Abstract
Carbon dioxide (CO2), a major greenhouse gas, capture has recently become a crucial technological solution to reduce atmospheric emissions from fossil fuel burning. Thereafter, many efforts have been put forwarded to reduce the burden on climate change by capturing and separating CO2, especially from larger power plants and from the air through the utilization of different technologies (e.g., membrane, absorption, microbial, cryogenic, chemical looping, and so on). Those technologies have often suffered from high operating costs and huge energy consumption. On the right side, physical process, such as adsorption, is a cost-effective process, which has been widely used to adsorb different contaminants, including CO2. Henceforth, this review covered the overall efficacies of CO2 adsorption from air at 196 K to 343 K and different pressures by the carbon-based materials (CBMs). Subsequently, we also addressed the associated challenges and future opportunities for CBMs. According to this review, the efficacies of various CBMs for CO2 adsorption have followed the order of carbon nanomaterials (i.e., graphene, graphene oxides, carbon nanotubes, and their composites) < mesoporous -microporous or hierarchical porous carbons < biochar and activated biochar < activated carbons.
1. Introduction
Fossil fuels supply more than 98% of the world’s energy demands [1]. Due to the burning of fossil fuels in industrial activities, the concentration of CO2 has been increasing in the atmosphere significantly [2]. For example, CO2 concentration hits up to 415.26 ppm at the Mauna Loa Observatory in Hawaii [3]. It is estimated that, in 2050, the atmospheric CO2 concentration will reach up to 550 ppm [3]. Therefore, the increased concentration of CO2 in the atmosphere causes global warming and significant environmental problems [3,4,5]. Hence, there is a great urgency to reduce the CO2 level from the atmosphere through the utilization of different technologies. The intergovernmental panel on climate change has recommended three fundamental steps for carbon capture and storage for combating carbon dioxide emissions. These involve (i) separation through capture, (ii) transportation, and (iii) storage of CO2 [6]. Although enough progress has been made on transportation and storage of CO2, progress is still going on the capture of CO2 through different processes [7]. Membrane separation techniques have been utilized for the capture of CO2 at low pressure. However, these kind of technologies often suffers from high operating costs, and they are non-energy efficient to compress the feed gas [8]. Technologies for the removal of CO2 from ambient air have been recently demonstrated using different solid or liquid sorbents, which can contribute to “negative carbon emission”, although there remains much room for their improvements [9,10]. On the other hand, porous-based materials are very promising materials to adsorb CO2. Hence, compared to the liquid adsorption-based technology (such as amine-based adsorption technology), CO2 capture via solid-state materials (e.g., adsorption technology) is very cost-effective, easy to design, has a functional surface, hydrophobicity, need low energy consumption, simple operation, and easy regeneration of adsorbents [11,12,13,14,15]. Solid adsorbents are alkaline metal oxides and hydroxides, zeolite, metal-organic frameworks, porous polymers, and carbon-based materials (CBMs), such as activated carbon, biochar, nanocarbons (carbon nanotubes (CNTs) and graphene), mesoporous, and microporous carbons, and so on. Among them, CBMs have great potential in the capture of CO2 due to their high surface area, well-defined porosities, larger pore volume, chemical stability, and easy handling [3,11,12,13,14,15,16,17,18,19].
Scheme 1 demonstrates a brief summary of CBMs, which are used for the adsorption of CO2. Although there are many reviews on CO2 capture, however, to our best knowledge, none of them has discussed the overall efficacy of CMBs for adsorption of CO2. Therefore, the main objective of this review was to demonstrate the comparative analysis of the efficacies of different CBMs for CO2 adsorption from the air at different temperatures and pressures. The subsequent objective of this review was to provide an overview of the performance of CBMs together with the major associated challenges and future opportunities for the potential applications of CBMs as CO2 adsorbents. Hence, we believed that this review would be very helpful for the different researchers and stockholders for the understanding of the recent trends of CBMs performances for CO2 capture through adsorption technology.

Scheme 1.
CBMs (carbon-based materials) for CO2 capture through adsorption technology.
2. Efficacy of CBMs for CO2 Capture
CBMs are considered as the top performance material for CO2 adsorption from the air [15]. CBMs have specific properties, which are highly required for efficient CO2 capture. There are many types of carbon-based adsorbents, but they can be broadly classified as biochar, nanocarbons materials (e.g., graphene, CNTs, nanocarbons), activated carbons (ACs), different microporous, mesoporous, and hierarchical carbons with or without doping with other inorganic, organics, metal components, or metal atoms, and so on. All of these CBMs have a significant surface area, pore density, volume, pore size, high stability, and sustainability properties, which are prime requirements for efficient CO2 capture. Therefore, this review has covered the performances of biochar, different nanomaterials, such as graphene, graphene oxides, and carbon nanotubes (CNTs), ACs, microporous, mesoporous, and hierarchical porous carbon materials together with their composites. The following subsections have addressed CO2 capture efficacies using those CBMs.
2.1. Biochar for CO2 Capture
Recently, among various adsorptive materials (e.g., AC, graphene, carbon fibers, etc.), biochar has gained considerable attention as an eco-friendly and cost-effective material for CO2 capture and sequestration, as catalysts, as greenhouse gas capturing material, as water treatment and, as soil remediation materials [20,21,22]. Biochar is a carbon-rich material, which is prepared from natural resources having high surface area, hydrophobic nature, and easy regeneration capability [23]. These properties make the biochar an attractive material for the researcher’s various applications [24,25]. Biochar can be synthesized from cheap and easily available biomass feedstocks and wastes from different industries (e.g., dairy manure, forestry, agricultural) and many other bio-wastes [26,27,28]. Biomass resources are composed of C, O, H structures and some of the inorganic materials in their complex matrix together with different heteroatoms (e.g., N, P, or S) [21,29]. However, the quality and yield of biochar depend on several parameters, such as feedstock material and operational conditions.
Biochar can be prepared through different processes, such as gasification (where different biochar, gaseous fuel, such as syngas, and tar (oil) are produced); torrefaction (where biomass is thermally treated for a short period at low-temperature sally 473–573 K); hydrothermal carbonization (where biochar is produced in the presence of water, low oxygen content, high pressure, usually 14–22 MPa, and low temperature at 393–573 K); pyrolysis process (where biomass is thermally converted into its basic graphitic structure at 473_1473 K in a limited or inert atmosphere) [30,31,32]. Figure 1 shows a simple overview of biochar production from biomass using different thermochemical processes. Hence, the porous biochar is produced [24,33,34].
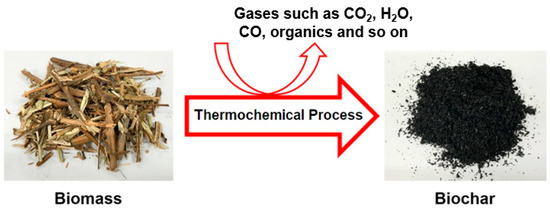
Figure 1.
A general overview of biochar production from different biomasses.
Owing to the unique structure and surface properties of biochar, it can act as an excellent adsorbent for the capture of several gases. In a study, Mohd et al. [35] reported that adsorption of toxic gases on biochar surface took place mainly through the physisorption process. The surface of biochar contains macro and micropores, which act as a storage place for gas molecules [35]. Table 1 shows the CO2 intake capacity of biochar at 1 bar atmospheric pressure and two different temperatures. It is clear from the table that chemically activated biochar prepared from Vine shoots were capable of adsorbing a higher amount of CO2 (6.08 mmol/g at 1 bar and 273 K) compared to physically activated biochar (4.07 mmol/g at 1 bar and 273 K) [36,37]. In another study, Ello et al. [37] prepared biochar and biochar activated with KOH at 1133 K for 1 h from Africa palm shells. They reported higher CO2 adsorption capacities (6.3 mmol/g at 273K and 4.4 mmol/g at 298 K and 1 bar, respectively). On the other hand, different CO2 intake capacities were also reported for chemically activated biochars from rice husk (3.71 mmol/g) [38], pine nutshell (5.0 mmol/g) [39], wheat flour (3.48 mmol/g) [40], vine shoots (2.46 mmol/g) [36], coconut shells (4.23 mmol/g) [11], Jujun grass (hydrochar, 4.9 mmol/g) [41], and Camellia Japonica (Hydrochar, 5.0 mmol/g) [41] at 298 K and 1 bar pressure. Moreover, single-step pyrolysis and activation of various biomasses to produce biochar and activated biochar were also reported by Serafin et al. [42]. They found that CO2 adsorption capacities of pomegranate peels, carrot peels, and fern leaves were 4.00, 4.18, and 4.12 mmol/g at 298 K, respectively, and 6.89, 5.64, and 4.52 mmol/g at 273 K, respectively, at 1 bar. Zhang et al. [43] produced amine functional group doped activated biochar from black locust. They reported a CO2 adsorption capacity of 5.05 mmol/g at 298 K and 1 bar. Similarly, Rouzitalab et al. [44] used urea to synthesize amine-functionalized activated biochar from the walnut shell in the presence of KOH, and they observed record CO2 adsorption capacity of 7.42 mmol/g at 298 K and 1 bar.

Table 1.
CO2 capture performances by top performance biochar produced from different biomasses and at different conditions. The surface area is based on Brunauer–Emmett–Teller (BET).
However, CO2 adsorption capacity can significantly vary with the changing of the surface morphology of biochar, i.e., the surface area, micropore volume, and size, together with the effects of temperature and pressure [24,42]. For example, Deng et al. [39] reported that biochar having a pore size of 0.33–0.63 nm played an important role in the higher CO2 adsorption. It was also reported that the control of micropores had greater importance for adsorbing high CO2 compared to surface area and total pore volume [39,42]. Figure 2 shows the presence of functional groups and porous structures (mesoporosity and microporosity) of biochar materials. Metal oxyhydroxide biochar composites have also been used to increase the adsorption capacity of biochar. For example, Lahijani et al. [47] reported that Mg-loaded biochar showed a higher CO2 adsorption capacity (3.7 mmol/g) than that of raw biochar (3.2 mmol/g) at 298 K and 1 atm. This phenomenon can be explained by the fact that the incorporation of metals (i.e., Mg, Al, Ni, and Fe) onto the biochar surface will increase basic sites on the surface of biochar, which enhances the adsorption capacity of acidic CO2 [47].
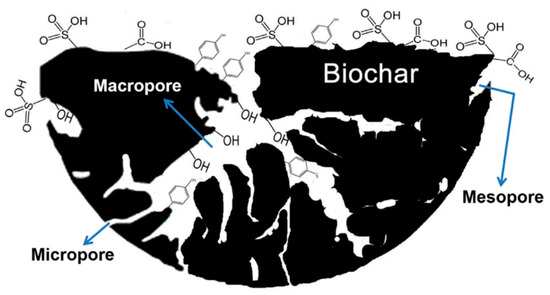
Figure 2.
Morphology and the presence of functional groups in biochar. Reproduced with permission from [24]; Elsevier and Copyright Clearance Center, 2017.
Therefore, it can be summarized that biochar and activated biochar/biochar-based adsorbents are low-cost, renewable, and promising materials for the adsorption of CO2. However, still there remain various challenges, especially which can prevent the practical and large-scale application of biochar-based adsorbents for CO2 removal, which need to be addressed. First of all, the robustness and stability of biochar-based adsorbents have not been fully demonstrated, despite the fact that high adsorption capacities and long-term cyclic operation are critical to ensure the economics and practicality of the technology [48]. Secondly, the production process should be simple, cost-efficient, and eco-friendly to develop highly efficient CO2 adsorbents. Thirdly, both physical and chemical modification methods have been carried out in laboratory-scale experiments. However, most studies are explorative in nature, and the effectiveness of the methods for large-scale biochar modification and application is still unclear. Finally, a new type of modified biochar should keep continuing to develop with larger surface area, well-defined porosity, together with surface functional groups, and it is also necessary to produce biochar from low-cost materials, such as agricultural wastes.
2.2. Graphene, Graphene Oxide, and Carbon Nanotubes (CNTs) for CO2 Capture
CBMs can be dimension-less and less than 100 nm but in many forms. Nanomaterials are extensively used for different applications, owing to their downsized unique properties. They can be used as catalysts supports, adsorption, energy conversion, charge storage device preparation, filtration, electrode materials, conductive materials, and so many [49]. Graphene-based nanomaterials are also used for CO2 capture [49]. The development of new adsorbents with high capacity and high selectivity for reducing energy-related CO2 emissions is a topic of utmost global importance because of its implications in climate change mitigation. Recent advances in materials science and engineering suggest that graphene-based adsorbents are wonder material with many attractive properties and can deliver viable solutions to the challenges of developing cost-effective, energy-efficient, and high-volume adsorption-based CO2 capture technologies. To date, a wide range of graphene materials has been investigated to curb CO2 emissions from static sources of fossil fuel combustion. Table 2 represents the CO2 adsorption performance by graphene, graphene oxide, CNTs, and composite materials. Graphene-based materials, such as graphene oxide, have different oxygen-containing functional groups, which can show higher chemical reactivity over pristine graphene [50]. The introduction of different heteroatoms (e.g., N, boron B, aluminum Al, sulfur S, and so on) in graphene can increase the adsorption capacity of CO2. For example, Liu et al. [51] prepared N and B-doped graphene aerogels, which showed CO2 capture capacities of 2.9 mmol/g at 273 K and 1.0 bar pressure. On the other hand, Bhanja et al. [52] did a modification of graphene oxide with 2,6-diformyl-4-methyl phenol. They reported that this material could capture CO2 up to 8.10 mmol/g at 273 K. Recently, graphene-based monoliths have been prepared following a one-step water-based method, which has shown an excellent CO2 capture performance of 2.1 mmol/g at 298 K and 1 bar [53]. On the other hand, Huang et al. [54] synthesized a hybrid composite based on polyethyleneimine (PEI)-modified graphene oxide and ZIF-8. This composite showed a higher CO2 capture capacity of 8.08 mmol/g at 273 K and 1 bar. Rahimi et al. synthesized bundles of double-walled CNTs with an inner diameter of 8 nm, and they reported excellent CO2 adsorption capacity (i.e., 3.5 mmol/g at 308 K and 1 bar) [55]. An improved innovative hydrate-based CO2 capture was observed by the rational surface modification of CNTs by Zhao et al. [56]. However, the maximum CO2 capture performance (up to 8.75 mmol/g at 196 K and 1 bar) was observed by Jonathan et al. [57] by synthesizing a new composite based on single-walled carbon nanotube (SWCNT@HKUST-1).

Table 2.
CO2 capture performances, recently reported by graphene, graphene oxide, carbon nanotubes (CNTs), and their composites.
On the other hand, Alhwaige et al. [63] synthesized chitosan aerogels with graphene oxide nanosheets, which showed CO2 capture capability up to 4.14 mmol/g. Few other aerogels and cross-linked composites have been also reported, which have shown CO2 adsorption capacity up to 5.72 mmol/g at 298 K and 1 bar [62].
Therefore, based on the above description, it can be clearly said that graphene, graphene oxide, and CNTs have CO2 capture ability, specifically in terms of high storage, excellent selectivity, rapid uptake, easy regeneration, and good reproducibility and stability. However, the maximum adsorption capacity comes from polyethyleneimine-modified graphene and graphene oxide compared to other graphene, graphene oxides, and CNTs. In comparison to other competing adsorbents, a key advantage of these material systems is that many different functional groups or heteroatoms can be attached to their surface, allowing custom-tailoring of surface properties without sacrificing the remarkable intrinsic characteristics of the graphene core. However, a number of technological limitations and practical challenges have to be tackled in order to produce next- generation graphene-based adsorbents with the capability of being applied on an industrial scale for efficient and effective CO2 separation from flue gases. Henceforth, future applications of such kinds of materials for CO2 capture need further consideration with mainly focusing on the significant improvement in the adsorption capacity as well as the low-cost production of these materials.
2.3. Activated Carbons (ACs) for CO2 Capture
Activated carbon is a high-porosity material, which is useful in adsorption and separation of many gas mixtures [64,65]. Perhaps, ACs have widely been used for CO2 capture compared to other types of CBMs. This is because they have high surface area (SA), pore-volume, and submicroscopic pores [5,66,67]. ACs are not degraded in acidic and basic conditions [68]. Hence, they possess excellent performance in CO2 uptake. Table 3 summarizes the CO2 capture performances by different ACs.

Table 3.
CO2 capture performances by different activated carbons (ACs).
ACs can be derived from biomass through pyrolysis but requires either physical or chemical activation. Physical activation can be performed using steam/water vapor, air, or CO2. On the other hand, carbon can also be chemically activated by various chemicals to increase the surface area, as well as add (or remove) specific surface functional groups. When carbon is activated with ammonia at high temperatures, nitrogenous groups are added, and acidic oxygen groups are removed, which significantly improves basicity (Shafeeyan et al., 2020) [81].
However, different precursors, such as biomasses, coal, and petroleum pitch, are used for the production of ACs. However, mostly used precursors are biomasses, coal, and petroleum pitch [82]. For example, Shao et al. [71] synthesized ACs from coal tar pitch with an extremely high surface area of 3537 m2/g. This AC could capture CO2 up to 20.66 mmol/g at 298 K and 18 bar. On the other hand, ACs can also be prepared from different biomass precursors. For instance, Chen et al. [67] synthesized N-doped microporous-ACs from coconut shells by using urea as an activating agent. They found the CO2 capture capacity of 7.0 and 4.8 mmol/g at 273 and 298 K, respectively, at 1 bar. An ultrahigh-surface area of ACs (3350 m2/g) was achieved by using starch as a source of a precursor. These ACs could capture CO2 up to 3.4 mmol/g at 298 K and 1 bar [72]. On the other hand, polyurethane foam-based AC was synthesized by Ge et al. [67], whose adsorption capacity was 5.85 mmol/g at 273K and 1 bar. In another study, the CO2 removal capacity of polyacrylonitrile-based AC fibers at 298K and 1 bar was reported to be 2.74 mmol/g [72]. It is reported that each year, around 140 billion metric tons of biomasses are produced from agriculture resources [73]. So, the proper utilization of agricultural wastes together with other biomass sources, such as food residues, nutshells, cellulose craft, lignin, sawdust, rice husk, chips, logs, wood processing residues, marine microalgae, and pitch, for the production of ACs in an environmentally friendly, as well as an economic way, could be an alternative solution. Such an example is given in Figure 3, where celtuce leaves were pyrolyzed at a high temperature, followed by a chemical activation process [74].
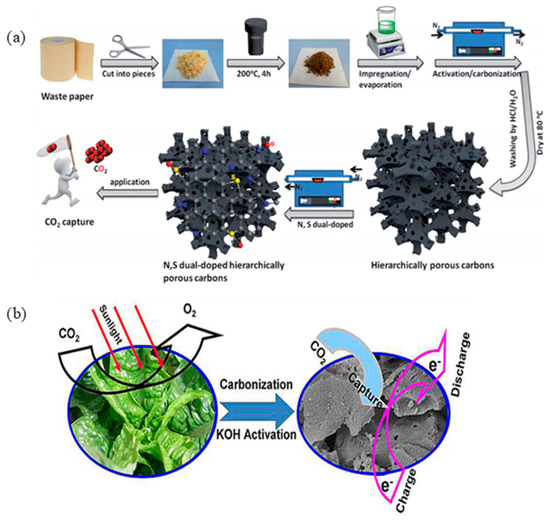
Figure 3.
ACs (activated carbons) preparation from (a) waste paper and (b) biomass [73,74]. Reproduced with permissions from the references of [73,74]; Copyright © 2019, Royal Society of Chemistry and Copyright © 2012, American Chemical Society; respectively.
In summary, it can be mentioned that ACs materials are excellent materials for the adsorption of CO2 with higher adsorption capacity, as well as they can be prepared from low-cost materials. ACs have higher potential for commercial applications as they have higher adsorption capacity, high surface area, microporosity, mesoporosity, and stability. Hence, AC is one of the top performance CBMs for the CO2 capture.
2.4. Microporous, Mesoporous, and Hierarchical Porous Carbons for CO2 Capture
Porous carbon materials have versatile properties, such as high Brunauer–Emmett–Teller (BET) surface area, adjustable pore structure, cost-effective, and easy regeneration [83]. Generally, there are three different types of porous carbon materials, i.e., microporous (<2 nm), mesoporous (2–50 nm), and macroporous (>50 nm), but hierarchical porous carbon (HPC) consists all of these properties [84]. For example, Lizen et al. [85] synthesized super porous carbon materials with 95% mesoporosity using polypyrrole as a precursor material. They mentioned about the ultra-high surface area (i.e., 2800–4000 m2/g) and pore volume (i.e., 2.5–3.6 cm3/g). However, their CO2 capture capacity was found to be 2.8 mmol/g at 298 K. On the other hand, it was found that the mesoporosity was significantly increased by using sodium amide (NaNH2) during activation and doping with magnesium (Mg) and nitrogen (N2). These materials showed excellent CO2 uptake performance (3.68–6.31 mmol/g at 273 K) [86,87,88]. On the other hand, Park et al. [89] synthesized 3D ordered mesoporous carbon and observed the CO2 capture capacity of 5.53 mmol/g. Recently, a newly designed porous geopolymer template was developed by Pei et al. [90], which was based on the metakaolin. This AC had an excellent CO2 capture performance of 26.30 mmol/g at 273 K and 30 bar (Table 4). HPC ordered materials have great potential for high CO2 capture as they have great interest due to their many advantages, such as high microporosity, high surface area, higher microporous quantity, and so on. For example, HPC with a prominent BET surface area up to 2734 m2/g had higher CO2 capture performance up to 27 mmol/g at 30 bar and 300 K [91]. Hence, carbon nanomaterials can possess a hierarchical porous structure and contain both macropores and micropores structure. These properties of carbon, together with the high surface area, are very important for higher CO2 capture [92].

Table 4.
CO2 capture performances by microporous, mesoporous, and hierarchical porous carbons.
Porous carbon materials have drawn great attention due to the remarkable pore structure, high specific surface area, large pore volume, excellent property of adsorption, and separation. When the material is highly microporous, it may result in a long equilibrium time for CO2 adsorption. Large mesopores enable faster transfer of gas from the bulk phase to micropores and, thus, result in faster equilibrium [113,114]. Although microporous and mesoporous content has been found to be the best indicator of CO2 capture performance, a large pore volume values originating from a distinct large mesoporous peak can improve CO2 performance as well. So, utilizing the hierarchical porous carbon materials by adjusting various templates and catalysis with large pore volume and high surface area would be the best candidate for reducing the emission of CO2 to the environment.
3. Comparative Analysis of CBMs Performances
CBMs are found to be very effective in the capture of CO2 at various conditions with varying degree of adsorption capacity. We know that different adsorbents have been produced at different conditions using different precursors. Based on rough estimation, it can be mentioned that biochar and activated biochar are cheap materials compared to any other CBMS. Table 5 lists the rough lower and higher prices of each CBMs, although the actual cost may vary depending on several factors, such as purity, quality, quantity, and so on. Based on the table, it can be seen that carbon-based nanomaterials, such as graphene, graphene oxide, and CNTs, have a higher cost compared to other types of CBMs. Besides, the further modification of those materials can significantly increase the cost, such as composite materials preparation and fabrication for the end-use. However, their average CO2 adsorption capacity values were 5.13 ± 1.62 and 3.23 ± 1.13 mmol/g, respectively, at 273 and 298 K, which was even lower than that of cheap materials, such as biochar at both temperatures (Figure 4). These results indicate that graphene, graphene oxide, and CNTs have lower CO2 adsorption capacity compared to biochar and activated biochar and even compared with other types of CBMs.

Table 5.
Rough prices of different carbon-based adsorbents [115]. Price varies based on purity, quantity, quality, and type of materials.
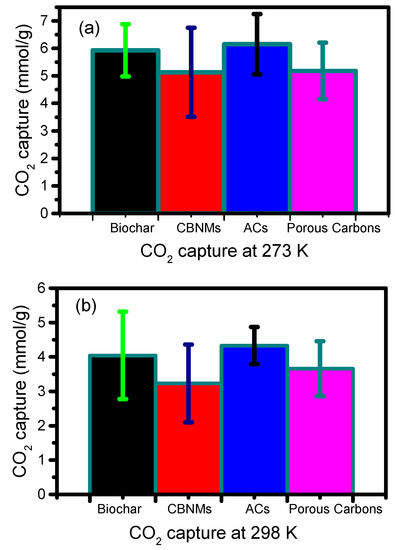
Figure 4.
Average (with standard deviation) CO2 capture performance by different carbon-based materials at two different temperatures, i.e., 273 K and 298 K, respectively. Biochar refers to biochar and activated biochar; CBNMs refers to graphene, graphene oxide, CNTs, and their composites; porous carbon refers to micro, meso, and hierarchical porous carbons. Each set of data refers to the average value (with standard deviation) at the adsorption capacities of each type of material, which was generated from Table 1, Table 2, Table 3 and Table 4.
On the other hand, biochar and activated biochar have higher CO2 capture performance over graphene, graphene oxides, and CNTs, although some special cases can cease this estimation. On the right side, different meso-micro and hierarchical porous carbons have slightly lower CO2 adsorption capacities than that of biochar, and they have higher efficacy over graphene, graphene oxides, and CNTs. Hence, biochar and activated biochar have a higher potential for the capture of CO2 than hierarchical porous carbons.
However, ACs have been found very effective among all types of CBMs with the higher average CO2 capture performances (6.15 ± 1.10, 4.33 ± 0.54 mmol/g, respectively, at 273 and 298 K) at both temperatures (Figure 4). These average values indicate that ACs have higher CO2 capacities over biochar, activated biochar, hierarchical porous carbons, graphene, graphene oxide, and CNTs. These are mainly due to their high surface area and the properties of ultra-microporous structures. Therefore, ACs are the top performance materials for the capture of CO2. However, there might have some other form of carbons that can overcome these estimations, but grossly ACs are the highly efficient materials for CO2 capture. Hence, for CO2 capture, CBMs follow the order of carbon nanomaterials (i.e., graphene, graphene oxides, CNTs, and their composites) < meso-micro or hierarchical porous carbons < biochar and activated biochar < activated carbons.
4. Future Challenges and Opportunities
Although enough progress has been done towards the synthesis of CBMs and application for CO2 capture, still there is a lack of studies. For example, it is highly necessary to consider the effects of different parameters, such as the presence of moisture, foreign ions, environmental conditions, neutral and ionic species, and so many, for the effective capture of CO2 and to measure the overall efficacy of CBMs from the atmosphere [116]. Therefore, further investigations are needed in many areas. They are:
- Developments of the novel composite to improve the capture performance of CO2 of CBMs.
- A need to properly understate the CO2 interactions with CBMs. For this reason, new analytical tools are needed to develop.
- Ensuring the regeneration efficiency for repeatable applications. Regeneration mechanisms also need to study in detail.
- Development of new technologies for the efficient capture of CO2.
- A highly efficient carbon-based catalyst needs to develop for the conversion of CO2 into valuable fuels, such as methane.
- Low-cost materials with high adsorption capacity need to develop.
- Most of the CBMs have been used for CO2 capture on a lab-scale basis, i.e., from ambient air. However, studies are not enough. Therefore, more studies are required.
- Other types of materials, such as metal-organic frameworks, porous silica, resin, amine derivatives sorbents, and new types of materials, need to produce with lower cost for the scale-up process.
- These coatings of sorbents can help for faster heat and mass transfer, as well as can reduce energy losses. Therefore, these kinds of sorbents need to develop.
- Detailed kinetics of sorption and mechanisms need to be focused on more clearly.
- Combining together and application of the different existing technologies can reduce the cost of the capture of CO2.
5. Conclusions
CBMs are very efficient in the capture of CO2 from the air at different temperatures and pressures due to their specific properties, including high surface area, mesoporosity, microporosity, micropore volume, well-defined pore size distributions, and high stability, at different environmental conditions. Among different CBMs, activated carbons and activated biochar are found to be the top performance materials for the capture of CO2 in an environmentally friendly way. Although extensive research has been carried out for the development of different suitable carbon-based materials for CO2 capture, still there is a lack of research for future studies on the development of low-cost suitable adsorbent material. In our opinion, CBMs have a good future for CO2 capture if all the properties can be merged into one material, which can compete with metal-organic frameworks. Therefore, the future focus should be given on the increase in the adsorption capacity, as well as materials properties, in order to sustain in the long future.
Author Contributions
T.K., M.S.H., M.A.H. and M.B.A. have collected data, designed the manuscript and written the main text. P.K.D., and M.S.R., have collected data and revised the manuscript. M.B.A., and M.A.H., have monitored, revised, designed and written the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Siriwardane, R.V.; Shen, M.; Fisher, E.P.; Poston, J.A. Adsorption of CO2 on molecular sieves and activated carbon. Energy Fuels 2001, 15, 279–284. [Google Scholar] [CrossRef]
- Lopes, F.V.S.; Grande, C.A.; Ribeiro, A.M.; Loureiro, J.M.; Evaggelos, O.; Nikolakis, V.; Rodrigues, A.E. Adsorption of H2, CO2, CH4, CO, N2 and H2O in activated carbon and zeolite for hydrogen production. Sep. Sci. Technol. 2009, 44, 1045–1073. [Google Scholar] [CrossRef]
- Haque, E.; Islam, M.M.; Pourazadi, E.; Sarkar, S.; Harris, A.T.; Minett, A.I.; Yanmaz, E.; Alshehri, S.M.; Ide, Y.; Wu, K.C.W. Boron-functionalized graphene oxide-organic frameworks for highly efficient CO2 capture. Chem. Asian. J. 2017, 12, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Creamer, A.E.; Gao, B. Carbon-based adsorbents for postcombustion CO2 capture: A critical review. Environ. Sci. Technol. 2016, 50, 7276–7289. [Google Scholar] [CrossRef]
- Alam, M.M.; Hossain, M.A.; Hossain, M.D.; Johir, M.; Hossen, J.; Rahman, M.S.; Zhou, J.L.; Hasan, A.; Karmakar, A.K.; Ahmed, M.B. The potentiality of rice husk-derived activated carbon: From synthesis to application. Processes 2020, 8, 203. [Google Scholar] [CrossRef]
- Rubin, E.; De Coninck, H. IPCC special report on carbon dioxide capture and storage. In TNO (2004): Cost Curves for CO2 Storage, Part 2; Cambridge University Press: Cambridge, UK, 2005; Volume 2, p. 14. [Google Scholar]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Lackner, K.S.; Chen, X. Capture CO2 from ambient air using nanoconfined ion hydration. Angew. Chem. 2016, 128, 4094–4097. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 99, 6984–7006. [Google Scholar] [CrossRef]
- Ren, X.; Li, H.; Chen, J.; Wei, L.; Modak, A.; Yang, H.; Yang, Q. N-doped porous carbons with exceptionally high CO2 selectivity for CO2 capture. Carbon 2017, 114, 473–481. [Google Scholar] [CrossRef]
- Hao, G.P.; Jin, Z.Y.; Sun, Q.; Zhang, X.Q.; Zhang, J.T.; Lu, A.H. Porous carbon nanosheets with precisely tunable thickness and selective CO2 adsorption properties. Energy Environ. Sci. 2013, 6, 3740–3747. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, W.C.; Tang, L.; Wang, Q.G.; Hu, Q.T.; Zhang, Y.; Lu, A.H. Primary amine modulated synthesis of two-dimensional porous nanocarbons with tunable ultramicropores. J. Mater. Chem. A 2018, 6, 24285–24290. [Google Scholar] [CrossRef]
- Qian, D.; Lei, C.; Wang, E.M.; Li, W.C.; Lu, A.H. A method for creating microporous carbon materials with excellent CO2-adsorption capacity and selectivity. ChemSusChem 2014, 7, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Drage, T.C.; Kozynchenko, O.; Pevida, C.; Plaza, M.G.; Rubiera, F.; Pis, J.; Snape, C.E.; Tennison, S. Developing activated carbon adsorbents for pre-combustion CO2 capture. Energy Proced. 2009, 1, 599–605. [Google Scholar] [CrossRef]
- Lu, C.; Bai, H.; Wu, B.; Su, F.; Hwang, J.F. Comparative study of CO2 capture by carbon nanotubes, activated carbons, and zeolites. Energy Fuels 2008, 22, 3050–3056. [Google Scholar] [CrossRef]
- Yu, C.H.; Huang, C.H.; Tan, C.S. A review of CO2 capture by absorption and adsorption. Aerosol Air Qual. Res. 2012, 12, 745–769. [Google Scholar] [CrossRef]
- Kim, I.; Svendsen, H.F. Heat of absorption of carbon dioxide (CO2) in monoethanolamine (MEA) and 2-(aminoethyl) ethanolamine (AEEA) solutions. Ind. Eng. Chem. 2007, 46, 5803–5809. [Google Scholar] [CrossRef]
- Choi, S.; Drese, J.H.; Jones, C.W. Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem: Chem. Sust. Energy Mater. 2009, 2, 796–854. [Google Scholar] [CrossRef]
- Qian, K.; Kumar, A.; Zhang, H.; Bellmer, D.; Huhnke, R. Recent advances in utilization of biochar. Renew. Sust. Energy Rev. 2015, 42, 1055–1064. [Google Scholar] [CrossRef]
- Tan, X.F.; Liu, S.B.; Liu, Y.G.; Gu, Y.L.; Zeng, G.M.; Hu, X.J.; Wang, X.; Liu, S.H.; Jiang, L.H. Biochar as potential sustainable precursors for activated carbon production: Multiple applications in environmental protection and energy storage. Bioresour. Technol. 2017, 227, 359–372. [Google Scholar] [CrossRef]
- Woolf, D.; Amonette, J.E.; Street-Perrott, F.A.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Insight into biochar properties and its cost analysis. Biomass Bioenerg. 2016, 84, 76–86. [Google Scholar] [CrossRef]
- Lee, J.; Kim, K.H.; Kwon, E.E. Biochar as a catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- You, S.; Ok, Y.S.; Chen, S.S.; Tsang, D.C.; Kwon, E.E.; Lee, J.; Wang, C.H. A critical review on sustainable biochar system through gasification: Energy and environmental applications. Bioresour. Technol. 2017, 246, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.; Gaunt, J.; Rondon, M. Bio-char sequestration in terrestrial ecosystems–a review. Mitig. Adapt. Strat. Glob. Change 2006, 11, 403–427. [Google Scholar] [CrossRef]
- Balahmar, N.; Mitchell, A.C.; Mokaya, R. Generalized Mechanochemical Synthesis of Biomass-derived sustainable carbons for high performance CO2 storage. Adv. Energy Mater. 2015, 5. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Nanda, S.; Dalai, A.K.; Berruti, F.; Kozinski, J.A. Biochar as an exceptional bioresource for energy, agronomy, carbon sequestration, activated carbon and specialty materials. Waste Biomass Valori. 2016, 7, 201–235. [Google Scholar] [CrossRef]
- Guizani, C.; Haddad, K.; Jeguirim, M.; Colin, B.; Limousy, L. Combustion characteristics and kinetics of torrefied olive pomace. Energy 2016, 107, 453–463. [Google Scholar] [CrossRef]
- Rousset, P.; Macedo, L.; Commandre, J.M.; Moreira, A. Biomass torrefaction under different oxygen concentrations and its effect on the composition of the solid by-product. J. Anal. Appl. Pyrol. 2012, 96, 86–91. [Google Scholar] [CrossRef]
- Ullah, H.; Liu, G.J.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Zhou, C.C. Combustion characteristics and retention-emission of selenium during co-firing of torrefied biomass and its blends with high ash coal. Bioresour. Technol. 2017, 245, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Jiang, H.; Yu, H.Q. Development of biochar-based functional materials: Toward a sustainable platform carbon material. Chem. Rev. 2015, 115, 12251–12285. [Google Scholar] [CrossRef] [PubMed]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic molecular structure of plant biomass-derived black carbon (biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Mohd, A.; Ghani, W.A.W.A.; Resitanim, N.Z.; Sanyang, L. A Review: Carbon dioxide capture: Biomass-derived-biochar and its applications. J. Disper. Sci. Technol. 2013, 34, 974–984. [Google Scholar] [CrossRef]
- Manyà, J.J.; González, B.; Azuara, M.; Arner, G. Ultra-microporous adsorbents prepared from vine shoots-derived biochar with high CO2 uptake and CO2/N2 selectivity. Chem. Eng. 2018, 345, 631–639. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.; Trokourey, A.; Jaroniec, M. Development of microporous carbons for CO2 capture by KOH activation of African palm shells. J. CO2 Util. 2013, 2, 35–38. [Google Scholar] [CrossRef]
- Li, D.W.; Ma, T.F.; Zhang, R.L.; Tian, Y.Y.; Qiao, Y.Y. Preparation of porous carbons with high low-pressure CO2 uptake by KOH activation of rice husk char. Fuel 2015, 139, 68–70. [Google Scholar] [CrossRef]
- Deng, S.B.; Wei, H.R.; Chen, T.; Wang, B.; Huang, J.; Yu, G. Superior CO2 adsorption on pine nut shell-derived activated carbons and the effective micropores at different temperatures. Chem. Eng. 2014, 253, 46–54. [Google Scholar] [CrossRef]
- Hong, S.M.; Jang, E.; Dysart, A.D.; Pol, V.G.; Lee, K.B. CO2 capture in the sustainable wheat-derived activated microporous carbon compartments. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Coromina, H.M.; Walsh, D.A.; Mokaya, R. Biomass-derived activated carbon with simultaneously enhanced CO2 uptake for both pre and post combustion capture applications. J. Mater. Chem. A 2016, 4, 280–289. [Google Scholar] [CrossRef]
- Serafin, J.; Narkiewicz, U.; Morawski, A.W.; Wrobel, R.J.; Michalkiewicz, B. Highly microporous activated carbons from biomass for CO2 capture and effective micropores at different conditions. J. CO2 Util. 2017, 18, 73–79. [Google Scholar] [CrossRef]
- Zhang, C.M.; Song, W.; Ma, Q.L.; Xie, L.J.; Zhang, X.C.; Guo, H. Enhancement of CO2 capture on biomass-based carbon from black locust by KOH activation and ammonia modification. Energy Fuels 2016, 30, 4181–4190. [Google Scholar] [CrossRef]
- Rouzitalab, Z.; Maklavany, D.M.; Rashidi, A.; Jafarinejad, S. Synthesis of N-doped nanoporous carbon from walnut shell for enhancing CO2 adsorption capacity and separation. J. Environ. Chem. Eng. 2018, 6, 6653–6663. [Google Scholar] [CrossRef]
- Zhu, B.J.; Shang, C.X.; Guo, Z.X. Naturally nitrogen and calcium-doped nanoporous carbon from pine cone with superior CO2 capture capacities. ACS Sustain. Chem. Eng. 2016, 4, 1050–1057. [Google Scholar] [CrossRef]
- Bamdad, H.; Hawboldt, K.; MacQuarrie, S. Nitrogen functionalized biochar as a renewable adsorbent for efficient CO2 removal. Energy Fuels 2018, 32, 11742–11748. [Google Scholar] [CrossRef]
- Lahijani, P.; Mohammadi, M.; Mohamed, A.R. Metal incorporated biochar as a potential adsorbent for high capacity CO2 capture at ambient condition. J. CO2 Util. 2018, 26, 281–293. [Google Scholar] [CrossRef]
- Dissanayake, P.D.; You, S.; Igalavithana, A.D.; Xia, Y.; Bhatnagar, A.; Gupta, S.; Kua, H.W.; Kim, S.; Kwon, J.H.; Tsang, D.C.W.; et al. Biochar-based adsorbents for carbon dioxide capture: A critical review. Renew. Sustain. Energy Rev. 2020, 119, 109582. [Google Scholar] [CrossRef]
- Chowdhury, S.; Balasubramanian, R. Three-dimensional graphene-based porous adsorbents for postcombustion CO2 capture. Ind. Eng. Chem. 2016, 55, 7906–7916. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Atarod, M.; Jaleh, B.; Gandomirouzbahani, M. In situ green synthesis of Ag nanoparticles on graphene oxide/TiO2 nanocomposite and their catalytic activity for the reduction of 4-nitrophenol, congo red and methylene blue. Ceram. Int. 2016, 42, 8587–8596. [Google Scholar] [CrossRef]
- Liu, Y.; Xiang, M.; Hong, L. Three-dimensional nitrogen and boron codoped graphene for carbon dioxide and oils adsorption. RSC Adv. 2017, 7, 6467–6473. [Google Scholar] [CrossRef]
- Bhanja, P.; Das, S.K.; Patra, A.K.; Bhaumik, A. Functionalized graphene oxide as an efficient adsorbent for CO2 capture and support for heterogeneous catalysis. RSC Adv. 2016, 6, 72055–72068. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cantador, L.S.; Cecilia, J.A.; Mehravar, E.; Tomovska, R. Graphene-based Monolithic Nanostructures for CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 8612–8621. [Google Scholar] [CrossRef]
- Huang, A.; Feng, B. Facile synthesis of PEI-GO@ ZIF-8 hybrid material for CO2 capture. Int. J. Hydrog. Energy 2018, 43, 2224–2231. [Google Scholar] [CrossRef]
- Rahimi, M.; Babu, D.J.; Singh, J.K.; Yang, Y.B.; Schneider, J.J.; Müller-Plathe, F. Double-walled carbon nanotube array for CO2 and SO2 adsorption. J. Chem. Phys. 2015, 143, 124701. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Bai, J.; Francisco, J.S.; Zeng, X.C. Formation of CO2 hydrates within single-walled carbon nanotubes at ambient pressure: CO2 capture and selective separation of a CO2/H2 mixture in water. J. Phys. Chem. 2018, 122, 7951–7958. [Google Scholar] [CrossRef]
- Cortés-Súarez, J.; Celis-Arias, V.; Beltrán, H.I.; Tejeda-Cruz, A.; Ibarra, I.A.; Romero-Ibarra, J.E.; Sánchez-González, E.; Loera-Serna, S. Synthesis and characterization of an SWCNT@ HKUST-1 composite: Enhancing the CO2 adsorption properties of HKUST-1. ACS Omega 2019, 4, 5275–5282. [Google Scholar] [CrossRef]
- Kemp, K.C.; Chandra, V.; Saleh, M.; Kim, K.S. Reversible CO2 adsorption by an activated nitrogen doped graphene/polyaniline material. Nanotechnology 2013, 24, 235703. [Google Scholar] [CrossRef]
- Shin, G.J.; Rhee, K.; Park, S.J. Improvement of CO2 capture by graphite oxide in presence of polyethylenimine. Int. J. Hydrog. Energy 2016, 41, 14351–14359. [Google Scholar] [CrossRef]
- Deng, M.; Park, H.G. Spacer-assisted amine-coiled carbon nanotubes for CO2 capture. Langmuir 2019, 35, 4453–4459. [Google Scholar] [CrossRef]
- Gromov, A.; Kulur, A.; Gibson, J.; Mangano, E.; Brandani, S.; Campbell, E. Carbon nanotube/PVA aerogels impregnated with PEI: Solid adsorbents for CO2 capture. Sustain. Energy Fuels 2018, 2, 1630–1640. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Agag, T.; Ishida, H.; Qutubuddin, S. Biobased chitosan hybrid aerogels with superior adsorption: Role of graphene oxide in CO2 capture. RSC Adv. 2013, 3, 16011–16020. [Google Scholar] [CrossRef]
- Alhwaige, A.A.; Ishida, H.; Qutubuddin, S. Carbon aerogels with excellent CO2 adsorption capacity synthesized from clay-reinforced biobased chitosan-polybenzoxazine nanocomposites. ACS Sustain. Chem. Eng. 2016, 4, 1286–1295. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T.C.; Rao, M.B. Activated carbon for gas separation and storage. Carbon 1996, 3, 1–12. [Google Scholar] [CrossRef]
- Hayashi, J.; Kazehaya, A.; Muroyama, K.; Watkinson, A.P. Preparation of activated carbon from lignin by chemical activation. Carbon 2000, 38, 1873–1878. [Google Scholar] [CrossRef]
- Ge, C.; Lian, D.; Cui, S.; Gao, J.; Lu, J. Highly selective CO2 capture on waste polyurethane foam-based activated carbon. Processes 2019, 7, 592. [Google Scholar] [CrossRef]
- Borhan, A.; Yusup, S.; Lim, J.W.; Show, P.L. Characterization and modelling studies of activated carbon produced from rubber-seed shell using KOH for CO2 adsorption. Processes 2019, 7, 855. [Google Scholar] [CrossRef]
- Basheer, O.A.; Hanafiah, M.M.; Abdulhakim Alsaadi, M.; Al-Douri, Y.; Malek, M.A.; Mohammed Aljumaily, M.; Saadi Fiyadh, S. Synthesis and characterization of natural extracted precursor date palm fibre-based activated carbon for aluminum removal by RSM optimization. Processes 2019, 7, 249. [Google Scholar] [CrossRef]
- Shao, X.; Feng, Z.; Xue, R.; Ma, C.; Wang, W.; Peng, X.; Cao, D. Adsorption of CO2, CH4, CO2/N2 and CO2/CH4 in novel activated carbon beads: Preparation, measurements and simulation. AIChE J. 2011, 57, 3042–3051. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 capture capacity of nitrogen-doped biomass-derived porous carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Rao, Y.; Zhao, X.; Wu, M. Superior CO2, CH4, and H2 uptakes over ultrahigh-surface-area carbon spheres prepared from sustainable biomass-derived char by CO2 activation. Carbon 2016, 105, 454–462. [Google Scholar] [CrossRef]
- Chiang, Y.C.; Yeh, C.Y.; Weng, C.H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Shi, W.; Wang, R.; Liu, H.; Chang, B.; Yang, B.; Zhang, Z. Biowaste-derived 3D honeycomb-like N and S dual-doped hierarchically porous carbons for high-efficient CO2 capture. RSC Adv. 2019, 9, 23241–23253. [Google Scholar] [CrossRef]
- Wang, R.; Wang, P.; Yan, X.; Lang, J.; Peng, C.; Xue, Q. Promising porous carbon derived from celtuce leaves with outstanding supercapacitance and CO2 capture performance. ACS Appl. Mater. 2012, 4, 5800–5806. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Chen, H.; Fu, N.; Chen, J.; Lan, G.; Qian, W.; Liu, Y.; Lin, H.; Han, S. Excellent electrochemical properties and large CO2 capture of nitrogen-doped activated porous carbon synthesised from waste longan shells. Electrochim. Acta 2017, 231, 403–411. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Johir, M.A.H.; Zhou, J.L.; Ngo, H.H.; Nghiem, L.D.; Richardson, C.; Moni, M.A.; Bryant, M.R. Activated carbon preparation from biomass feedstock: Clean production and carbon dioxide adsorption. J. Clean. Prod. 2019, 225, 405–413. [Google Scholar] [CrossRef]
- Ello, A.S.; de Souza, L.K.; Trokourey, A.; Jaroniec, M. Coconut shell-based microporous carbons for CO2 capture. Microporous Mesoporous Mater. 2013, 180, 280–283. [Google Scholar] [CrossRef]
- Parshetti, G.K.; Chowdhury, S.; Balasubramanian, R. Biomass derived low-cost microporous adsorbents for efficient CO2 capture. Fuel 2015, 148, 246–254. [Google Scholar] [CrossRef]
- Demir, M.; Tessema, T.D.; Farghaly, A.A.; Nyankson, E.; Saraswat, S.K.; Aksoy, B.; Islamoglu, T.; Collinson, M.M.; El-Kaderi, H.M.; Gupta, R.B. Lignin-derived heteroatom-doped porous carbons for supercapacitor and CO2 capture applications. Int. J. Energy Res. 2018, 42, 2686–2700. [Google Scholar] [CrossRef]
- Yu, D.; Hu, J.; Zhou, L.; Li, J.; Tang, J.; Peng, C.; Liu, H. Nitrogen-doped coal tar pitch based microporous carbons with superior CO2 capture performance. Energy Fuels 2018, 32, 3726–3732. [Google Scholar] [CrossRef]
- Shafeeyan, M.S.; Daud, W.M.A.W.; Houshmand, A.; Shamiri, A. A review on surface modification of activated carbon for carbon dioxide adsorption. J. Anal. Appl. Pyrolysis 2010, 89, 143–151. [Google Scholar] [CrossRef]
- Petrova, B.; Budinova, T.; Petrov, N.; Yardim, M.; Ekinci, E.; Razvigorova, M. Effect of different oxidation treatments on the chemical structure and properties of commercial coal tar pitch. Carbon 2005, 43, 261–267. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Li, W.C.; Lu, A.H. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Long, L.; Jiang, X.; Liu, J.; Han, D.; Xiao, M.; Wang, S.; Meng, Y. In situ template synthesis of hierarchical porous carbon used for high performance lithium–sulfur batteries. RSC Adv. 2018, 8, 4503–4513. [Google Scholar] [CrossRef]
- Cox, M.; Mokaya, R. Ultra-high surface area mesoporous carbons for colossal pre combustion CO2 capture and storage as materials for hydrogen purification. Sustain. Energy Fuels 2017, 1, 1414–1424. [Google Scholar] [CrossRef]
- Huang, K.; Chai, S.H.; Mayes, R.T.; Tan, S.; Jones, C.W.; Dai, S. Significantly increasing porosity of mesoporous carbon by NaNH2 activation for enhanced CO2 adsorption. Microporous Mesoporous Mater. 2016, 230, 100–108. [Google Scholar] [CrossRef]
- Lu, J.; Jiao, C.; Majeed, Z.; Jiang, H. Magnesium and Nitrogen Co-Doped Mesoporous Carbon with Enhanced Microporosity for CO2 Adsorption. Nanomaterials 2018, 8, 275. [Google Scholar] [CrossRef]
- Yaumi, A.; Bakar, M.A.; Hameed, B. Reusable nitrogen-doped mesoporous carbon adsorbent for carbon dioxide adsorption in fixed-bed. Energy 2017, 138, 776–784. [Google Scholar] [CrossRef]
- Park, D.H.; Lakhi, K.S.; Ramadass, K.; Kim, M.K.; Talapaneni, S.N.; Joseph, S.; Ravon, U.; Al-Bahily, K.; Vinu, A. Energy efficient synthesis of ordered mesoporous carbon nitrides with a high nitrogen content and enhanced CO2 capture capacity. Chem. Eur. J. 2017, 23, 10753–10757. [Google Scholar] [CrossRef]
- Pei, Y.R.; Choi, G.; Asahina, S.; Yang, J.H.; Vinu, A.; Choy, J.H. A novel geopolymer route to porous carbon: High CO2 adsorption capacity. Chem. Comm. 2019, 55, 3266–3269. [Google Scholar] [CrossRef]
- Srinivas, G.; Krungleviciute, V.; Guo, Z.X.; Yildirim, T. Exceptional CO2 capture in a hierarchically porous carbon with simultaneous high surface area and pore volume. Energy Environ. Sci. 2014, 7, 335–342. [Google Scholar] [CrossRef]
- Lu, A.H.; Hao, G.P.; Zhang, X.Q. Porous carbons for carbon dioxide capture. In Porous Materials for Carbon Dioxide Capture; Springer: Berlin, Germany, 2014; pp. 15–77. [Google Scholar]
- Yuan, B.; Wu, X.; Chen, Y.; Huang, J.; Luo, H.; Deng, S. Adsorption of CO2, CH4, and N2 on ordered mesoporous carbon: Approach for greenhouse gases capture and biogas upgrading. Environ. Sci. Technol. 2013, 47, 5474–5480. [Google Scholar] [CrossRef]
- Zhang, Z.; Luo, D.; Lui, G.; Li, G.; Jiang, G.; Cano, Z.P.; Deng, Y.P.; Du, X.; Yin, S.; Chen, Y. In-situ ion-activated carbon nanospheres with tunable ultramicroporosity for superior CO2 capture. Carbon 2019, 143, 531–541. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Wang, G.; Du, J.; Zhang, Y.; Fu, X.; Chen, A. Synthesis of mesoporous carbon nanospheres via “pyrolysis-deposition” strategy for CO2 capture. J. Mater. Sci. 2017, 52, 9640–9647. [Google Scholar] [CrossRef]
- Zhou, J.; Li, Z.; Xing, W.; Zhu, T.; Shen, H.; Zhuo, S. N-doped microporous carbons derived from direct carbonization of K+ exchanged meta-aminophenol–formaldehyde resin for superior CO2 sorption. Chem. Comm. 2015, 51, 4591–4594. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Z.; Jia, Z.; Zhao, L.; Zhang, T.; Xing, W.; Komarneni, S.; Subhan, F.; Yan, Z. New strategy to prepare ultramicroporous carbon by ionic activation for superior CO2 capture. Chem. Eng. J. 2018, 337, 290–299. [Google Scholar] [CrossRef]
- Robertson, C.; Mokaya, R. Microporous activated carbon aerogels via a simple subcritical drying route for CO2 capture and hydrogen storage. Microporous Mesoporous Mater. 2013, 179, 151–156. [Google Scholar] [CrossRef]
- Xing, W.; Liu, C.; Zhou, Z.; Zhang, L.; Zhou, J.; Zhuo, S.; Yan, Z.; Gao, H.; Wang, G.; Qiao, S.Z. Superior CO2 uptake of N-doped activated carbon through hydrogen-bonding interaction. Energy Environ. Sci. 2012, 5, 7323–7327. [Google Scholar] [CrossRef]
- Chang, B.; Sun, L.; Shi, W.; Zhang, S.; Yang, B. Cost-Efficient Strategy for Sustainable Cross-Linked Microporous Carbon Bead with Satisfactory CO2 Capture Capacity. ACS Omega 2018, 3, 5563–5573. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Manmuanpom, N.; Thubsuang, U.; Dubas, S.T.; Wongkasemjit, S.; Chaisuwan, T. Enhanced CO2 capturing over ultra-microporous carbon with nitrogen-active species prepared using one-step carbonization of polybenzoxazine for a sustainable environment. J. Environ. Manag. 2018, 223, 779–786. [Google Scholar] [CrossRef]
- Seema, H.; Kemp, K.C.; Le, N.H.; Park, S.-W.; Chandra, V.; Lee, J.W.; Kim, K.S. Highly selective CO2 capture by S-doped microporous carbon materials. Carbon 2014, 66, 320–326. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef]
- Zhang, L.H.; Li, W.C.; Liu, H.; Wang, Q.G.; Tang, L.; Hu, Q.T.; Xu, W.J.; Qiao, W.H.; Lu, Z.Y.; Lu, A.H. Thermoregulated Phase-Transition Synthesis of Two-Dimensional Carbon Nanoplates Rich in sp2 Carbon and Unimodal Ultramicropores for Kinetic Gas Separation. Angew. Chem. Int. Ed. 2018, 57, 1632–1635. [Google Scholar] [CrossRef]
- Shen, W.; He, Y.; Zhang, S.; Li, J.; Fan, W. Yeast-based microporous carbon materials for carbon dioxide capture. ChemSusChem 2012, 5, 1274–1279. [Google Scholar] [CrossRef]
- Guo, L.P.; Hu, Q.T.; Zhang, P.; Li, W.C.; Lu, A.H. Polyacrylonitrile-Derived Sponge-Like Micro/Macroporous Carbon for Selective CO2 Separation. Chem. Eur. J. 2018, 24, 8369–8374. [Google Scholar] [CrossRef]
- Li, Q.; Yang, J.; Feng, D.; Wu, Z.; Wu, Q.; Park, S.S.; Ha, C.-S.; Zhao, D. Facile synthesis of porous carbon nitride spheres with hierarchical three-dimensional mesostructures for CO2 capture. Nano Res. 2010, 3, 632–642. [Google Scholar] [CrossRef]
- Estevez, L.; Barpaga, D.; Zheng, J.; Sabale, S.; Patel, R.L.; Zhang, J.G.; McGrail, B.P.; Motkuri, R.K. Hierarchically porous carbon materials for CO2 capture: The role of pore structure. Ind. Eng. Chem. Res. 2018, 57, 1262–1268. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Ma, C.; Qiao, W.; Ling, L. Fabrication of hierarchical carbon nanosheet-based networks for physical and chemical adsorption of CO2. J. Colloid Interf. Sci. 2019, 534, 72–80. [Google Scholar] [CrossRef]
- Chang, B.; Shi, W.; Yin, H.; Zhang, S.; Yang, B. Poplar catkin-derived self-templated synthesis of N-doped hierarchical porous carbon microtubes for effective CO2 capture. Chem. Eng. J. 2019, 358, 1507–1518. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, X.; Wang, B.; Cao, J.; Yang, J.; Wei, J. Waste wool derived nitrogen-doped hierarchical porous carbon for selective CO2 capture. RSC Adv. 2018, 8, 19818–19826. [Google Scholar] [CrossRef]
- Gao, A.; Guo, N.; Yan, M.; Li, M.; Wang, F.; Yang, R. Hierarchical porous carbon activated by CaCO3 from pigskin collagen for CO2 and H2 adsorption. Microporous Mesoporous Mater. 2018, 260, 172–179. [Google Scholar] [CrossRef]
- Marszewska, J.; Jaroniec, M. Tailoring porosity in carbon spheres for fast carbon dioxide adsorption. J. Colloid Interf. Sci. 2017, 487, 162–174. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Zhou, J.L.; Ngo, H.H.; Guo, W. Adsorptive removal of antibiotics from water and wastewater: Progress and challenges. Sci. Total Environ. 2015, 532, 112–126. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Liao, X.; Armstrong, M.; Chen, X.; Lackner, K.S. Humidity effect on ion behaviors of moisture-driven CO2 sorbents. J. Chem. Phys. 2018, 149, 164708. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).