Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Precursor of SBAC
2.2. Preparation of SBAC
2.3. Characteristics of SBAC
2.4. Adsorption Tests
2.5. Analytical Methods
3. Results and Discussion
3.1. Characterizations of the SBAC
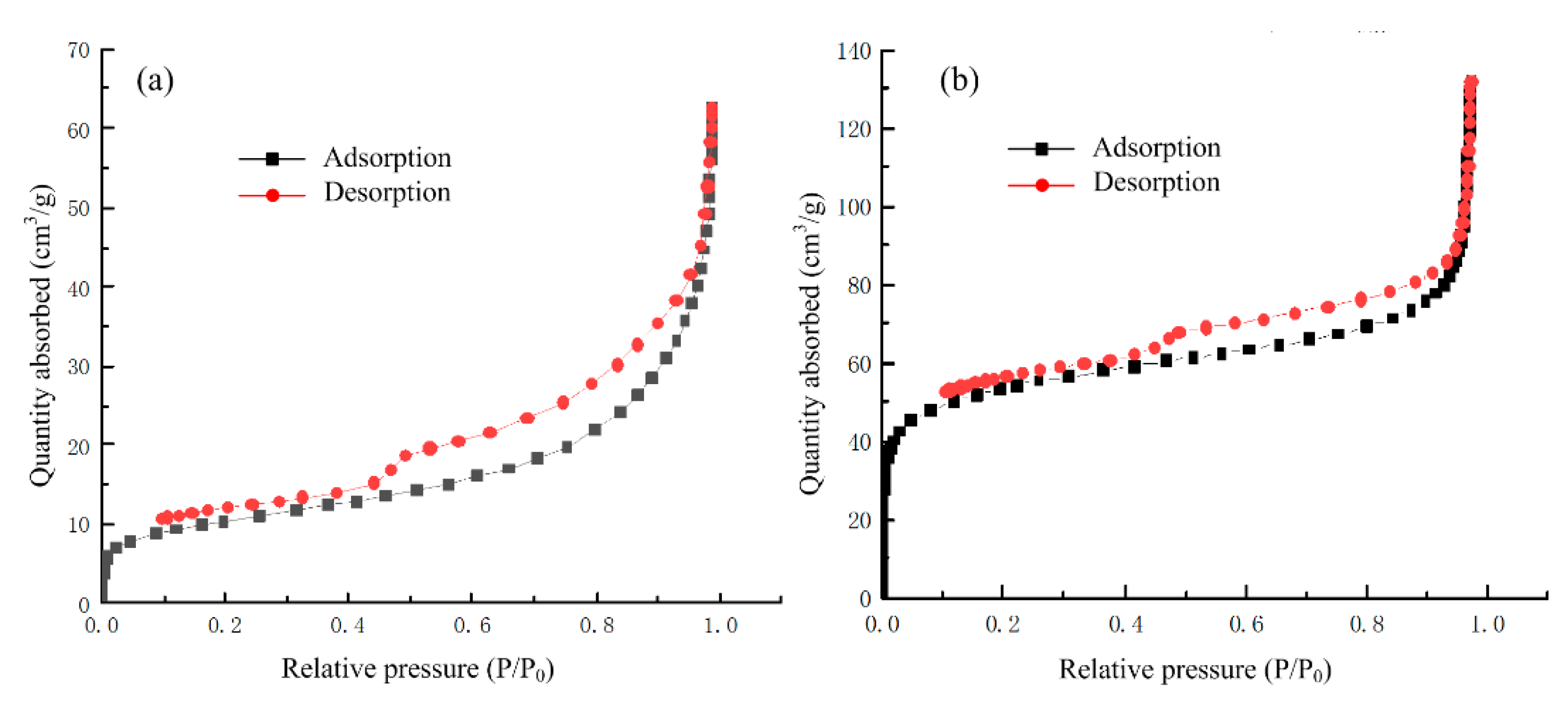
3.1.1. BET Surface Area and Pore Structure
3.1.2. SEM Analyses
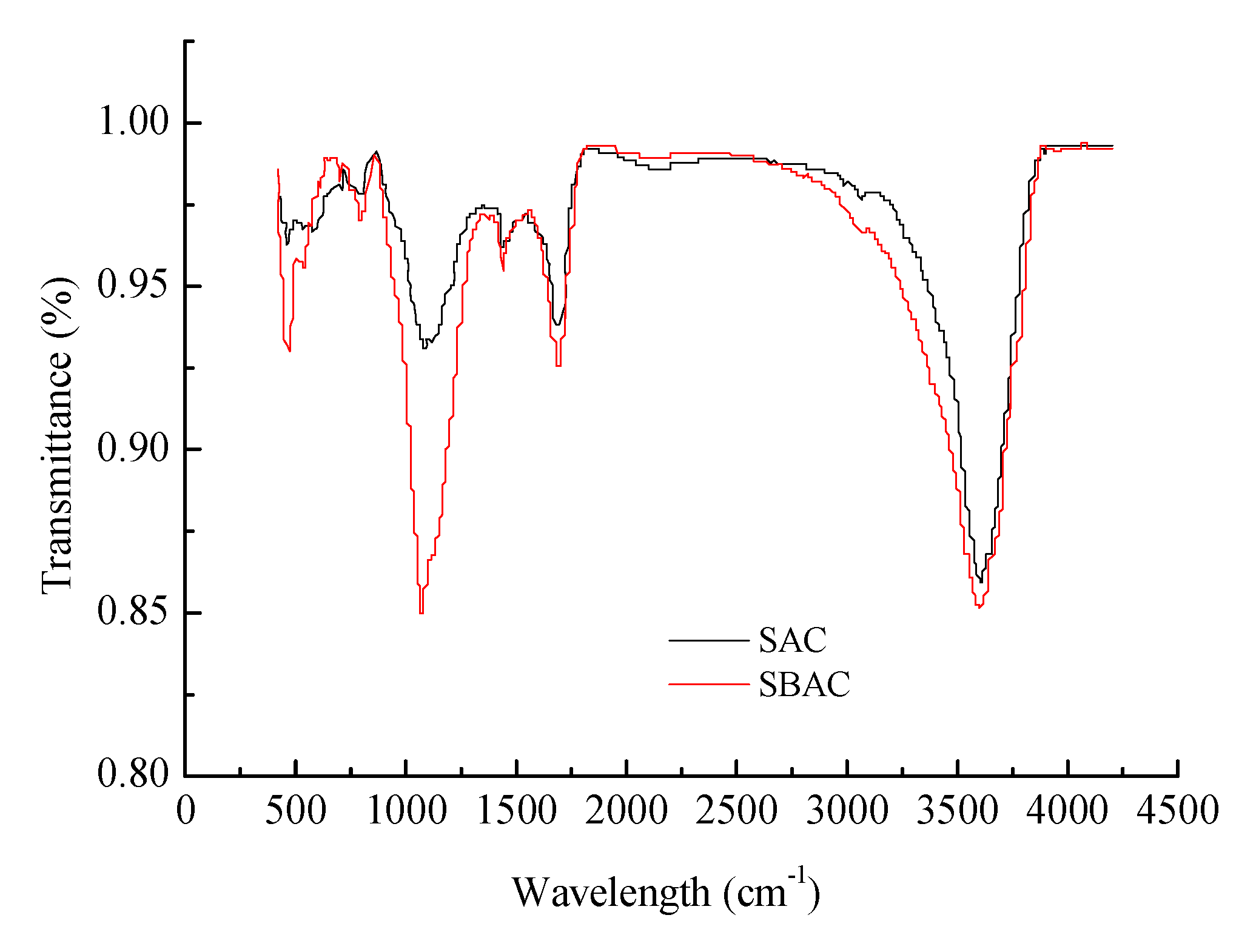
3.1.3. FTIR Analysis
3.2. Adsorption Performances of SBAC
3.2.1. Effect of Initial pH
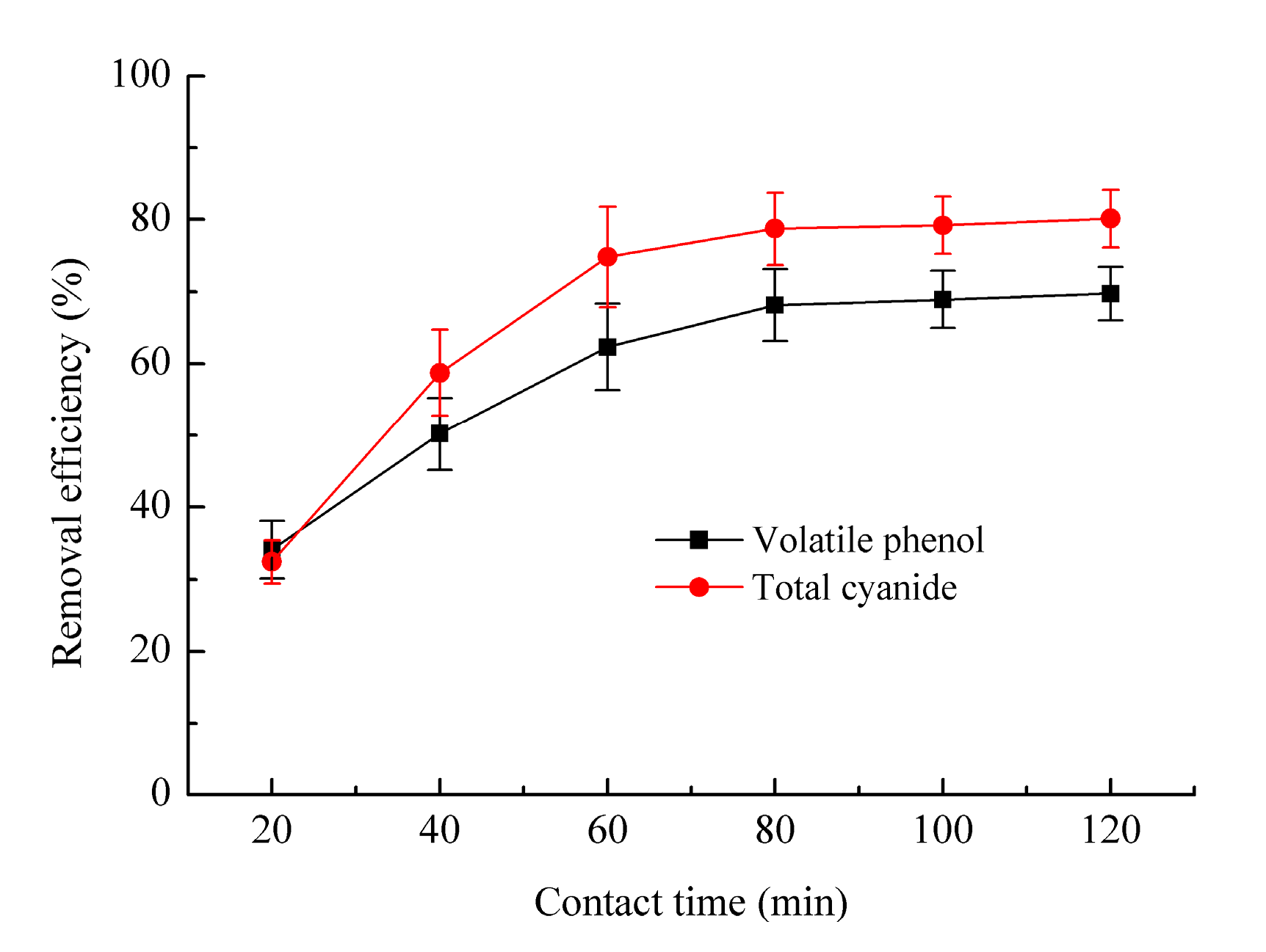
3.2.2. Effect of Contact Time
3.2.3. Effect of SBAC Dosage
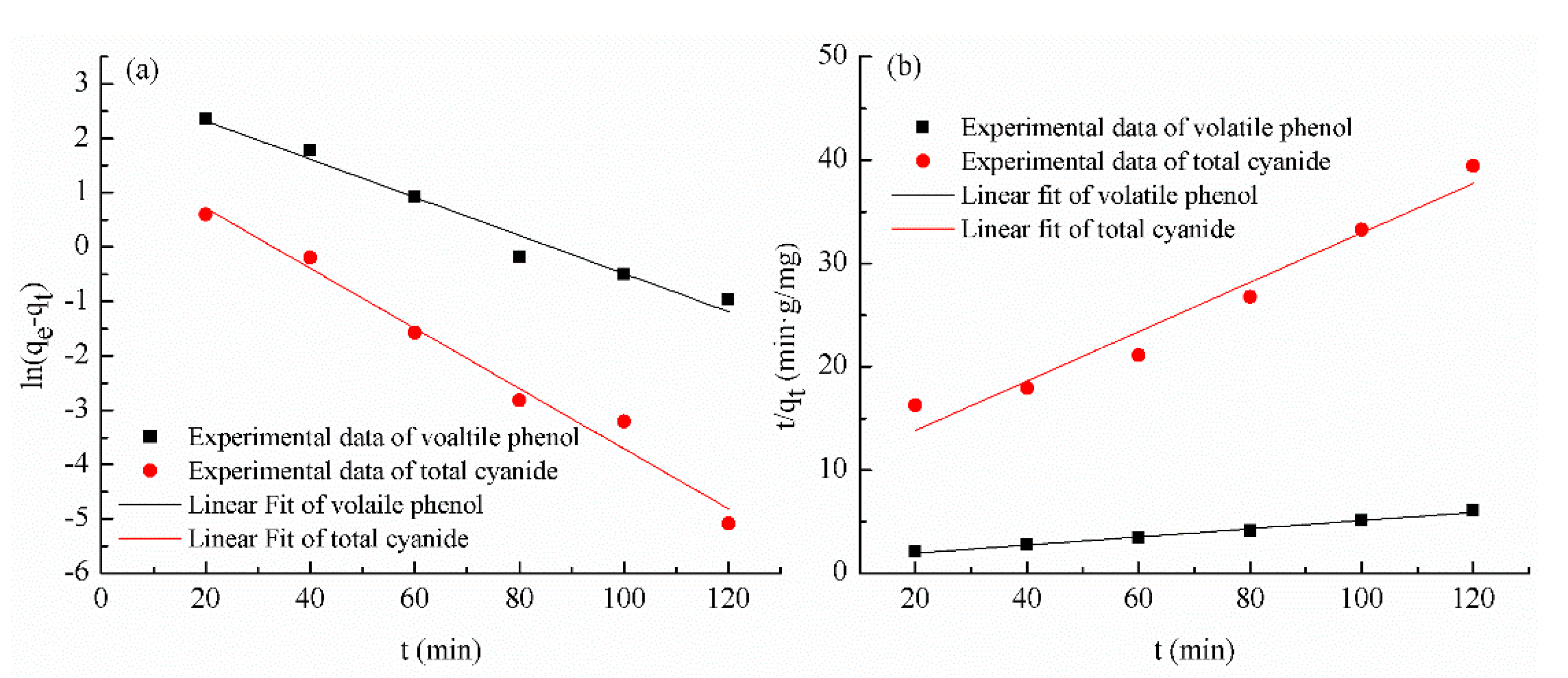
3.3. Kinetics of Volatile Phenol and Total Cyanide
3.4. Cost Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, C.; Wu, H.; Kong, Q.; Wei, J.; Feng, C.; Qiu, G.; Wei, C.; Li, F. Residual chemical oxygen demand (COD) fractionation in bio-treated coking wastewater integrating solution property characterization. J. Environ. Manag. 2019, 246, 324–333. [Google Scholar] [CrossRef]
- Zhang, Y.; Wei, C.; Yan, B. Emission characteristics and associated health risk assessment of volatile organic compounds from a typical coking wastewater treatment plant. Sci. Total Environ. 2019, 693, 133417–133427. [Google Scholar] [CrossRef] [PubMed]
- Martinkova, L.; Chmatal, M. The integration of cyanide hydratase and tyrosinase catalysts enables effective degradation of cyanide and phenol in coking wastewaters. Water Res. 2016, 102, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Razaviarani, V.; Zazo, J.A.; Casas, J.A.; Jaffe, P.R. Coupled fenton-denitrification process for the removal of organic matter and total nitrogen from coke plant wastewater. Chemosphere 2019, 224, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Zhou, M.; Zhang, Q.; Xu, X.; Li, Y.; Su, P.; Paidar, M.; Bouzek, K. Cost-efficient improvement of coking wastewater biodegradability by multi-stages flow through peroxi-coagulation under low current load. Water Res. 2019, 154, 336–348. [Google Scholar] [CrossRef]
- Sun, G.; Wan, J.; Sun, Y.; Li, H.; Chang, C.; Wang, Y. Enhanced removal of nitrate and refractory organic pollutants from bio-treated coking wastewater using corncobs as carbon sources and biofilm carriers. Chemosphere 2019, 237, 124520. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Pal, P. A novel forward osmosis-nano filtration integrated system for coke-oven wastewater reclamation. Chem. Eng. Res. Des. 2015, 100, 542–553. [Google Scholar] [CrossRef]
- Xie, R.; Wu, M.; Qu, G.; Ning, P.; Cai, Y.; Lv, P. Treatment of coking wastewater by a novel electric assisted micro-electrolysis filter. J. Environ. Sci. China 2018, 66, 165–172. [Google Scholar] [CrossRef]
- Zhou, X.; Hou, Z.; Lv, L.; Song, J.; Yin, Z. Electro-Fenton with peroxi-coagulation as a feasible pre-treatment for high-strength refractory coke plant wastewater: Parameters optimization, removal behavior and kinetics analysis. Chemosphere 2020, 238, 124649. [Google Scholar] [CrossRef]
- Pueyo, N.; Miguel, N.; Ovelleiro, J.L.; Ormad, M.P. Limitations of the removal of cyanide from coking wastewater by ozonation and by the hydrogen peroxide-ozone process. Water Sci. Technol. 2016, 74, 482–490. [Google Scholar] [CrossRef]
- Zhou, H.; Wei, C.; Zhang, F.; Hu, Y.; Wu, H.; Kraslawski, A. A comprehensive evaluation method for sludge pyrolysis and adsorption process in the treatment of coking wastewater. J. Environ. Manag. 2019, 235, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Devi, P.; Saroha, A.K. Utilization of sludge based adsorbents for the removal of various pollutants: A review. Sci. Total Environ. 2017, 578, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Peng, Y.; He, R.; Xu, Y.; Yu, T.; Hu, J. Poplar leaves reclamation for porous granules and their application in nitrobenzene removal from aqueous solution. Desalin. Water Treat. 2016, 57, 449–458. [Google Scholar] [CrossRef]
- Wang, Z. Efficient adsorption of dibutyl phthalate from aqueous solution by activated carbon developed from phoenix leaves. Int. J. Environ. Sci. Technol. 2015, 12, 1923–1932. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Balomajumder, C. Simultaneous removal of phenol and cyanide from aqueous solutionby adsorption onto surface modified activated carbon prepared fromcoconut shell. J. Water Process Eng. 2016, 9, 233–245. [Google Scholar] [CrossRef]
- Liu, Y.; Kang, X.; Li, X.; Wang, Z.; Jing, Z. Performance and mechanism of sludge dewaterability enhanced by potassium ferrate pretreatment and calcium chloride addition. J. Water Reuse Desalin. 2017, 7, 136–141. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Lin, S.; Pile, L.S.; Fang, Z.; Wang, G.G. Effect of potassium permanganate and pyrolysis temperature on the biochar production from rice straw and suitability of biochars for heavy metal (Cd & Pb) immobilizaiton in paper sludge. Fresenius Environ. Bull. 2018, 27, 9008–9018. [Google Scholar]
- Silva, T.L.; Cazetta, A.L.; Souza, P.S.C.; Zhang, T.; Asefa, T.; Almeida, V.C. Mesoporous activated carbon fibers synthesized from denim fabric waste: Efficient adsorbents for removal of textile dye from aqueous solutions. J. Clean. Prod. 2018, 171, 482–490. [Google Scholar] [CrossRef]
- Anisuzzaman, S.M.; Joseph, C.G.; Krishnaiah, D.; Bono, A.; Suali, E.; Abang, S.; Fai, L.M. Removal of chlorinated phenol from aqueous media by guava seed (Psidium guajava) tailored activated carbon. Water Resour. Ind. 2016, 16, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Castro, C.S.; Abreu, A.L.; Silva, C.L.T.; Guerreiro, M.C. Phenol adsorption by activated carbon produced from spent coffee grounds. Water Sci. Technol. 2011, 64, 2059–2065. [Google Scholar] [CrossRef]
- Jin, J.; Wang, M.; Cao, Y.; Wu, S.; Liang, P.; Li, Y.; Zhang, J.; Zhang, J.; Wong, M.H.; Shan, S.; et al. Cumulative effects of bamboo sawdust addition on pyrolysis of sewage sludge: Biochar properties and environmental risk from metals. Bioresour. Technol. 2017, 228, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Lou, L.; Luo, L.; Cao, R.; Duan, D.; Chen, Y. Effect of bamboo biochar on pentachlorophenol leachability and bioavailability in agricultural soil. Sci. Total Environ. 2012, 414, 727–731. [Google Scholar]
- Al-Wabel, M.I.; Al-Omran, A.; El-Naggar, A.H.; Nadeem, M.; Usman, A.R.A. Pyrolysis temperature induced changes in characteristics and chemical composition of biochar produced from conocarpus wastes. Bioresour. Technol. 2013, 131, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Mu’azu, N.D.; Jarrah, N.; Zubair, M.; Alagha, O. Removal of Phenolic Compounds from Water Using Sewage Sludge-Based Activated Carbon Adsorption: A Review. Int. J. Environ. Res. Public Health 2017, 14, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, P. Adsorption isotherms of microporous-mesoporous solids revisited. Appl. Catal. A Gen. 1995, 129, 157–165. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Liu, Q.S.; Zheng, T.; Wang, P.; Jiang, J.P.; Li, N. Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 2010, 157, 348–356. [Google Scholar] [CrossRef]
- Xi, X.; Guo, X. Preparation of bio-charcoal from sewage sludge and its performance on removal of Cr (VI) from aqueous solutions. J. Mol. Liq. 2013, 183, 26–30. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 20th ed.; American Public Health Association: Washington, DC, USA, 2001. [Google Scholar]
- Zhang, D.; Huo, P.; Liu, W. Behavior of phenol adsorption on thermal modified activated carbon. Chin. J. Chem. Eng. 2016, 24, 446–452. [Google Scholar] [CrossRef]
- Salame, I.I.; Bandosz, T.J. Role of surface chemistry in adsorption of phenol on activated carbons. J. Colloid Interface Sci. 2003, 264, 307–312. [Google Scholar] [CrossRef]
- Gokce, Y.; Aktas, Z. Nitric acid modification of activated carbon produced from waste tea and adsorption of methylene blue and phenol. Appl. Surf. Sci. 2014, 313, 352–359. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Martin-Martinez, M.; Gomes, H.T.; Ovejero, G.; García, J. Enhancement of p-nitrophenol adsorption capacity through N2-thermal-based treatment of activated carbons. Appl. Surf. Sci. 2017, 414, 424–434. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, B.; Balomajumder, C.; Thakur, P.K. Simultaneous co-adsorptive removal of phenol and cyanide from binary solution using granular activated carbon. Chem. Eng. J. 2013, 228, 655–664. [Google Scholar] [CrossRef]
- Feng, J.; Qiao, K.; Pei, L.; Lv, J.; Xie, S. Using activated carbon prepared from Typha orientails Presl to remove phenol from aqueous solutions. Ecol. Eng. 2015, 84, 209–217. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. K. Sven. Vetensk. Handl. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Ahmaruzzaman, M. Industrial wastes as low-cost potential adsorbents for the treatment of wastewater laden with heavy metals. Adv. Colloid Interface Sci. 2011, 166, 36–59. [Google Scholar] [CrossRef]
- Xu, G.; Yang, X.; Spinosa, L. Development of sludge-based adsorbents: Preparation, characterization, utilization and its feasibility assessment. J. Environ. Manag. 2015, 151, 221–232. [Google Scholar] [CrossRef]



| Parameters | Volatile Solids (VS) (dw %) | Ash (dw %) | Water Content (ww %) | C (dw %) | H (dw %) | N (dw %) |
|---|---|---|---|---|---|---|
| MS | 62.01 | 37.99 | 78.86 | 26.5 | 6.24 | 4.08 |
| BW | 98.64 | 1.36 | 9.94 | 48.5 | 3.35 | 0.75 |
| Parameters | Coking Wastewater |
|---|---|
| Chemical oxygen demand (COD) (mg/L) | 2457.6 ± 125.4 |
| Ammonia nitrogen (mg/L) | 316.3 ± 24.2 |
| Total cyanide (mg/L) | 35.4 ± 1.5 |
| Volatile phenol (mg/L) | 227.5 ± 7.6 |
| Suspended solids (SS) (mg/L) | 76.9 ± 6.5 |
| pH | 8.1 ± 0.8 |
| Samples | SBET (m2/g) | Micropore Surface Area (m2/g) | VT (cm3/g) | Vmic (cm3/g) | Dp (nm) |
|---|---|---|---|---|---|
| SAC | 70.921 | 18.568 | 0.194 | 0.016 | 10.941 |
| SBAC | 289.578 | 70.523 | 0.267 | 0.049 | 3.688 |
| Kinetic Modeling Parameters | Pseudo-First-Order | Pseudo-Second-Order | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Volatile phenol | 0.0351 | 20.46 | 0.9737 | 1.29 | 0.0013 | 25.38 | 0.9912 | 25.64 | 20.02 |
| Total cyanide | 0.0553 | 6.17 | 0.9804 | 89.26 | 0.0063 | 4.18 | 0.9605 | 28.22 | 3.26 |
| Materials and Energy | Consumption | Unit Cost | Cost (USD) |
|---|---|---|---|
| ZnCl2 (recovery rate of 70%) | 0.315 kg | 1.227 (USD/kg) | 0.387 |
| HCl | 0.169 kg | 45.984 (USD/t) | 0.008 |
| Water | 51.240 L | 0.859 (USD/kg) | 0.044 |
| Electricity | 6.5 kWh | 0.104 (USD/kWh) | 0.676 |
| BW | 0.562 kg | 0.359 (USD/kg) | 0.202 |
| By-product tar | 0.52 kg | 308.955 (USD/t) | −0.306 |
| Sludge treatment compensation | 7.460 kg | 22.992 (USD/t) | −0.171 |
| Total (USD/kg) | 0.840 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cheng, H.; He, Y. Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater. Processes 2020, 8, 82. https://doi.org/10.3390/pr8010082
Liu Y, Cheng H, He Y. Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater. Processes. 2020; 8(1):82. https://doi.org/10.3390/pr8010082
Chicago/Turabian StyleLiu, Yali, Han Cheng, and Yueting He. 2020. "Application and Mechanism of Sludge-Based Activated Carbon for Phenol and Cyanide Removal from Bio-Treated Effluent of Coking Wastewater" Processes 8, no. 1: 82. https://doi.org/10.3390/pr8010082