |
|---|---|---|
| Acids (mmol × g−1) | Basics (mmol × g−1) | |
| 0.28 | 0.17 | 3.95 |
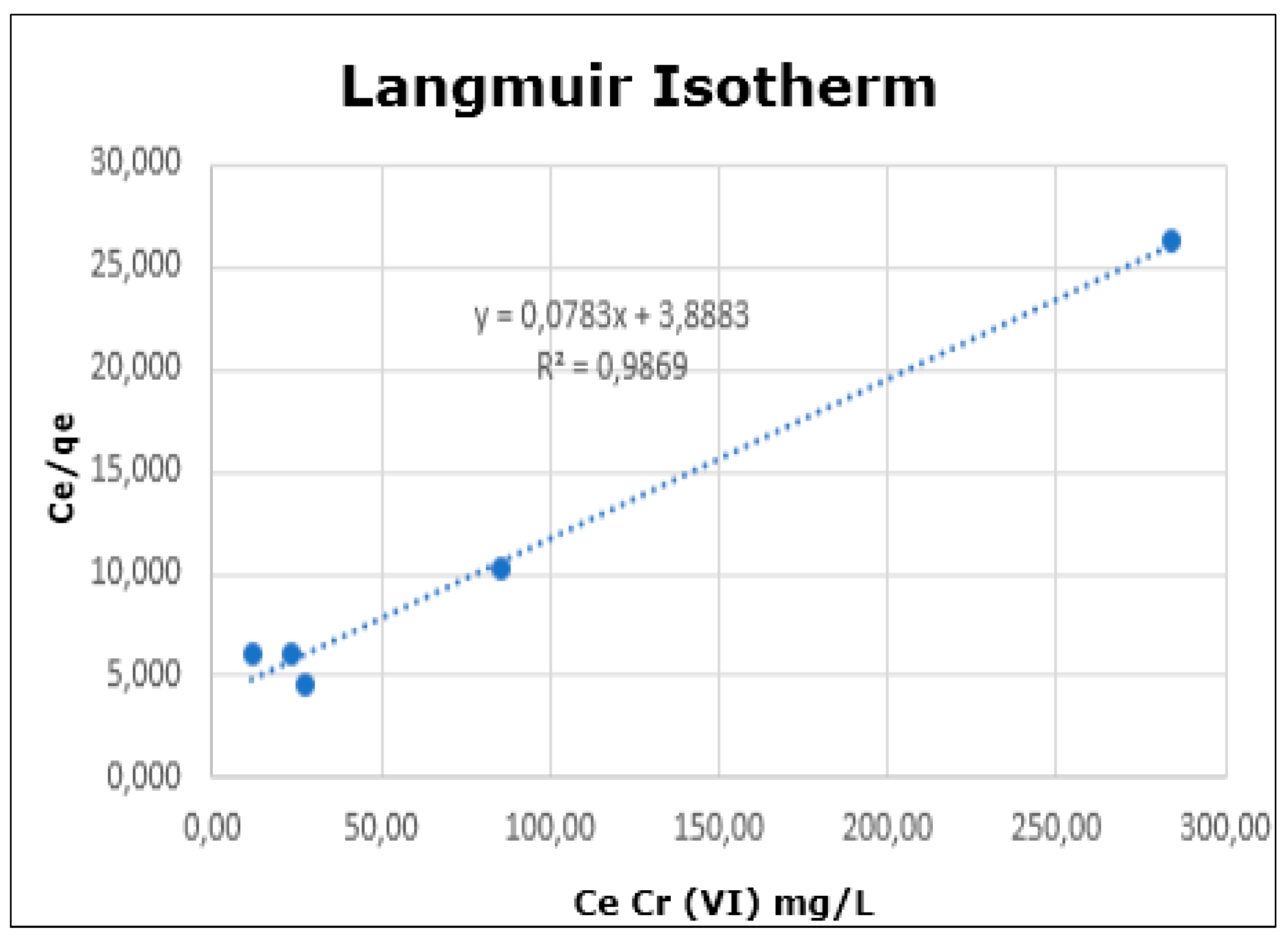
| Adsorption Isotherms | Separation Factor (RL) Model Langmuir | Maximum Adsorption Capacity (qmáx) (mg × g−1) | ||
|---|---|---|---|---|
| Langmuir R2 | Freundlich R2 | Henry R2 | ||
| 0.9869 | 0.8420 | 0.7180 | 0.12–0.77 | 13.48 |
| Mixture | Mixture Ratio (g) Agricultural Waste (PP:CP) | Optimum pH of Adsorption (pH Units) | Vol. (mL) | % Removal |
|---|---|---|---|---|
| 1 | 0.250:0.250 | 2.0 | 25 | 59.13 |
| 2 | 0.125:0.250 | 59.28 | ||
| 3 | 0.0825:0.250 | 58.86 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Aguilar, D.L.; Rodríguez Miranda, J.P.; Esteban Muñoz, J.A.; Betancur P., J.F. Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters. Processes 2019, 7, 403. https://doi.org/10.3390/pr7070403
Gómez Aguilar DL, Rodríguez Miranda JP, Esteban Muñoz JA, Betancur P. JF. Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters. Processes. 2019; 7(7):403. https://doi.org/10.3390/pr7070403
Chicago/Turabian StyleGómez Aguilar, Dora Luz, Juan Pablo Rodríguez Miranda, Javier Andrés Esteban Muñoz, and Jhon Fredy Betancur P. 2019. "Coffee Pulp: A Sustainable Alternative Removal of Cr (VI) in Wastewaters" Processes 7, no. 7: 403. https://doi.org/10.3390/pr7070403






