Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Chemicals and Solvents
2.3. Sample Preparation
2.4. Spectrophotometric Analysis
2.4.1. Determination of Total Phenolic and Flavonoid Content
2.4.2. Determination of Antioxidant Activity
2.5. Chromatographic Studies
2.6. Statistical Analysis
3. Results and Discussion
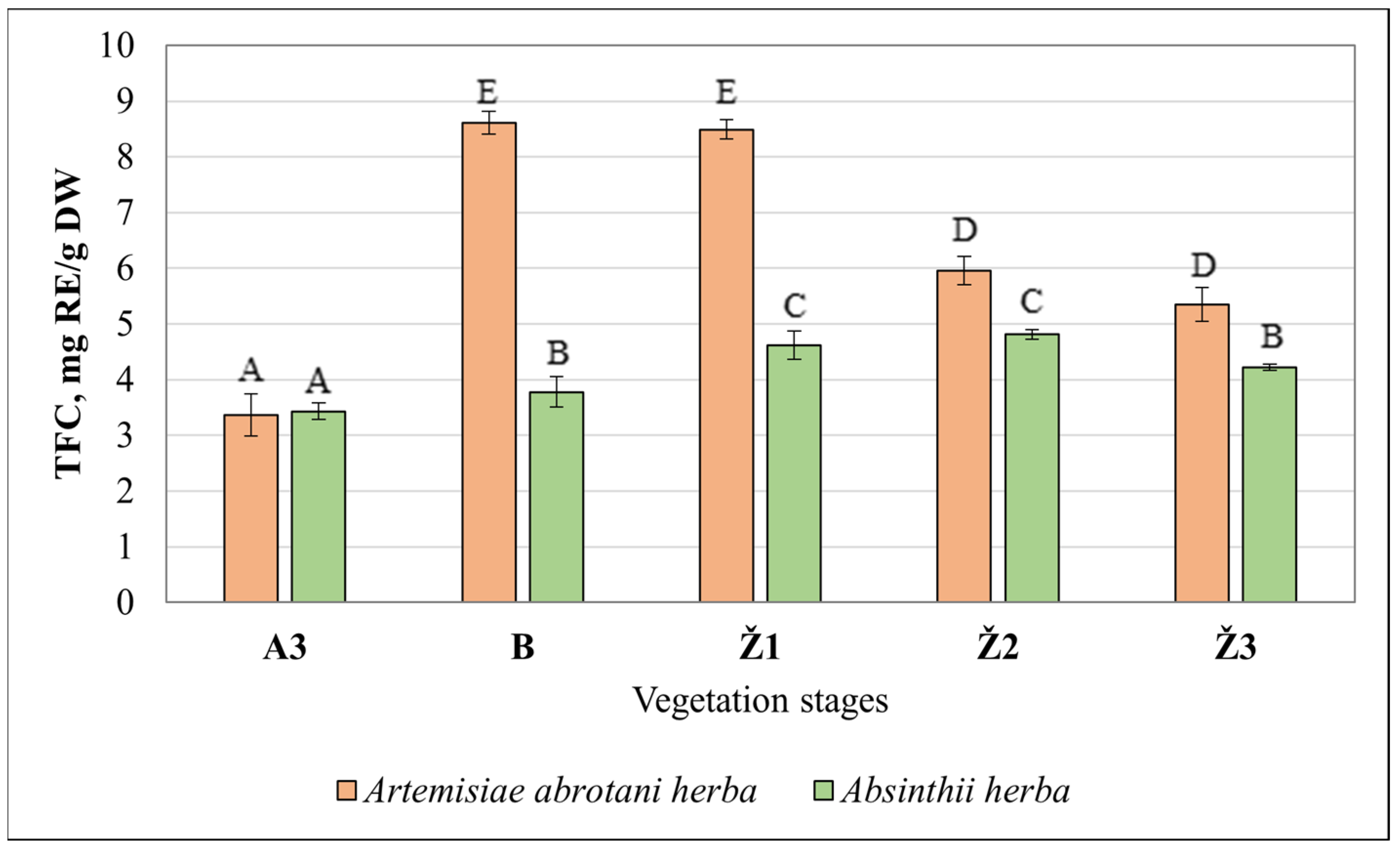
3.1. Determination of Total Phenolic and Flavonoid Content
3.2. Quantitative and Qualitative Composition of Phenolic Compounds
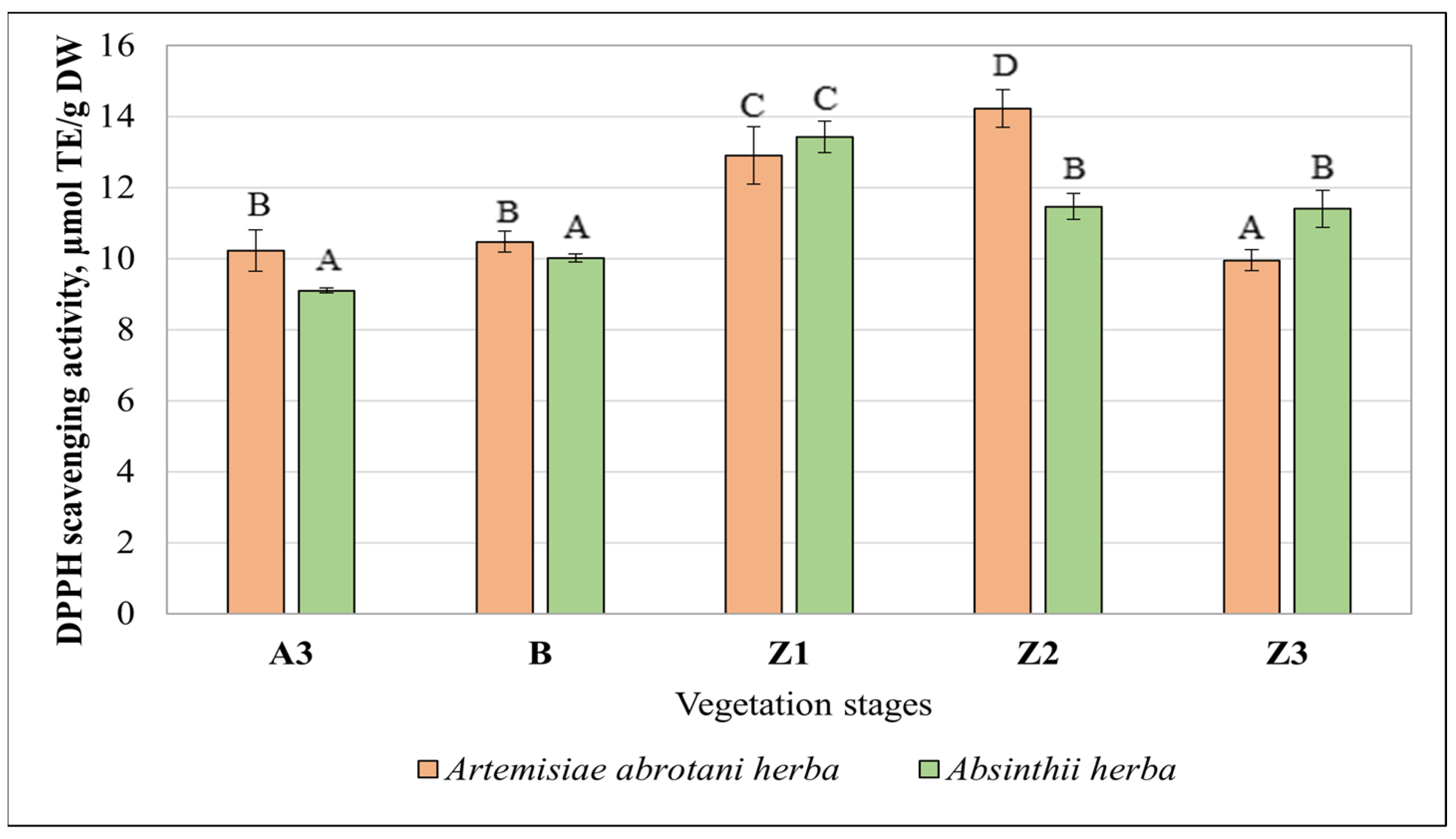
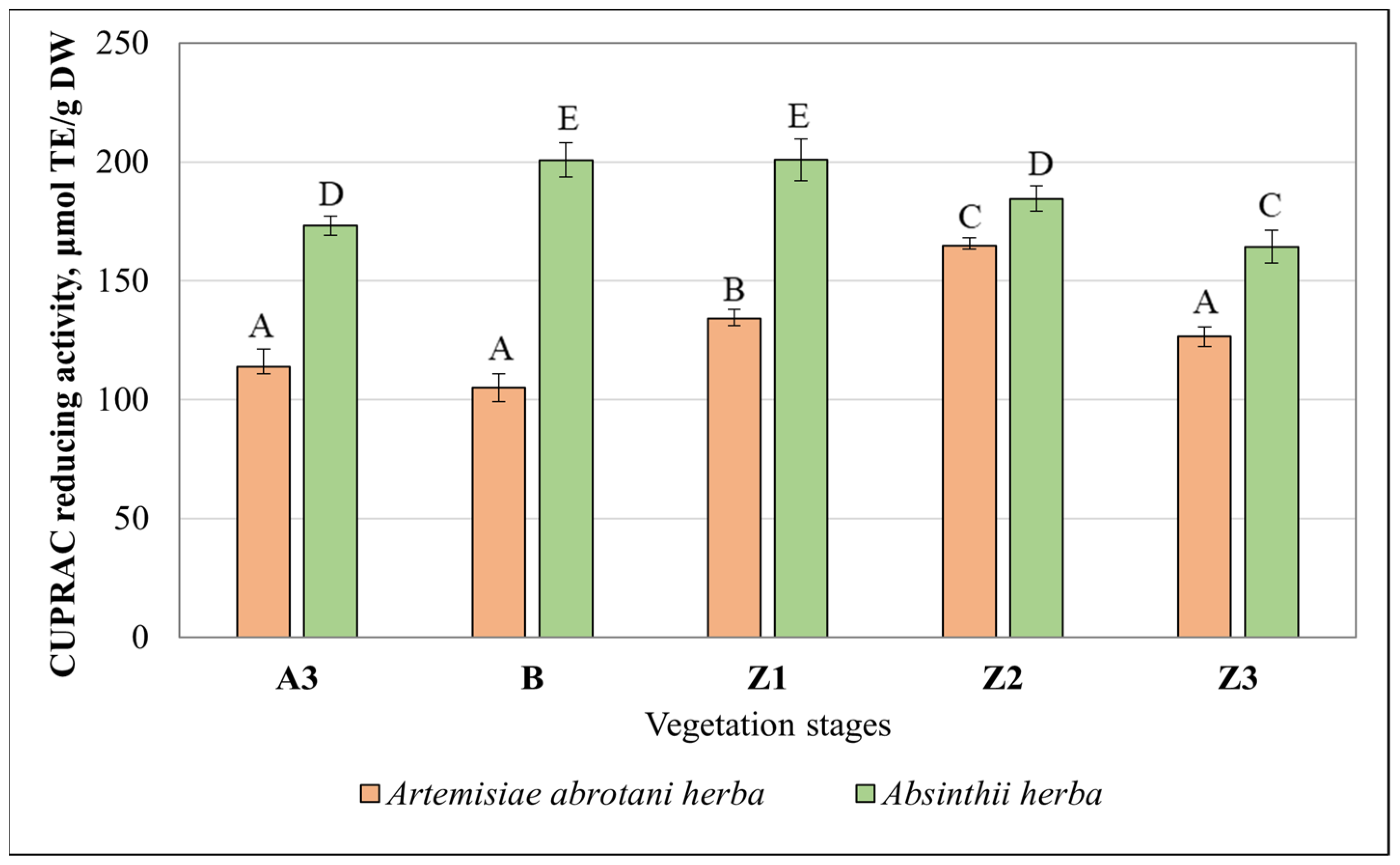
3.3. Antioxidant Activity of Artemisia Extracts
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Bisht, D.; Kumar, D.; Kumar, D.; Dua, K.; Chellappan, D.K. Phytochemistry and pharmacological activity of the genus Artemisia. Arch. Pharmacal Res. 2021, 44, 439–474. [Google Scholar] [CrossRef] [PubMed]
- Judžentienė, A.; Budienė, J. Compositional Variation in Essential Oils of Wild Artemisia absinthium from Lithuania. J. Essent. Oil-Bear. Plants 2010, 13, 275–285. [Google Scholar] [CrossRef]
- Judžentienė, A.; Budienė, J. Chemical Polymorphism of Essential Oils of Artemisia vulgaris Growing Wild in Lithuania. Chem. Biodivers. 2018, 15, e1700257. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.; Huang, Y.; Ouyang, Y.; Zhang, Y.; Xi, L.; Chu, X.; Wang, Y.; Wang, C.; Zhang, L. Artemisia annua-sublingual immunotherapy for seasonal allergic rhinitis: A randomized controlled trial. Allergy Eur. J. Allergy Clin. Immunol. 2020, 75, 2026–2036. [Google Scholar] [CrossRef] [PubMed]
- El Gaber, S.B.; Beshbishy, A.M.; Tayebwa, D.S.; Adeyemi, O.S.; Yokoyama, N.; Igarashi, I. Anti-piroplasmic potential of the methanolic Peganum harmala seeds and ethanolic Artemisia absinthium leaf extracts. J. Protozool. Res. 2019, 29, 8–27. [Google Scholar] [CrossRef]
- Szopa, A.; Pajor, J.; Klin, P.; Rzepiela, A.; Elansary, H.O.; Al-Mana, F.; Mattar, M.A.; Ekiert, H. Artemisia absinthium L.—Importance in the History of Medicine, the Latest Advances in Phytochemistry and Therapeutical, Cosmetological and Culinary Uses. Plants 2020, 9, 1063. [Google Scholar] [CrossRef]
- Ekiert, H.; Pajor, J.; Klin, P.; Rzepiela, A.; Ślesak, H.; Szopa, A. Significance of Artemisia vulgaris L. (Common Mugwort) in the history of medicine and its possible contemporary applications substantiated by phytochemical and pharmacological studies. Molecules 2020, 25, 4415. [Google Scholar] [CrossRef]
- Ekiert, H.; Świątkowska, J.; Klin, P.; Rzepiela, A.; Szopa, A. Artemisia annua—Importance in Traditional Medicine and Current State of Knowledge on the Chemistry, Biological Activity and Possible Applications. Planta Med. 2021, 87, 584–599. [Google Scholar] [CrossRef]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical Profiling, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Effects of Essential Oils Isolated from the Leaves of Artemisia scoparia and Artemisia absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Romeilah, R.M.; El-Beltagi, H.S.; Shalaby, E.A.; Younes, K.M.; El Moll, H.; Rajendrasozhan, S.; Mohamed, H.I. Antioxidant and cytotoxic activities of Artemisia monosperma L. and Tamarix aphylla L. essential oils. Not. Bot. Horti Agrobot. Cluj-Napoca 2021, 49, 12233. [Google Scholar] [CrossRef]
- Nigam, M.; Atanassova, M.; Mishra, P.A.; Pezzani, R.; Devkota, P.H.; Plygun, S.; Salehi, B.; Setzer, N.W.; Rad, S.J. Bioactive Compounds and Health Benefits of Artemisia Species. Nat. Prod. Commun. 2019, 14, 1934578X19850354. [Google Scholar] [CrossRef]
- Nurbek, S.; Murata, T.; Suganuma, K.; Ishikawa, Y.; Buyankhishig, B.; Kikuchi, T.; Byambajav, T.; Davaapurev, B.O.; Sasaki, K.; Batkhuu, J. Isolation and evaluation of trypanocidal activity of sesquiterpenoids, flavonoids, and lignans in Artemisia sieversiana collected in Mongolia. J. Nat. Med. 2020, 74, 750–757. [Google Scholar] [CrossRef]
- Sultan, M.H.; Moni, S.S. Spectral analysis and antibacterial effect of cold methanolic extract of Artemisia absinthium L. Braz. J. Biol. 2022, 84, e264869. [Google Scholar] [CrossRef]
- Song, X.; Wen, X.; He, J.; Wang, J.; Li, S.; Wang, M. Phytochemical components and biological activities of Artemisia argyi. J. Funct. Foods. 2019, 52, 648–662. [Google Scholar] [CrossRef]
- Han, B.; Xin, Z.; Ma, S.; Liu, W.; Zhang, B.; Ran, L.; Yi, L.; Ren, D. Comprehensive characterization and identification of antioxidants in Folium Artemisiae Argyi using high-resoliution tandem mass spectrometry. J. Chromatogr. B 2017, 1063, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Li, K.M.; Dong, X.; Ma, Y.N.; Wu, Z.H.; Yan, Y.M.; Cheng, Y.X. Antifungal coumarins and lignans from Artemisia annua. Fitoterapia 2019, 134, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Chi, N.J.; Liu, Y.; Aisa, H.A. Tetrahydrofuran lignans and flavonoids from Artemisia absinthium. Chem. Nat. Compd. 2012, 48, 666–667. [Google Scholar] [CrossRef]
- Orege, J.I.; Adeyemi, S.B.; Tiamiyu, B.B.; Akinyemi, T.O.; Ibrahim, Y.A.; Orege, O.B. Artemisia and Artemisia-based products for COVID-19 management: Current state and future perspective. Adv. Tradit. Med. 2023, 23, 85–96. [Google Scholar] [CrossRef]
- Zahnit, W.; Smara, O.; Bechki, L.; Bensouici, C.; Messaoudi, M.; Benchikha, N.; Larkem, I.; Awuchi, C.G.; Sawicka, B.; Simal-Gandara, J. Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae 2022, 8, 914. [Google Scholar] [CrossRef]
- Rashid, M.U.; Alamzeb, M.; Ali, S.; Ullah, Z.; Shah, Z.A.; Naz, I.; Khan, M.R. The chemistry and pharmacology of alkaloids and allied nitrogen compounds from Artemisia species: A review. Phytother. Res. 2019, 33, 2661–2684. [Google Scholar] [CrossRef]
- Singh, A.; Jindal, S.; Longchar, B.; Khan, F.; Gupta, V. Overexpression of Artemisia annua sterol C-4 methyl oxidase gene, AaSMO1, enhances total sterols and improves tolerance to dehydration stress in tobacco. Plant Cell Tissue Organ Cult. 2015, 121, 167–181. [Google Scholar] [CrossRef]
- Hbika, A.; Daoudi, N.E.; Bouyanzer, A.; Bouhrim, M.; Mohti, H.; Loukili, E.H.; Mechchate, H.; Al-Salahi, R.; Nasr, F.A.; Bnouham, M.; et al. Artemisia absinthium L. Aqueous and Ethyl Acetate Extracts: Antioxidant Effect and Potential Activity In Vitro and In Vivo Against Pancreatic α-Amylase and Intestinal α-Glucosidase. Pharmaceutics 2022, 14, 481. [Google Scholar] [CrossRef] [PubMed]
- Pieracci, Y.; Vento, M.; Pistelli, L.; Lombardi, T.; Pistelli, L. Halophyte Artemisia caerulescens L.: Metabolites from In Vitro Shoots and Wild Plants. Plants 2022, 11, 1081. [Google Scholar] [CrossRef]
- Hrytsyk, R.A.; Kutsyk, R.V.; Yurchyshyn, O.I.; Struk, O.A.; Kireev, I.V.; Grytsyk, A.R. The investigation of antimicrobial and antifungal activity of some Artemisia L. species. Pharmacia 2021, 68, 93–100. [Google Scholar] [CrossRef]
- Singh, R.; Verma, P.K.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101–104. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and efflux pump inhibitory activity of caffeoylquinic acids from Artemisia absinthium against gram-positive pathogenic bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef] [PubMed]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; El-Ansary, D.O.; Al-Mana, F.A.; Mahmoud, E.A. Polyphenol content and biological activities of Ruta graveolens L. and Artemisia abrotanum L. in Northern Saudi Arabia. Processes 2020, 8, 531. [Google Scholar] [CrossRef]
- Remberg, P.; Björk, L.; Hedner, T.; Sterner, O. Characteristics, clinical effect profile and tolerability of a nasal spray preparation of Artemisia abrotanum L. for allergic rhinitis. Phytomedicine 2004, 11, 36–42. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [PubMed]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef] [PubMed]
- Adegbaju, O.D.; Otunola, G.A.; Afolayan, A.J. Effects of growth stage and seasons on the phytochemical content and antioxidant activities of crude extracts of Celosia argentea L. Heliyon 2020, 6, e04086. [Google Scholar] [CrossRef] [PubMed]
- Council of Europe. Disintegration of Tablets and Capsules. In European Pharmacopoeia 6.0; Monograph: 2.9.1.; Council of Europe: Strasbourg, France, 2007. [Google Scholar]
- Riahi, L.; Ghazghazi, H.; Ayari, B.; Aouadhi, C.; Klay, I.; Chograni, H.; Cherif, A.; Zoghlami, N. Effect of environmental conditions on chemical polymorphism and biological activities among Artemisia absinthium L. essential oil provenances grown in Tunisia. Ind. Crops Prod. 2015, 66, 96–102. [Google Scholar] [CrossRef]
- Moacă, E.A.; Pavel, I.Z.; Danciu, C.; Crăiniceanu, Z.; Minda, D.; Ardelean, F.; Antal, D.S.; Ghiulai, R.; Cioca, A.; Derban, M.; et al. Romanian Wormwood (Artemisia absinthium L.): Physicochemical and Nutraceutical Screening. Molecules 2019, 24, 3087. [Google Scholar] [CrossRef]
- Suseela, V.; Gopalakrishnan, V.K.; Varghese, S. In vitro Antioxidant Studies of Fruits of Artemisia nilagirica (Clarke) Pamp. Indian J. Pharm. Sci. 2010, 72, 644–649. [Google Scholar] [CrossRef]
- Messaili, S.; Colas, C.; Fougère, L.; Destandau, E. Combination of molecular network and centrifugal partition chromatography fractionation for targeting and identifying Artemisia annua L. antioxidant compounds. J. Chromatogr. A 2020, 1615, 460785. [Google Scholar] [CrossRef]
- Mumivand, H.; Babalar, M.; Tabrizi, L.; Craker, L.E.; Shokrpour, M.; Hadian, J. Antioxidant properties and principal phenolic phytochemicals of Iranian tarragon (Artemisia dracunculus L.) accessions. Hortic. Environ. Biotechnol. 2017, 58, 414–422. [Google Scholar] [CrossRef]
- Koyuncu, I. Evaluation of anticancer, antioxidant activity and phenolic compounds of Artemisia absinthium L. Extract. Cell. Mol. Biol. 2018, 64, 25–34. [Google Scholar] [CrossRef]
- Raudone, L.; Puzeryte, V.; Vilkickyte, G.; Niekyte, A.; Lanauskas, J.; Viškelis, J.; Viskelis, P. Sea Buckthorn Leaf Powders: The Impact of Cultivar and Drying Mode Antioxidant, Phytochemical, and Chromatic Profile of Valuable Resource. Molecules 2021, 26, 4765. [Google Scholar] [CrossRef]
- Bordean, M.E.; Ungur, R.A.; Toc, D.A.; Borda, I.M.; Marțiș, G.S.; Pop, C.R.; Filip, M.; Vlassa, M.; Nasui, B.A.; Pop, A.; et al. Antibacterial and Phytochemical Screening of Artemisia Species. Antioxidants 2023, 12, 596. [Google Scholar] [CrossRef] [PubMed]
- Msaada, K.; Salem, N.; Bachrouch, O.; Bousselmi, S.; Tammar, S.; Alfaify, A.; Sane, K.A.; Ammar, W.B.; Azeiz, S.; Brahim, A.H.; et al. Chemical Composition and Antioxidant and Antimicrobial Activities of Wormwood (Artemisia absinthium L.) Essential Oils and Phenolics. J. Chem. 2015, 2, 804658. [Google Scholar] [CrossRef]
- Bhat, M.Y.; Gul, M.Z.; Lohamror, L.R.; Qureshi, I.A.; Ghazi, I.A. An in vitro Study of the Antioxidant and Antiproliferative Properties of Artemisia absinthium—A Potent Medicinal Plant. Free Radic. Antioxid. 2017, 8, 18–25. [Google Scholar]
- Minda, D.; Ghiulai, R.; Banciu, C.D.; Pavel, I.Z.; Danciu, C.; Racoviceanu, R.; Soica, C.; Budu, O.D.; Muntean, D.; Diaconeasa, Z.; et al. Phytochemical Profile, Antioxidant and Wound Healing Potential of Three Artemisia Species: In Vitro and In Ovo Evaluation. Appl. Sci. 2022, 12, 1359. [Google Scholar] [CrossRef]
- Deka, H.; Barman, T.; Dutta, J.; Devi, A.; Tamuly, P.; Paul, R.K.; Karak, T. Catechin and caffeine content of tea (Camelia sinensis L.) leaf significantly differ with seasonal variation: A study on popular cultivars in North East India. J. Food Compos. Anal. 2021, 96, 103684. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six Artemisia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Trifan, A.; Zengin, G.; Sinan, K.I.; Sieniawska, E.; Sawicki, R.; Maciejewska-Turska, M.; Skalikca-Woźniak, K.; Luca, S.V. Unveiling the Phytochemical Profile and Biological Potential of Five Artemisia Species. Antioxidants 2022, 11, 1017. [Google Scholar] [CrossRef]
- Sharma, K.R.; Adhikari, S. Phytochemical analysis and biological activities of Artemisia vulgaris grown in different altitudes of Nepal. Int. J. Food Prop. 2023, 26, 414–427. [Google Scholar] [CrossRef]
- Baiceanu, E.; Vlase, L.; Baiceanu, A.; Nanes, M.; Rusu, D.; Crisan, G. New Polyphenols Identified in Artemisiae abrotani herba Extract. Molecules 2015, 20, 11063–11075. [Google Scholar] [CrossRef]
- Beigh, Y.A.; Ganai, A.M. Potential of Wormwood (Artemisia absinthium Linn.) herb for use as additive in livestock feeding: A review. J. Pharm. Innov. 2017, 6, 176–187. [Google Scholar]
- Jahani, R.; Khaledyan, D.; Jahani, A.; Jamshidi, E.; Kamalinejad, M.; Khoramjouy, M.; Faizi, M. Evaluation and comparison of the antidepressant-like activity of Artemisia dracunculus and Stachys lavandulifolia ethanolic extracts: An in vivo study. Res. Pharm. Sci. 2019, 14, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Kreft, I.; Germ, M.; Golob, A.; Vombergar, B.; Bonafaccia, F.; Luthar, Z. Impact of Rutin and Other Phenolic Substances on the Digestibility of Buckwheat Grain Metabolites. Int. J. Mol. Sci. 2022, 23, 3923. [Google Scholar] [CrossRef] [PubMed]
- Ahamad, J.; Mir, S.; Amin, S.A.A. Pharmacognostic Review on Artemisia absinthium. Int. Res. J. Pharm. 2019, 10, 25–31. [Google Scholar] [CrossRef]
- Hadi, A.; Hossein, N.; Shirin, P.; Najmeh, N.; Abolfazl, M. Anti-inflammatory and Analgesic Activities of Artemisia absinthium and Chemical Composition of its Essential Oil. Int. J. Pharm. Sci. Rev. Res. 2014, 24, 237–244. [Google Scholar]
- Gonzalez-Coloma, A.; Bailen, M.; Diaz, C.E.; Fraga, B.M.; Martínez-Díaz, R.; Zuñiga, G.E.; Contreras, R.A.; Cabrera, R.; Burillo, J. Major components of Spanish cultivated Artemisia absinthium populations: Antifeedant, antiparasitic, and antioxidant effects. Ind. Crops Prod. 2012, 37, 401–407. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, L.; Hu, X.; Miao, X.; Chen, X.; Nan, S.; Fu, H. Genome–Scale Transcriptome Analysis of the Desert Shrub Artemisia sphaerocephala. PLoS ONE 2016, 11, e0154300. [Google Scholar] [CrossRef]
- Balčiūnaitienė, A.; Viškelis, P.; Viškelis, J.; Štreimikytė, P.; Liaudanskas, M.; Bartkienė, E.; Zavistanavičiūtė, P.; Zokaitytė, E.; Starkutė, V.; Ruzauskas, M.; et al. Green Synthesis of Silver Nanoparticles Using Extract of Artemisia absinthium L., Humulus lupulus L. and Thymus vulgaris L., Physico-Chemical Characterization, Antimicrobial and Antioxidant Activity. Processes 2021, 9, 1304. [Google Scholar] [CrossRef]
- Skowyra, M.; Gallego, M.G.; Segovia, F.; Almajano, M.P. Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants 2014, 3, 116–128. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Popov, A.D.; Chupakhina, N.U.; Skrypnik, L.N.; Larina, V.V.; Babich, O.O. Comparative characteristics of spectrophotometric methods for determining the in vitro antioxidant activity of plant extracts. IOP Conf. Ser. Earth Environ. Sci. 2023, 1154, 012081. [Google Scholar] [CrossRef]
- Taherkhani, M.; Rustaiyan, A.; Rasooli, I.; Taherkhani, T. Chemical composition, antimicrobial activity, antioxidant and total phenolic content within the leaves essential oil of Artemisia absinthium L. growing wild in Iran. Afr. J. Pharm. Pharmacol. 2013, 7, 30–36. [Google Scholar] [CrossRef]




| Raw Material | Compound | Vegetation Stages | The Coefficient of Variation (CV) | ||||
|---|---|---|---|---|---|---|---|
| Intensive Growth | Butonization | The Beginning of Flowering | Intensive Flowering | The End of Flowering | |||
| Artemisiae abrotani herba | 4,5-Dicaffeoylquinic acid | 56.28 ± 0.34 e | 46.45 ± 0.22 d | 32.16 ± 0.01 b | 35.08 ± 0.05 c | 27.85 ± 0.02 a | 0.26 |
| Absinthii herba | NDE | NDE | NDE | NDE | NDE | - | |
| Artemisiae abrotani herba | 4-O-Caffeoylquinic acid | 16.42 ± 0.42 c | 11.88 ± 0.60 b | 4.98 ± 0.24 a | 4.92 ± 0.07 a | 4.54 ± 0.49 a | 4.43 |
| Absinthii herba | 1.07 ± 0.06 a | 4.54 ± 0.06 e | 1.89 ± 0.04 c | 1.89 ± 0.04 c | 1.16 ± 0.05 b | 3.06 | |
| Artemisiae abrotani herba | 3,5-Dicaffeoylquinic acid | 136.39 ± 1.16 e | 132.69 ± 0.14 d | 86.63 ± 1.38 b | 97.86 ± 0.34 c | 67.84 ± 0.62 a | 0.76 |
| Absinthii herba | 13.57 ± 0.58 a | 36.48 ± 1.12 b | 37.95 ± 1.48 c | 100.33 ± 1.59 e | 47.55 ± 0.01 d | 4.28 | |
| Artemisiae abrotani herba | 3,4-Dicaffeoylquinic acid | 102.71 ± 0.05 d | 109.13 ± 0.52 e | 49.15 ± 0.36 b | 85.71 ± 1.02 c | 36.51 ± 0.05 a | 0.54 |
| Absinthii herba | 6.78 ± 0.07 a | 21.08 ± 0.23 c | 24.05 ± 0.16 d | 28.72 ± 0.19 e | 12.15 ± 0.01 b | 1.20 | |
| Artemisiae abrotani herba | Caffeic acid | 3.62 ± 0.05 d | 4.19 ± 0.05 e | 1.54 ± 0.03 a | 3.04 ± 0.02 c | 1.65 ± 0.03 b | 1.27 |
| Absinthii herba | 0.48 ± 0.02 c | 1.16 ± 0.09 e | 0.61 ± 0.03 d | 0.84 ± 0.02 a | 0.35 ± 0.06 b | 5.54 | |
| Artemisiae abrotani herba | Chlorogenic acid | 203.37 ± 1.63 d | 217.39 ± 0.36 e | 96.53 ± 2.54 b | 169.95 ± 0.79 c | 86.12 ± 1.16 a | 1.08 |
| Absinthii herba | 11.26 ± 0.01 a | 118.66 ± 0.52 e | 20.31 ± 0.58 b | 54.30 ± 0.06 d | 28.84 ± 0.11 c | 0.76 | |
| Artemisiae abrotani herba | Neochlorogenic acid | 18.02 ± 2.23 d | 14.78 ± 0.11 c | 5.52 ± 0.35 a | 9.32 ± 0.14 b | 5.66 ± 0.02 a | 4.23 |
| Absinthii herba | 4.06 ± 0.12 c | 8.51 ± 0.03 e | 6.62 ± 0.27 d | 3.15 ± 0.14 b | 1.59 ± 0.01 a | 0.71 | |
| Artemisiae abrotani herba | Isorhamnetin-3-rutinoside | 1.36 ± 0.01 a | 5.58 ± 0.11 c | 3.69 ± 0.01 b | 8.71 ± 0.05 d | 3.90 ± 0.31 b | 2.23 |
| Absinthii herba | 1.27 ± 0.04 b | 2.91 ± 0.11 d | 3.68 ± 0.19 e | 2.19 ± 0.08 c | 0.80 ± 0.02 a | 0.22 | |
| Artemisiae abrotani herba | Luteolin-7-glycoside | 3.69 ± 0.14 e | 2.64 ± 0.08 c | 2.41 ± 0.05 b | 1.36 ± 0.06 a | 2.80 ± 0.06 d | 2.03 |
| Absinthii herba | 2.55 ± 0.06 a | 4.16 ± 0.04 c | 3.68 ± 0.02 b | 1.77 ± 0.10 e | 0.65 ± 0.11 d | 0.44 | |
| Artemisiae abrotani herba | Luteolin-7-rutinoside | 3.87 ± 0.03 d | 5.43 ± 0.26 e | 1.98 ± 0.06 b | 2.45 ± 0.03 c | 1.37 ± 0.12 a | 2.01 |
| Absinthii herba | 1.02 ± 0.03 c | 1.56 ± 0.06 d | 2.01 ± 0.02 e | 0.43 ± 0.01 b | 0.22 ± 0.03 a | 0.17 | |
| Artemisiae abrotani herba | Rutin | 60.79 ± 0.40 a | 121.72 ± 90.20 abc | 75.52 ± 0.47 a | 165.37 ± 0.11 c | 81.44 ± 0.23 a | 15.15 |
| Absinthii herba | 1.31 ± 0.04 b | 2.32 ± 0.01 d | 2.99 ± 0.08 e | 2.05 ± 0.06 c | 0.96 ± 0.02 a | 1.93 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saunoriūtė, S.; Ragažinskienė, O.; Ivanauskas, L.; Marksa, M.; Laužikė, K.; Raudonė, L. Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages. Separations 2023, 10, 545. https://doi.org/10.3390/separations10100545
Saunoriūtė S, Ragažinskienė O, Ivanauskas L, Marksa M, Laužikė K, Raudonė L. Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages. Separations. 2023; 10(10):545. https://doi.org/10.3390/separations10100545
Chicago/Turabian StyleSaunoriūtė, Sandra, Ona Ragažinskienė, Liudas Ivanauskas, Mindaugas Marksa, Kristina Laužikė, and Lina Raudonė. 2023. "Phenolic Diversity and Antioxidant Activity of Artemisia abrotanum L. and Artemisia absinthium L. during Vegetation Stages" Separations 10, no. 10: 545. https://doi.org/10.3390/separations10100545






