Biocatalytic Approach for Novel Functional Oligoesters of ε-Caprolactone and Malic Acid
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Oligoester Synthesis in Organic Solvent Media
2.2.2. Oligoester Synthesis in Solvent-Free System
2.2.3. Time Course of the Oligomer Synthesis
2.2.4. Operational Stability of the Biocatalyst
2.2.5. HPLC Chromatography Analysis
2.2.6. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.7. MALDI-TOF MS (Matrix-Assisted Laser Desorption Ionization Time-of-Flight Mass Spectrometry) Analysis
2.2.8. Thermal Analysis
3. Results and Discussion
3.1. Characterization of the Oligoester Products by MALDI-TOF MS
3.2. The Effect of the ε-Caprolactone/Malic Acid Molar Ratio
3.3. The Influence of Organic Solvents on the Oligoester Synthesis
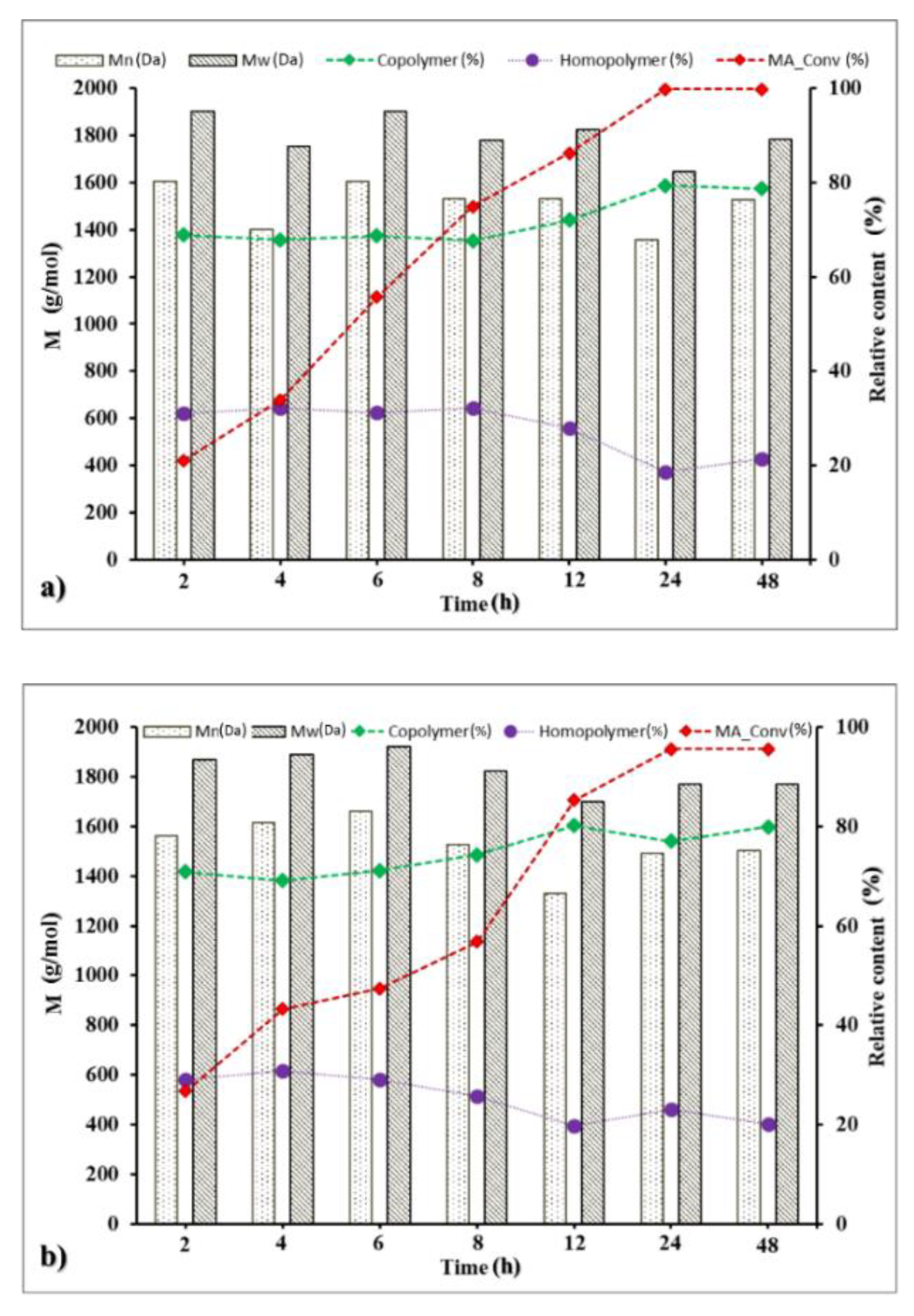
3.4. Time Course of the Oligomerization Reaction
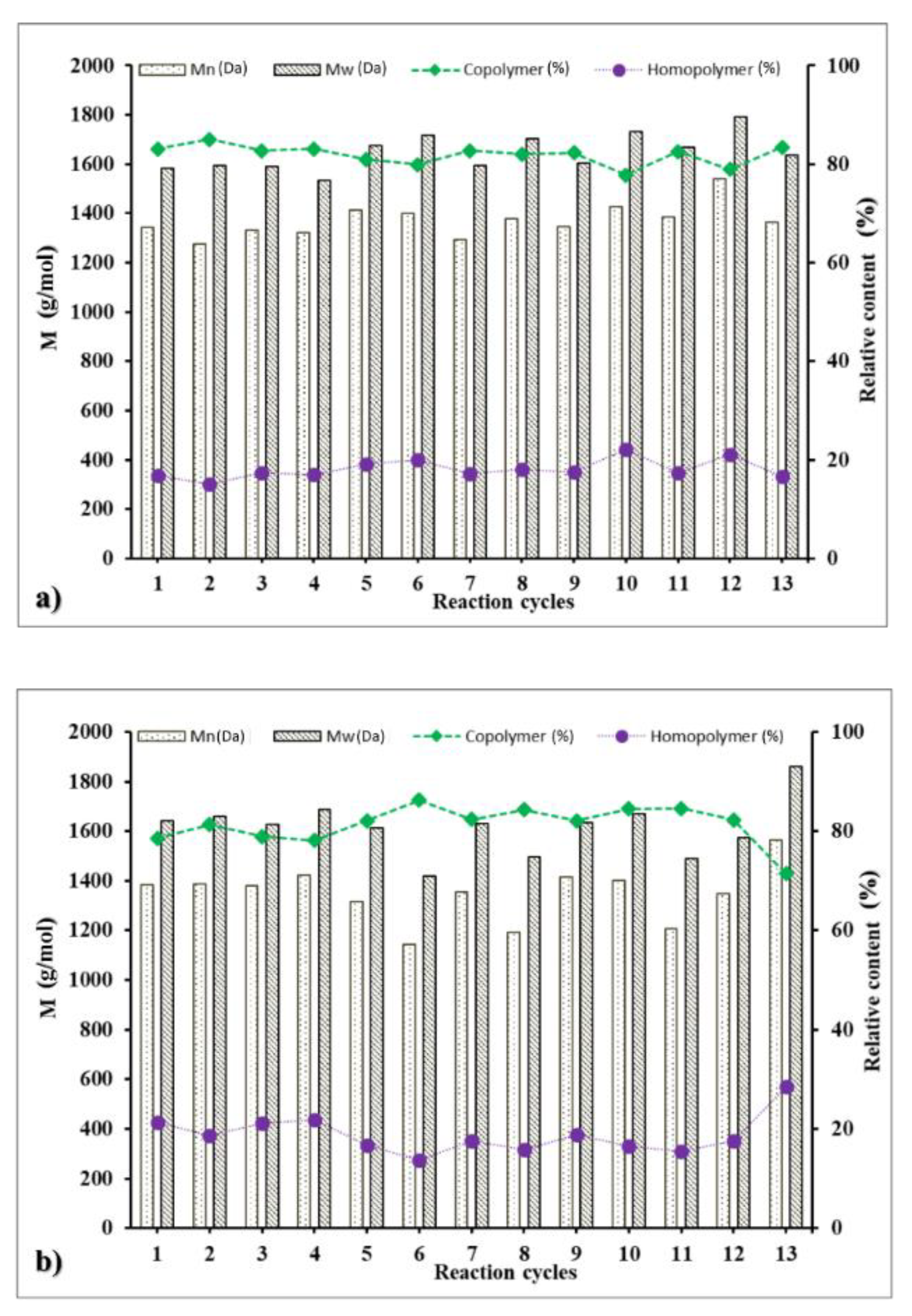
3.5. Operational Stability of the Biocatalyst in Repeated Reaction Cycles
3.6. Characterization of the Oligomerization Products by FTIR Spectroscopy
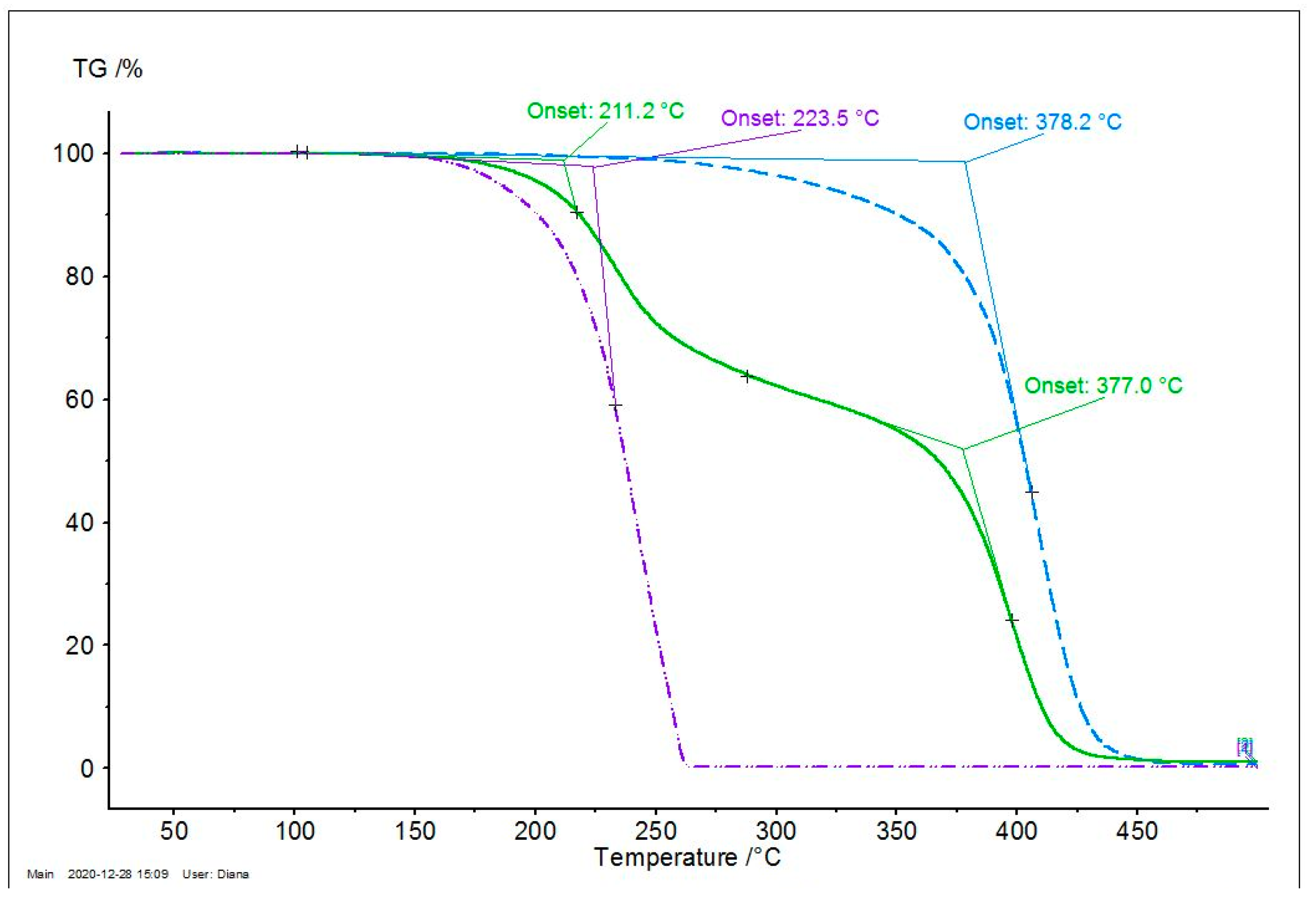
3.7. Thermal Stability of the Malic Acid-Based Oligoesters
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theriot, J.C.; McCarthy, B.G.; Lim, C.-H. Organocatalyzed atom transfer radical polymerization: Perspectives on catalyst design and performance. Macromol. Rapid Commun. 2017, 38, 1700040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goetjen, T.A.; Liu, J.; Wu, Y.; Sui, J.; Zhang, X.; Hupp, J.T.; Farha, O.K. Metal-organic framework (MOF) materials as polymerization catalysts: A review and recent advances. Chem. Commun. 2020, 56, 10409–10418. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.N.; Gross, R.A. Green polymer chemistry: Biocatalysis and biomaterials. In ACS Symposium Series; Cheng, H.N., Gross, R.A., Eds.; American Chemical Society: Washington, DC, USA, 2010; Volume 1043, pp. 1–14. [Google Scholar]
- Kobayashi, S. Enzymatic polymerization. In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; Volume 5, pp. 217–237. [Google Scholar]
- Kobayashi, S.; Uyama, H.; Kimura, S. Enzymatic polymerization. Chem. Rev. 2001, 101, 3793–3818. [Google Scholar] [CrossRef] [PubMed]
- Kourist, R.; Hollmann, F.; Nguyen, G.S. Lipases as sustainable biocatalysts for the sustainable industrial production of fine chemicals and cosmetics. JSM Biotechnol. Bioeng. 2014, 2, 1029. [Google Scholar]
- Filho, D.G.; Silva, A.G.; Guidini, C.Z. Lipases: Sources, immobilization methods, and industrial applications. Appl. Microbiol. Biotechnol. 2019, 103, 7399–7423. [Google Scholar] [CrossRef]
- Cimporescu, A.; Todea, A.; Badea, V.; Paul, C.; Peter, F. Efficient kinetic resolution of 1,5-dihydroxy-1,2,3,4-tetrahydronaphthalene catalyzed by immobilized Burkholderia cepacia lipase in batch and continuous-flow system. Process Biochem. 2016, 51, 2076–2083. [Google Scholar] [CrossRef]
- Angajala, G.; Pavan, P.; Subashini, R. Lipases: An overview of its current challenges and prospectives in the revolution of biocatalysis. Biocatal. Agric. Biotechnol. 2016, 7, 257–270. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa, S.R.; Arora, P.K. Microbial lipases and their industrial applications: A comprehensive review. Microb. Cell Fact. 2020, 19, 169. [Google Scholar] [CrossRef]
- Kobayashi, S. Lipase-catalyzed polyester synthesis-a green polymer chemistry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 338–365. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S. Enzymatic ring-opening polymerization and polycondensation for the green synthesis of polyesters. Polym. Adv. Technol. 2015, 26, 677–686. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, D.; Liu, C.; Zhao, Z.; Yang, Y.; Li, Q. Lipase/esterase-catalyzed synthesis of aliphatic polyesters via polycondensation: A review. Process Biochem. 2012, 47, 1027–1036. [Google Scholar] [CrossRef]
- Liu, Y.; Song, L.; Feng, N.; Jiang, W.; Jin, Y.; Li, X. Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase-catalyzed polymerization. RSC Adv. 2020, 10, 36230–36240. [Google Scholar] [CrossRef]
- Grobelny, Z.; Golba, S.; Jurek-Suliga, J. Mechanism of ε-caprolactone polymerization in the presence of alkali metal salts: Investigation of initiation course and determination of polymers structure by MALDI-TOF mass spectrometry. Polym. Bull. 2019, 76, 3501–3515. [Google Scholar] [CrossRef] [Green Version]
- Engel, J.; Cordellier, A.; Huang, L.; Kara, S. Enzymatic ring-opening polymerization of lactones: Traditional approaches and alternative strategies. ChemCatChem 2019, 11, 4983–4997. [Google Scholar] [CrossRef]
- Huang, Y.; Li, L.; Li, G. An enzyme-catalysed access to amphiphilic triblock copolymer of PCL-b-PEG-b-PCL: Synthesis, characterization and self-assembly properties. Des. Monomers Polym. 2015, 18, 799–806. [Google Scholar] [CrossRef]
- Todea, A.; Badea, V.; Nagy, L.; Keki, S.; Boeriu, C.G.; Peter, F. Biocatalytic synthesis of δ-gluconolactone and ε-caprolactone copolymers. Acta Biochim. Pol. 2014, 61, 205–210. [Google Scholar] [CrossRef] [Green Version]
- Todea, A.; Biró, E.; Badea, V.; Paul, C.; Cimporescu, A.; Nagy, L.; Kéki, S.; Bandur, G.; Boeriu, C.G.; Peter, F. Optimization of enzymatic ring-opening copolymerizations involving delta-gluconolactone as monomer by experimental design. Pure Appl. Chem. 2014, 86, 1781–1792. [Google Scholar] [CrossRef]
- Todea, A.; Aparaschivei, D.; Bîtcan, I.; Ledeti, I.V.; Bandur, G.; Peter, F.; Nagy, L.; Kéki, S.; Biró, E. Thermal behavior of oligo[(ε-caprolactone)-co-δ-gluconolactone] enzymatically synthesized in reaction conditions optimized by experimental design. J. Thermal Anal. Calor. 2020, 141, 1017–1026. [Google Scholar] [CrossRef]
- Todea, A.; Bîtcan, I.; Aparaschivei, D.; Păușescu, I.; Badea, V.; Peter, F.; Gherman, V.D.; Rusu, G.; Nagy, L.; Kéki, S. Biodegradable oligoesters of ε-caprolactone and 5-hydroxymethyl-2-furancarboxylic acid synthesized by immobilized lipases. Polymers 2019, 11, 1402. [Google Scholar] [CrossRef] [Green Version]
- Aparaschivei, D.; Todea, A.; Păușescu, I.; Badea, V.; Medeleanu, M.; Șișu, E.; Puiu, M.; Chirilă-Emandi, A.; Peter, F. Synthesis, characterization and enzymatic degradation of copolymers of ε-caprolactone and hydroxy-fatty acid. Pure Appl. Chem. 2016, 88, 1191–1201. [Google Scholar] [CrossRef]
- Todea, A.; Aparaschivei, D.; Badea, V.; Boeriu, C.G.; Peter, F. Biocatalytic route for the synthesis of oligoesters of hydroxy-fatty acids and ϵ-caprolactone. Biotechnol. J. 2018, 13, 1700629. [Google Scholar] [CrossRef] [PubMed]
- Kántor, I.; Aparaschivei, D.; Todea, A.; Biró, E.; Babos, G.; Szerényi, D.; Kakasi, B.; Péter, F.; Șișu, E.; Feczkó, T. Biocatalytic synthesis of poly[ε-caprolactone-co-(12-hydroxystearate)] copolymer for sorafenib nanoformulation useful in drug delivery. Cat. Today 2020. [Google Scholar] [CrossRef]
- Wedde, S.; Rommelmann, P.; Scherkus, C.; Schmidt, S.; Bornscheuer, U.T.; Liese, A.; Gröger, H. An alternative approach towards poly-ε-caprolactone through a chemoenzymatic synthesis: Combined hydrogenation, bio-oxidations and polymerization without the isolation of intermediates. Green Chem. 2017, 19, 1286–1290. [Google Scholar] [CrossRef]
- Zhang, J.; Lombardo, L.; Gözaydın, G.; Dyson, P.J.; Yan, N. Single-step conversion of lignin monomers to phenol: Bridging the gap between lignin and high-value chemicals. Chin. J. Cat. 2018, 39, 1445–1452. [Google Scholar] [CrossRef]
- Wahlberg, J.; Persson, P.V.; Olsson, T.; Hedenstrom, E.; Iversen, T. Structural characterization of a lipase-catalyzed copolymerization of ϵ-caprolactone and D,L-lactide. Biomacromolecules 2003, 4, 1068–1071. [Google Scholar] [CrossRef]
- Hollmann, F.; Grzebyk, P.; Heinrichs, V.; Doderer, K.; Thum, O. On the inactivity of Candida antartica lipase B towards strong acids. J. Mol. Cat. B Enzymatic 2009, 57, 257–261. [Google Scholar] [CrossRef]
- Kövilein, A.; Kubisch, C.; Cai, L.; Ochsenreither, K. Malic acid production from renewables: A review. J. Chem. Technol. Biotechnol. 2020, 95, 513–526. [Google Scholar] [CrossRef] [Green Version]
- Coulembier, O.; Degee, P.; Hedrick, J.L.; Dubois, P. From controlled ring-opening polymerization to biodegradable aliphatic polyester: Especially poly (b-malic acid) derivatives. Progress Polym. Sci. 2006, 31, 723–747. [Google Scholar] [CrossRef]
- Hahn, C.; Wesselbaum, S.; Keul, H.; Möller, M. OH-functional polyesters based on malic acid: Influence of the OH-groups onto the thermal properties. Eur. Polym. J. 2013, 49, 217–220. [Google Scholar] [CrossRef]
- Ljubimova, J.Y.; Fujita, M.; Khazenzon, N.M.; Lee, B.S.; Wachsmann-Hogiu, S.; Farkas, D.L.; Black, K.L.; Holler, E. Nanoconjugate based on polimalic acid for tumor targeting. Chem. Biol. Interact 2008, 171, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Telegdi, J.; Trif, L.; Mihaly, J.; Nagy, E.; Nyikos, L. Controlled synthesis and characterization of biodegradable, stereomer co-polycondensates of L-malic acid. J. Therm. Anal. Calorim. 2015, 121, 663–673. [Google Scholar] [CrossRef]
- Coulembier, O.; Degee, P.; Dubois, P. Synthesis and micellization properties of novel symmetrical poly(ε-caprolactone-b-[R,S] β-malic acid-b-ε-caprolactone) triblock copolymers. Macromol. Chem. Phys. 2006, 207, 484–491. [Google Scholar] [CrossRef]
- Yao, D.; Li, G.; Kuila, T.; Li, P.; Kim, N.H.; Kim, S.-I.; Lee, J.H. Lipase-catalyzed synthesis and characterization of biodegradable polyester containing L-malic acid unit in solvent system. J. Appl. Polym. Sci. 2011, 120, 1114–1120. [Google Scholar] [CrossRef]
- Li, G.; Yao, D.; Zong, M. Lipase-catalyzed synthesis of biodegradable copolymer containing malic acid units in solvent-free system. Eur. Polym. J. 2008, 44, 1123–1129. [Google Scholar] [CrossRef]
- Zappaterra, F.; Summa, D.; Semeraro, B.; Buzzi, R.; Trapella, C.; Ladero, M.; Costa, S.; Tamburini, E. Enzymatic esterification as potential strategy to enhance the sorbic acid behavior as food and beverage preservative. Fermentation 2020, 6, 96. [Google Scholar] [CrossRef]
- Ravelo, M.; Fuente, E.; Blanco, A.; Ladero, M.; Garcia-Ochoa, F. Esterification of glycerol and ibuprofen in solventless media catalyzed by free CALB: Kinetic modelling. Biochem. Eng. J. 2015, 101, 228–236. [Google Scholar] [CrossRef]
- Babasola, O.I.; Amsden, B.G. Surface eroding, liquid injectable polymers based on 5-ethylene ketal ε-caprolactone. Biomacromolecules 2011, 12, 3423–3431. [Google Scholar] [CrossRef]
- Utsunomia, C. Applications and perspectives of biobased oligomeric esters in the industry. J. Microbiol. Biotechnol. 2020, 5, 000159. [Google Scholar]
- Todea, A.; Otten, L.G.; Frissen, A.E.; Arends, I.W.C.E.; Peter, F.; Boeriu, C.G. Selectivity of lipases for estolides synthesis. Pure Appl. Chem. 2015, 87, 51–58. [Google Scholar] [CrossRef]
- Szajli, E.; Feher, T.; Medzihradszky, K.F. Investigating the quantitative nature of MALDI-TOF MS. Mol. Cell. Proteom. 2008, 7, 2410–2418. [Google Scholar] [CrossRef] [Green Version]
- Kornhauser, A.; Coelho, S.G.; Hearing, V.J. Applications of hydroxy acids: Classification, mechanisms, and photoactivity. Clin. Cosmet. Investig. Dermatol. 2010, 3, 135–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brem, J.; Paizs, C.; Toşa, M.I.; Vass, E.; Irimie, F.D. Enzyme-catalyzed synthesis of (R)- and (S)-3-heteroaryl-3-hydroxy-propanoic acids and their derivatives. Tetrahedron Asym. 2009, 20, 489–496. [Google Scholar] [CrossRef]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, A.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380. [Google Scholar] [CrossRef] [Green Version]
- Alcantara, A.R.; de Maria, P.D. Recent advances on the use of 2-methyltetrahydrofuran (2-MeTHF) in biotransformations. Curr. Green Chem. 2018, 5, 86–103. [Google Scholar] [CrossRef]
- Bartha-Vári, J.H.; Toşa, M.I.; Irimie, F.-D.; Weiser, D.; Boros, Z.; Vértessy, B.G.; Paizs, C.; Poppe, L. Immobilization of phenylalanine ammonia-lyase on single-walled carbon nanotubes for stereoselective biotransformations in batch and continuous-flow modes. ChemCatChem 2015, 7, 1122–1128. [Google Scholar] [CrossRef] [Green Version]
- Weiser, D.; Nagy, F.; Bánóczi, G.; Oláh, M.; Farkas, A.; Szilágyi, A.; László, K.; Gellért, Á.; Marosi, G.; Kemény, S.; et al. Immobilization engineering—How to design advanced sol-gel systems for biocatalysis? Green Chem. 2017, 19, 3927–3937. [Google Scholar] [CrossRef] [Green Version]
- Huang, M.; Dong, X.; Wang, L.; Zhao, J.; Liu, G.; Wang, D. Two-way shape memory property and its structural origin of cross-linked poly(3-caprolactone). RSC Adv. 2014, 4, 55483–55494. [Google Scholar] [CrossRef]

 are linear oligoesters with 1 unit of MA,
are linear oligoesters with 1 unit of MA,  are cyclic oligoesters with 1 unit of MA,
are cyclic oligoesters with 1 unit of MA,  and are linear oligoesters with 2 units of MA).
and are linear oligoesters with 2 units of MA).
 are linear oligoesters with 1 unit of MA,
are linear oligoesters with 1 unit of MA,  are cyclic oligoesters with 1 unit of MA,
are cyclic oligoesters with 1 unit of MA,  and are linear oligoesters with 2 units of MA).
and are linear oligoesters with 2 units of MA).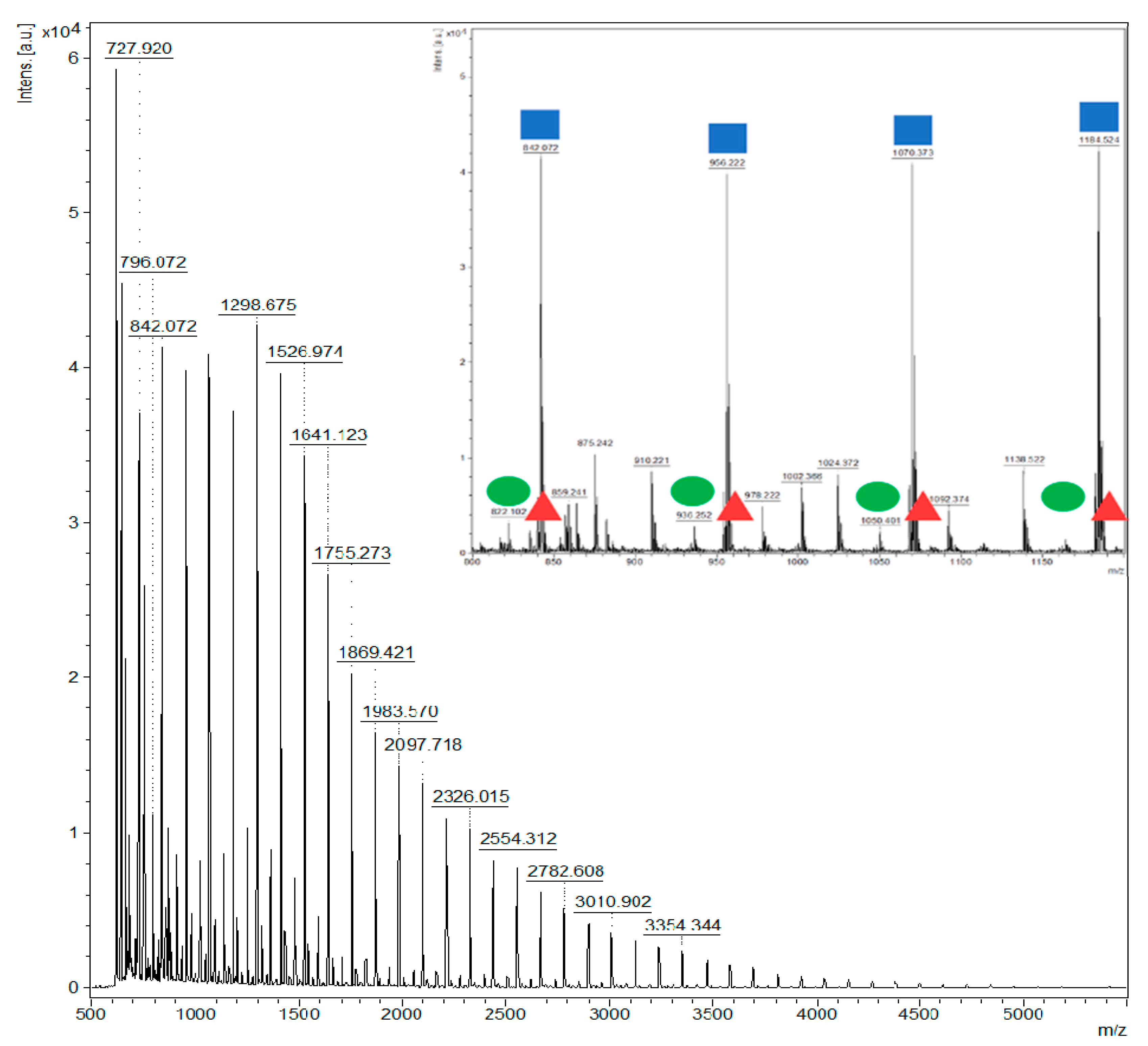

| Co-Monomer | Malic Acid Conversion (%) | Molar Ratio a | Mn (Da) | Mw (Da) | ĐM | Relative Composition of the Reaction Products (%) | DP Max f | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LC b | CC c | LH d | CH e | |||||||
| D,L-malic acid | 20.41 | 1:2 | 782 | 831 | 1.20 | 63.4 | 1.37 | 23.6 | 11.6 | 14 |
| 57.93 | 1:1 | 924 | 1038 | 1.12 | 88.6 | 2.6 | 2.1 | 6.7 | 18 | |
| 99.77 | 2:1 | 1343 | 1582 | 1.18 | 82.1 | 1.0 | 8.0 | 8.8 | 26 | |
| L-malic acid | 46.82 | 1:2 | 807 | 849 | 1.05 | 84.7 | 5.0 | 5.8 | 4.5 | 13 |
| 68.93 | 1:1 | 952 | 1053 | 1.11 | 86.6 | 1.3 | 1.3 | 10.9 | 16 | |
| 97.87 | 2:1 | 1079 | 1247 | 1.18 | 82.7 | 1.5 | 3.5 | 12.3 | 21 | |
| Co-Monomer | Malic Acid Conversion (%) | Molar Ratio a | Mn (Da) | Mw (Da) | ĐM | Relative Composition of the Reaction Products (%) | DP Max f | |||
|---|---|---|---|---|---|---|---|---|---|---|
| LC b | CC c | LH d | CH e | |||||||
| D,L-malic acid | 26.22 | 1:2 | 800 | 881 | 1.20 | 57.0 | 3.4 | 18.5 | 21.1 | 15 |
| 75.11 | 1:1 | 1166 | 1266 | 1.09 | 78.6 | 2.9 | 2.2 | 16.3 | 20 | |
| 95.61 | 2:1 | 1383 | 1642 | 1.19 | 78.0 | 0.6 | 11.4 | 9.9 | 29 | |
| L-malic acid | 45.00 | 1:2 | 936 | 1019 | 1.09 | 76.9 | 1.8 | - | 21.3 | 15 |
| 63.07 | 1:1 | 1068 | 1245 | 1.17 | 78.4 | 1.0 | 6.7 | 13.9 | 23 | |
| 94.91 | 2:1 | 1214 | 1523 | 1.25 | 78.3 | 0.5 | 9.7 | 11.4 | 30 | |
| Solvent | Malic Acid Conversion(%) | Mn (Da) | Mw (Da) | ĐM | Relative Composition of the Reaction Products (%) | DP Max e | |||
|---|---|---|---|---|---|---|---|---|---|
| LC a | CC b | LH c | CH d | ||||||
| - | 98.72 | 1357 | 1644 | 1.21 | 78.0 | 1.3 | 1.1 | 9.6 | 28 |
| Acetonitrile | 42.85 | 842 | 961 | 1.14 | 35.9 | 0.4 | 59.4 | 4.3 | 25 |
| Me-THF | 98.08 | 970 | 1053 | 1.09 | 53.4 | 0.96 | 36.4 | 9.0 | 17 |
| Toluene | 51.34 | 1023 | 1138 | 1.11 | 32.4 | 0.4 | 47.9 | 19.2 | 19 |
| Compound | Mass Loss (%) | |||
|---|---|---|---|---|
| 20–200 °C | 20–300 °C | 20–400 °C | 20–500 °C | |
| ECL_MA | 4.44 | 37.84 | 78.74 | 98.50 |
| PCL | 0.30 | 3.72 | 42.94 | 99.29 |
| MA | 9.64 | 99.79 | 99.79 | 99.79 |
| Compound | Ti (°C) | Tf (°C) | ΔT (°C) | Tpk (°C) | ΔHf (J/g) | χc (%) |
|---|---|---|---|---|---|---|
| MA | 122.0 | 144.5 | 22.5 | 137.5 | 305.4 | n.d. |
| PCL | 45.7 | 77.6 | 31.9 | 66.6 | 142.4 | 100 |
| ECL_MA | 34.6 113.3 | 64.3 135.7 | 29.7 22.4 | 58 126.9 | 72.29 57.35 | 50.76 40.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dreavă, D.M.; Benea, I.C.; Bîtcan, I.; Todea, A.; Șișu, E.; Puiu, M.; Peter, F. Biocatalytic Approach for Novel Functional Oligoesters of ε-Caprolactone and Malic Acid. Processes 2021, 9, 232. https://doi.org/10.3390/pr9020232
Dreavă DM, Benea IC, Bîtcan I, Todea A, Șișu E, Puiu M, Peter F. Biocatalytic Approach for Novel Functional Oligoesters of ε-Caprolactone and Malic Acid. Processes. 2021; 9(2):232. https://doi.org/10.3390/pr9020232
Chicago/Turabian StyleDreavă, Diana Maria, Ioana Cristina Benea, Ioan Bîtcan, Anamaria Todea, Eugen Șișu, Maria Puiu, and Francisc Peter. 2021. "Biocatalytic Approach for Novel Functional Oligoesters of ε-Caprolactone and Malic Acid" Processes 9, no. 2: 232. https://doi.org/10.3390/pr9020232






