Enzymatic Synthesis of Poly(alkylene succinate)s: Influence of Reaction Conditions
Abstract
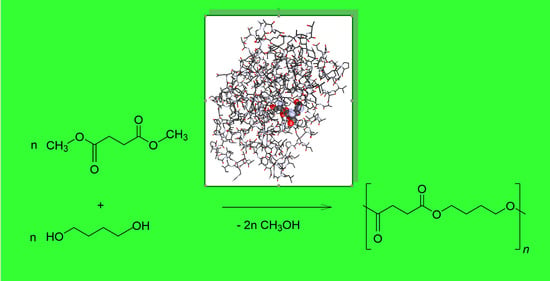
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Procedures
2.3. Methods
2.3.1. Determination of Enzyme Activity
2.3.2. Solution Viscosity
2.3.3. Size Exclusion Chromatography
2.3.4. NMR Spectroscopy
2.3.5. Matrix-Assisted Laser Desorption Ionization–Time-of-Flight Mass Spectrometry (MALDI-TOF MS)
2.3.6. Differential Scanning Calorimetry (DSC)
2.3.7. Wide-Angle X-ray Scattering (WAXS)
3. Results and Discussion
3.1. Activity of the Lipases
3.2. Polycondensation Results
3.3. Influence of Polycondensation Conditions on the Solid State Structure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CALB | Candida antarctica Lipase B |
| PBA | poly(butylene adipate) |
| PBS | poly(butylene succinate) |
| N435 | Novozyme 435, immobilized lipase B from Candida Antarctica, produced by Novozymes (DK) |
| Ti(OBu)4 | titanium(IV)n-butoxide |
| Sb2O3 | antimony trioxide |
| BD | 1,4-butanediol |
| DMS | dimethyl succinate |
| DMA | dimethyl adipate |
| SEC | size exclusion chromatography |
| NMR | nuclear magnetic resonance |
| MALDI-TOF MS | matrix-assisted laser desorption ionization–time-of-flight mass spectrometry |
| WAXS | wide-angle X-ray scattering |
| DSC | differential scanning calorimetry |
| Mw | weight average molar mass |
| Mn | number average molar mass |
References
- Varma, I.K.; Albertsson, A.C.; Rajkhowa, R.; Srivastava, R.K. Enzyme catalyzed synthesis of polyesters. Prog. Polym. Sci. 2005, 30, 949–981. [Google Scholar] [CrossRef]
- Albertsson, A.C.; Varma, I.K. Aliphatic polyesters: Synthesis, properties and applications. Adv. Polym. Sci. 2002, 157, 1–40. [Google Scholar] [CrossRef]
- Uyama, H.; Kobayashi, S. Enzymatic synthesis and properties of polymers from polyphenols. Adv. Polym. Sci. 2006, 194, 51–67. [Google Scholar] [CrossRef]
- Xu, P.; Singh, A.; Kaplan, D.L. Enzymatic catalysis in the synthesis of polyanilines and derivatives of polyanilines. Adv. Polym. Sci. 2006, 194, 69–94. [Google Scholar] [CrossRef]
- Behabtu, N.; Kralj, S. Enzymatic Polymerization Routes to Synthetic-Natural Materials: A Review. ACS Sustain. Chem. Eng. 2020, 8, 9947–9954. [Google Scholar] [CrossRef]
- Gustini, L.; Lavilla, C.; Janssen, W.W.T.J.; Martínez De Ilarduya, A.; Muñoz-Guerra, S.; Koning, C.E. Green and selective polycondensation methods toward linear sorbitol-based polyesters: Enzymatic versus organic and metal-based catalysis. ChemSusChem 2016, 9, 2250–2260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A.; Kaplan, D.L. In vitro enzyme-induced vinyl polymerization. Adv. Polym. Sci. 2006, 194, 211–224. [Google Scholar] [CrossRef]
- Matsumura, S. Enzymatic synthesis of polyesters via Ring-Opening Polymerization. Adv. Polym. Sci. 2006, 194, 95–132. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Schwarz, G. Poly(thioester)s. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 625–649. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z. Novozym 435-Catalyzed Synthesis of Well-Defined Hyperbranched Aliphatic Poly(β-thioether ester). Moledcules 2020, 25, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.-X. Lipase-catalyzed synthesis and post-polymerization modi fi cation of new fully bio-based poly(hexamethylene γ-ketopimelate) and poly(hexamethylene γ-ketopimelate-co-hexamethylene adipate) copolyesters. E-Polymers 2020, 20, 214–225. [Google Scholar] [CrossRef]
- Uyama, H.; Kobayashi, S. Enzymatic synthesis of polyesters via polycondensation. Adv. Polym. Sci. 2006, 194, 133–158. [Google Scholar] [CrossRef]
- Namekawa, S.; Uyama, H.; Kobayashi, S.; Kricheldorf, H.R. Lipase-catalyzed ring-opening polymerization and copolymerization of cyclic dicarbonates. Macromol. Chem. Phys. 2000, 201, 261–264. [Google Scholar] [CrossRef]
- Kato, M.; Toshima, K.; Matsumura, S. Preparation of aliphatic poly(thioester) by the lipase-catalyzed direct polycondensation of 11-mercaptoundecanoic acid. Biomacromolecules 2005, 6, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Loos, K. Enzymatic synthesis of biobased polyesters and polyamides. Polymers 2016, 8, 243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Song, L.; Feng, N.; Jiang, W.; Jin, Y.; Li, X. Recent advances in the synthesis of biodegradable polyesters by sustainable polymerization: Lipase-catalyzed polymerization. RSC Adv. 2020, 10, 36230–36240. [Google Scholar] [CrossRef]
- Douka, A.; Vouyiouka, S.; Papaspyridi, L.M.; Papaspyrides, C.D. A review on enzymatic polymerization to produce polycondensation polymers: The case of aliphatic polyesters, polyamides and polyesteramides. Prog. Polym. Sci. 2018, 79, 1–25. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Lopez, J.; Beckman, E.J.; Russell, A.J. Biocatalytic solvent-free polymerization to produce high molecular weight polyesters. Biotechnol. Prog. 1997, 13, 318–325. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Beckman, E.J.; Russell, A.J. Biocatalytic polyester synthesis: Analysis of the evolution of molecular weight and end group functionality. Biotechnol. Bioeng. 1997, 55, 227–239. [Google Scholar] [CrossRef]
- Chaudhary, A.K.; Beckman, E.J.; Russell, A.J. Nonequal Reactivity Model for Biocatalytic Polytransesterification. AIChE J. 1998, 44, 753–764. [Google Scholar] [CrossRef]
- Skoczinski, P.; Espinoza Cangahuala, M.K.; Maniar, D.; Albach, R.W.; Bittner, N.; Loos, K. Biocatalytic Synthesis of Furan-Based Oligomer Diols with Enhanced End-Group Fidelity. ACS Sustain. Chem. Eng. 2020, 8, 1068–1086. [Google Scholar] [CrossRef]
- Fodor, C.; Golkaram, M.; Woortman, A.J.J.; Van Dijken, J.; Loos, K. Enzymatic approach for the synthesis of biobased aromatic-aliphatic oligo-/polyesters. Polym. Chem. 2017, 8, 6795–6805. [Google Scholar] [CrossRef]
- Japu, C.; Martínez De Ilarduya, A.; Alla, A.; Jiang, Y.; Loos, K.; Muñoz-Guerra, S. Copolyesters Made from 1,4-Butanediol, Sebacic Acid, and D-Glucose by Melt and Enzymatic Polycondensation. Biomacromolecules 2015, 16, 868–879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Corici, L.; Pellis, A.; Ferrario, V.; Ebert, C.; Cantone, S.; Gardossi, L. Understanding Potentials and Restrictions of Solvent-Free Enzymatic Polycondensation of Itaconic Acid: An Experimental and Computational Analysis. Adv. Synth. Catal. 2015, 357, 1763–1774. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, A.; Cutifani, V.; Cespugli, M.; Pellis, A.; Vassallo, R.; Asaro, F.; Ebert, C.; Gardossi, L. Functionalization of Enzymatically Synthesized Rigid Poly(itaconate)s via Post-Polymerization Aza-Michael Addition of Primary Amines. Adv. Synth. Catal. 2019, 361, 2559–2573. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Ferrario, V.; Cespugli, M.; Corici, L.; Guarneri, A.; Zartl, B.; Herrero Acero, E.; Ebert, C.; Guebitz, G.M.; Gardossi, L. Fully renewable polyesters: Via polycondensation catalyzed by Thermobifida cellulosilytica cutinase 1: An integrated approach. Green Chem. 2017, 19, 490–502. [Google Scholar] [CrossRef]
- Pellis, A.; Gardossi, L. Integrating Computational and Experimental Methods for Efficient Biocatalytic Synthesis of Polyesters, 1st ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; Volume 627, ISBN 9780128170953. [Google Scholar]
- Vouyiouka, S.N.; Topakas, E.; Katsini, A.; Papaspyrides, C.D.; Christakopoulos, P. A green route for the preparation of aliphatic polyesters via lipase-catalyzed prepolymerization and low-temperature postpolymerization. Macromol. Mater. Eng. 2013, 298, 679–689. [Google Scholar] [CrossRef]
- Sarmah, N.; Revathi, D.; Sheelu, G.; Rani, K.Y.; Sridhar, S.; Mehtab, V.; Sumana, C. Recent Advances and Industrial Applications of Microencapsulation. Biocatal. Bioreact. Des. 2017. [Google Scholar] [CrossRef]
- Busto, E.; Gotor-Fernández, V.; Gotor, V. Hydrolases: Catalytically promiscuous enzymes for non-conventional reactions in organic synthesis. Chem. Soc. Rev. 2010, 39, 4504–4523. [Google Scholar] [CrossRef]
- Bisswanger, H. Enzyme—Struktur, Kinetik und Anwendungen; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Kobayashi, S. Lipase-catalyzed polyester synthesis—A green polymer chemistry. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 338–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulschewski, T. Modellierung der Candida antarctica Lipase B unter Nicht-Natürlichen Bedingungen. Ph.D. Thesis, Universität Stuttgart, Stuttgart, Germany, 2015. [Google Scholar]
- Mahapatro, A.; Kumar, A.; Kalra, B.; Gross, R.A. Solvent-Free Adipic Acid/1,8-Octanediol Condensation Polymerizations Catalyzed by Candida antartica Lipase B. Macromolecules 2004, 37, 35–40. [Google Scholar] [CrossRef]
- Ren, L.; Wang, Y.; Ge, J.; Lu, D.; Liu, Z. Enzymatic Synthesis of High-Molecular-Weight Poly(butylene succinate) and its Copolymers. Macromol. Chem. Phys. 2015, 216, 636–640. [Google Scholar] [CrossRef]
- Nasr, K.; Meimoun, J.; Favrelle-Huret, A.; De Winter, J.; Raquez, J.M.; Zinck, P. Enzymatic polycondensation of 1,6-hexanediol and diethyl adipate: A statistical approach predicting the key-parameters in solution and in bulk. Polymers 2020, 12, 1907. [Google Scholar] [CrossRef] [PubMed]
- Gkountela, C.; Rigopoulou, M.; Barampouti, E.M.; Youyiouka, S. Enzymatic prepolymerization combined with post-polymerization towards the production of bio-based polyesters: The case of poly(butylene succinate). Eur. Polym. J. 2021, 143, 110197. [Google Scholar] [CrossRef]
- Azim, H.; Dekhterman, A.; Jiang, Z.; Gross, R.A. Candida antarctica Lipase B catalyzed synthesis of poly(butylene succinate): Shorter chain building blocks also work. ACS Symp. Ser. 2008, 999, 285–293. [Google Scholar] [CrossRef]
- Binns, F.; Roberts, S.M.; Taylor, A.; Williams, C.F. Enzymic polymerisation of an unactivated diol/diacid system. J. Chem. Soc. Perkin Trans. 1 1993, 899–904. [Google Scholar] [CrossRef]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies of lipase-catalyzed polyesterification of an unactivated diacid/diol system. J. Polym. Sci. Part. A Polym. Chem. 1998, 36, 2069–2079. [Google Scholar] [CrossRef]
- Binns, F.; Harffey, P.; Roberts, S.M.; Taylor, A. Studies leading to the large scale synthesis of polyesters using enzymes. J. Chem. Soc. Perkin Trans. 1 1999, 2671–2676. [Google Scholar] [CrossRef]
- Linko, Y.Y.; Wang, Z.L.; Seppälä, J. Lipase-catalyzed synthesis of poly(1,4-butyl sebacate) from sebacic acid or its derivatives with 1,4-butanediol. J. Biotechnol. 1995, 40, 133–138. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Spinella, S.; Xie, W.; Cai, J.; Yang, Y.; Wang, Y.Z.; Gross, R.A. Polymeric triglyceride analogs prepared by enzyme-catalyzed condensation polymerization. Eur. Polym. J. 2013, 49, 793–803. [Google Scholar] [CrossRef]
- Sonseca, A.; McClain, A.; Puskas, J.E.; El Fray, M. Kinetic studies of biocatalyzed copolyesters of poly(butylene succinate)(PBS)containing fully bio-based dilinoleic diol. Eur. Polym. J. 2019, 116, 515–525. [Google Scholar] [CrossRef]
- Sokołowska, M.; Stachowska, E.; Czaplicka, M.; El Fray, M. Effect of enzymatic versus titanium dioxide/silicon dioxide catalyst on crystal structure of ‘green’ poly[(butylene succinate)-co-(dilinoleic succinate)] copolymers. Polym. Int. 2020. [Google Scholar] [CrossRef]
- Sonseca, A.; El Fray, M. Enzymatic synthesis of an electrospinnable poly(butylene succinate-co-dilinoleic succinate) thermoplastic elastomer. RSC Adv. 2017, 7, 21258–21267. [Google Scholar] [CrossRef] [Green Version]
- Sonseca, A.; Sahay, R.; Stepien, K.; Bukala, J.; Wcislek, A.; McClain, A.; Sobolewski, P.; Sui, X.M.; Puskas, J.E.; Kohn, J.; et al. Architectured helically coiled scaffolds from elastomeric poly(butylene succinate) (PBS) copolyester via wet electrospinning. Mater. Sci. Eng. C 2020, 108, 110505. [Google Scholar] [CrossRef]
- Uyama, H.; Yaguchi, S.; Kobayashi, S. Enzymatic Synthesis of Aromatic Polyesters by Lipase-Catalyzed Polymerization of Dicarboxylic Acid Divinyl Esters and Glycols. Polym. J. 1999, 31, 380–383. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Chisti, Y.; Chand, U. and Applications of Lipases. Biotechnol. Adv. 2001, 19, 627–662. [Google Scholar] [CrossRef] [Green Version]
- Miletic, N. Improved Biocatalysts Based on Candida antarctica Lipase B Immobilization. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2009. [Google Scholar]
- Ortiz, C.; Ferreira, M.L.; Barbosa, O.; Dos Santos, J.C.S.; Rodrigues, R.C.; Berenguer-Murcia, Á.; Briand, L.E.; Fernandez-Lafuente, R. Novozym 435: The “perfect” lipase immobilized biocatalyst? Catal. Sci. Technol. 2019, 9, 2380–2420. [Google Scholar] [CrossRef] [Green Version]
- Vera, M.; Fodor, C.; Garcia, Y.; Pereira, E.; Loos, K.; Rivas, B.L. Multienzymatic immobilization of laccases on polymeric microspheres: A strategy to expand the maximum catalytic efficiency. J. Appl. Polym. Sci. 2020, 137. [Google Scholar] [CrossRef]
- Gigli, M.; Fabbri, M.; Lotti, N.; Gamberini, R.; Rimini, B.; Munari, A. Poly(butylene succinate)-based polyesters for biomedical applications: A review in memory of our beloved colleague and friend Dr. Lara Finelli. Eur. Polym. J. 2016, 75, 431–460. [Google Scholar] [CrossRef]
- Peng, S.; Bu, Z.; Wu, L.; Li, B.G.; Dubois, P. High molecular weight poly(butylene succinate-co-furandicarboxylate) with 10 mol% of BF unit: Synthesis, crystallization-melting behavior and mechanical properties. Eur. Polym. J. 2017, 96, 248–255. [Google Scholar] [CrossRef]
- Adamopoulou, E. Poly(butylene succinate): A Promising Biopolymer. Ph.D. Thesis, University of Athens, Athens, Greece, 2012; p. 137. [Google Scholar]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability. Polymers 2020, 12, 1558. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Aliphatic Polyesters: Synthesis, properties and Applications. In Advances in Polymer Science; Springer: Berlin/Heidelberg, Germany, 2002; Volume 157, pp. 2–35. ISBN 4842684712. [Google Scholar]
- Jacquel, N.; Freyermouth, F.; Fenouillot, F.; Rousseau, A.; Pascault, J.P.; Fuertes, P.; Saint-Loup, R. Synthesis and properties of poly(butylene succinate): Efficiency of different transesterification catalysts. J. Polym. Sci. Part A Polym. Chem. 2011, 49, 5301–5312. [Google Scholar] [CrossRef]
- Pospiech, D.; Korwitz, A.; Komber, H.; Jehnichen, D.; Häussler, L.; Scheibner, H.; Liebmann, M.; Jähnichen, K.; Voit, B. Biobased Aliphatic Polyesters with DOPO Substituents for Enhanced Flame Retardancy. Macromol. Chem. Phys. 2015, 216. [Google Scholar] [CrossRef]
- Dai, S.; Xue, L.; Zinn, M.; Li, Z. Enzyme-catalyzed polycondensation of polyester macrodiols with divinyl adipate: A green method for the preparation of thermoplastic block copolyesters. Biomacromolecules 2009, 10, 3176–3181. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Rathi, P.; Gupta, R. Simplified para-nitrophenyl palmitate assay for lipases and esterases. Anal. Biochem. 2002, 311, 98–99. [Google Scholar] [CrossRef]
- Bragg, W.H.; Bragg, W.L. X-rays and Crystal Structure; G. Bell & Sons: London, UK, 1915. [Google Scholar]
- Alexander, L.E. X-ray Diffraction Methods in Polymer Science; John Wiley: New York, NY, USA, 1970. [Google Scholar]
- Mezoul, G.; Lalot, T.; Brigodiot, M.; Maréchal, E. Enzyme-catalyzed synthesis of aliphatic polyesters in organic media: Study of transesterification equilibrium shift and characterization of cyclic compounds. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 2691–2698. [Google Scholar] [CrossRef]
- Uyama, H.; Takamoto, T.; Kobayashi, S. Enzymatic Synthesis of Polyesters in Ionic Liquids. Polym. J. 2002, 34, 94–96. [Google Scholar] [CrossRef] [Green Version]
- Pellis, A.; Corici, L.; Sinigoi, L.; D’Amelio, N.; Fattor, D.; Ferrario, V.; Ebert, C.; Gardossi, L. Towards feasible and scalable solvent-free enzymatic polycondensations: Integrating robust biocatalysts with thin film reactions. Green Chem. 2015, 17, 1756–1766. [Google Scholar] [CrossRef] [Green Version]
- Mesiano, A.J.; Beckman, E.J.; Russell, A.J. Biocatalytic synthesis of fluorinated polyesters. Biotechnol. Prog. 2000, 16, 64–68. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Uyama, H.; Kobayashi, S. Enzymatic synthesis and curing of biodegradable crosslinkable polyesters. Macromol. Biosci. 2002, 2, 329–335. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Schwarz, G. Cyclic Polymers by Kinetically Controlled Step-Growth Polymerization. Macromol. Rapid Commun. 2003, 24, 359–381. [Google Scholar] [CrossRef]
- Debuissy, T.; Pollet, E.; Avérous, L. Synthesis and characterization of biobased poly(butylene succinate-ran-butylene adipate). Analysis of the composition-dependent physicochemical properties. Eur. Polym. J. 2017, 87, 84–98. [Google Scholar] [CrossRef]
- Jiang, Y.; Woortman, A.J.J.; Alberda Van Ekenstein, G.O.R.; Loos, K. Environmentally benign synthesis of saturated and unsaturated aliphatic polyesters via enzymatic polymerization of biobased monomers derived from renewable resources. Polym. Chem. 2015, 6, 5451–5463. [Google Scholar] [CrossRef]
- Schwarzer, M.; Korwitz, A.; Komber, H.; Häußler, L.; Dittrich, B.; Schartel, B.; Pospiech, D. Phosphorus-Containing Polymer Flame Retardants for Aliphatic Polyesters. Macromol. Mater. Eng. 2018, 303. [Google Scholar] [CrossRef]
- Garaleh, M.; Kricheldorf, H.R.; Weidner, S.M.; Yashiro, T.; Garaleh, M.; Kricheldorf, H.R.; Weidner, S.M.; Yashiro, T. Hafnium Chloride Catalyzed Polycondensation of α, ω -Alkanediol with Dicarboxylic Acids or Succinic Anhydride Hafnium Chloride Catalyzed Polycondensation of α, ω -Alkanediol with Dicarboxylic Acids or Succinic Anhydride. J. Macromol. Sci. Part A 2017, 47, 303–308. [Google Scholar] [CrossRef]
- Armelin, E.; Casas, M.T.; Puiggalõ, J. Structure of poly(hexamethylene sebacate). Polymer 2001, 42, 5695–5699. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kondo, H.; Igarashi, Y.; Noguchi, K.; Okuyama, K.; Washiyama, J. Crystal structures of a and b forms of poly(tetramethylene succinate). Polymer 2000, 41, 4719–4727. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Suzuki, J.; Washiyama, J.; Moteki, Y.; Nogiuchi, K.; Okuyama, K. Strain-induced crystal modification in poly(tetrameth ylene succinate). Polymer 1994, 35, 3338–3339. [Google Scholar] [CrossRef]
- Ichikawa, Y.; Kondo, H.; Igarashi, Y.; Noguchi, K.; Okuyama, K.; Washiyama, J. Corrigendum to “Crystal structures of α and β forms of poly(tetramethylene succinate)”. Polymer 2001, 42, 847. [Google Scholar] [CrossRef]
- Gan, Z.; Abe, H.; Doi, Y. Temperature-induced polymorphic crystals of poly(butylene adipate). Macromol. Chem. Phys. 2002, 203, 2369–2374. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Chen, Q.; Xi, Z.; Wang, C.; Chen, X.; Feng, X.; Liang, R.; Yang, J. Modulated crystallization behavior, polymorphic crystalline structure and enzymatic degradation of poly(butylene adipate): Effects of layered metal phosphonate. Eur. Polym. J. 2015, 72, 222–237. [Google Scholar] [CrossRef]
- Wang, M.; Vantasin, S.; Wang, J.; Sato, H.; Zhang, J.; Ozaki, Y. Distribution of Polymorphic Crystals in the Ring-Banded Spherulites of Poly(butylene adipate) Studied Using High-Resolution Raman Imaging. Macromolecules 2017, 50, 3377–3387. [Google Scholar] [CrossRef]
- Wu, M.C.; Woo, E.M. Effects of α-form or β-form nuclei on polymorphic crystalline morphology of poly(butylene adipate). Polym. Int. 2005, 54, 1681–1688. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. Polymorphic Structures of Poly(tetramethylene Adipate). J. Macromol. Sci. Part B 1979, 16, 407–417. [Google Scholar] [CrossRef]
- Minke, R.; Blackwell, J. Single Crystals of Poly(tetramethylene Adipate). J. Macromol. Sci. Part B 1980, 18, 233–255. [Google Scholar] [CrossRef]
- Woo, E.M.; Wu, M.C. Thermal and X-ray analysis of polymorphic crystals, melting, and crystalline transformation in poly(butylene adipate). J. Polym. Sci. Part. B Polym. Phys. 2005, 43, 1662–1672. [Google Scholar] [CrossRef]
- Yoo, E.S.; Im, S.S. Melting behavior of poly(butylene succinate) during heating scan by DSC. J. Polym. Sci. Part B Polym. Phys. 1999, 37, 1357–1366. [Google Scholar] [CrossRef]
- Charlon, S.; Delbreilh, L.; Dargent, E.; Follain, N.; Soulestin, J.; Marais, S. Influence of crystallinity on the dielectric relaxations of poly(butylene succinate) and poly[(butylene succinate)-co-(butylene adipate)]. Eur. Polym. J. 2016, 84, 366–376. [Google Scholar] [CrossRef]






| Sample | Description | A at 50 °C (U/g) |
|---|---|---|
| CALB1 | Stored 2 years | 1400 ± 100 |
| CALB2 | Stored 6 months | 3600 ± 300 |
| CALB3 | Fresh | 2900 ± 700 |
| CALB1r | Recovered after solution polycondensation at 80 °C | 500 |
| Entry | Ratio Ester/BD (mmol/mmol) | Cat. | Ratio mon./to-luene (g/mL) | t (h) | T (°C) | Mn,SEC (g/mol) | Mw,SEC (g/mol) | Ɖ = Mw/Mn | ηinh (dL/g) | Yield (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| PBA1 | 4.5/4.5 | CALB1 (dried) | 0.259 | 8 | 70 | 1400 | 4700 | 3.36 | n.d. | 43 |
| PBA2 | 4.5/4.5 | CALB1 | 0.259 | 8 | 70 | 1600 | 5000 | 3.12 | n.d. | 52 |
| PBA3 | 4.5/5.0 | CALB1 | 0.268 | 8 | 70 | 6400 | 9700 | 1.52 | 0.27 | 39 |
| PBA4 | 4.5/5.0 | CALB1 | 0.268 | 24 | 70 | 18,300 | 59,500 | 3.25 | 0.79 | 37 |
| PBA5 | 4.5/5.0 | CALB2 | 0.447 | 24 | 70 | 12,300 | 35,300 | 2.87 | 0.65 | 69 |
| PBA5-1 | 4.5/5.0 | CALB2 | 0.447 | 8 | 70 | 8100 | 14,800 | 1.84 | n.d. | 42 |
| PBA6 | 4.5/5.0 | CALB2 | melt | 24 | 70 | 7100 | 12,600 | 1.78 | n.d. | 33 |
| PBA7 | 9.0/9.0 | CALB2 | melt | 24 | 70 | 7100 | 31,000 | 4.37 | 0.41 | 74 |
| PBA8 | 4.5/5.0 | P.F.(b) | 0.447 | 24 | 70 | no react. | - | - | - | - |
| PBA9 (a) | 4.5/4.5 | Ti(OBu)4 | melt | 4 | 150–245 | 28,000 | 58,000 | 2.12 | 0.70 | 90 |
| PBS1 | 4.5/4.5 | Ti(OBu)4 | Melt(vac) | 3 | 245 | 40,500 | 93,000 | 2.29 | 0.90 | 92 |
| PBS1-2 | 4.5/4.5 | Ti(OBu)4 | Melt(vac) | 4 | 245 | 18,500 | 40,000 | 2.16 | 0.55 | 90 |
| PBS1-3 (c) | 4.5/4.5 | Ti(OBu)4 | Melt(vac) | 4 | 245 | 147,000 | 308,000 | 2.09 | 1.8 | 89 |
| PBS2 | 4.5/4.5 | CALB1 | 0.044 | 24 | 70 | 2900 | 3800 | 1.31 | n.d. | 51 |
| PBS3 | 4.5/4.5 | CALB2 | 0.044 | 24 | 70 | 5400 | 8400 | 1.56 | n.d. | 53 |
| PBS4 | 4.5/4.5 | CALB3 | 0.044 | 24 | 70 | 3800 | 5200 | 1.37 | n.d. | 50 |
| PBS5 | 4.5/4.5 | CALB3 | 0.044 | 24 | 80 | 11,000 | 21,300 | 1.93 | 0.31 | 56 |
| PBS6 | 4.5/4.5 | CALB3 | 0.213 | 24 | 70 | 5400 | 7100 | 1.31 | 0.87 | 40 |
| PBS7 | 4.5/4.5 | CALB2 | melt | 1 | 80 | 4300 | 5300 | 1.23 | 0.17 | 40 |
| PBS8 | 4.5/4.5 | CALB2 | melt | 1.50 0.25 | 80 150 | 11,000 | 19,700 | 1.79 | 0.35 | 75 |
| PBS9 | 4.5/4.5 | CALB2 | melt | 1.50 0.25 | 80 200 | 3900 | 5400 | 1.38 | 0.16 | 42 |
| PBS10 | 4.5/4.5 | CALB3 | Melt (vac) | 0.75 3.00 | 80 200 | 4600 | 6450 | 1.40 | 0.17 | 45 |
| PBS11 | 4.5/4.5 | CALB3 | Melt (vac) | 0.75 3 | 80 130 | 11,700 | 23,600 | 2.02 | 0.36 | 75 |
| Polymer | Polycondensation Conditions | Total Crystallinity cα+β | Crystallinity cβ |
|---|---|---|---|
| PBA4 | Solution, 70 °C, CALB | 0.58 | 0.36 |
| PBA5 | Solution, 70 °C, CALB | 0.53 | 0.13 |
| PBA5-1 | Solution, 70 °C, CALB | 0.52 | 0.06 |
| PBA7 | Melt, 70 °C, CALB | 0.36 | 0.01 |
| PBA9 | Melt, 245 °C, Ti(OBu)4 | 0.46 | 0.01 |
| PBS5 | Solution, 80 °C, CALB | 0.69 | - 1 |
| PBS7 | Melt, 80 °C, CALB | 0.74 | - 1 |
| PBS8 | Melt, 150 °C, CALB | 0.75 | - 1 |
| PBS1 | Melt, 245 °C, Ti(OBu)4 (stirring autoclave) | 0.50 | - 1 |
| PBS1-2 | Melt, 245 °C, Ti(OBu)4 (lab) | 0.57 | - 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pospiech, D.; Choińska, R.; Flugrat, D.; Sahre, K.; Jehnichen, D.; Korwitz, A.; Friedel, P.; Werner, A.; Voit, B. Enzymatic Synthesis of Poly(alkylene succinate)s: Influence of Reaction Conditions. Processes 2021, 9, 411. https://doi.org/10.3390/pr9030411
Pospiech D, Choińska R, Flugrat D, Sahre K, Jehnichen D, Korwitz A, Friedel P, Werner A, Voit B. Enzymatic Synthesis of Poly(alkylene succinate)s: Influence of Reaction Conditions. Processes. 2021; 9(3):411. https://doi.org/10.3390/pr9030411
Chicago/Turabian StylePospiech, Doris, Renata Choińska, Daniel Flugrat, Karin Sahre, Dieter Jehnichen, Andreas Korwitz, Peter Friedel, Anett Werner, and Brigitte Voit. 2021. "Enzymatic Synthesis of Poly(alkylene succinate)s: Influence of Reaction Conditions" Processes 9, no. 3: 411. https://doi.org/10.3390/pr9030411