Application of Choline Chloride-Based Deep Eutectic Solvents in the Synthesis of Hydrazones
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of DESs and Detecting Water Content
2.2. Conventional Method
2.3. Ultrasound Method
2.4. Recycling and Reuse of DESs
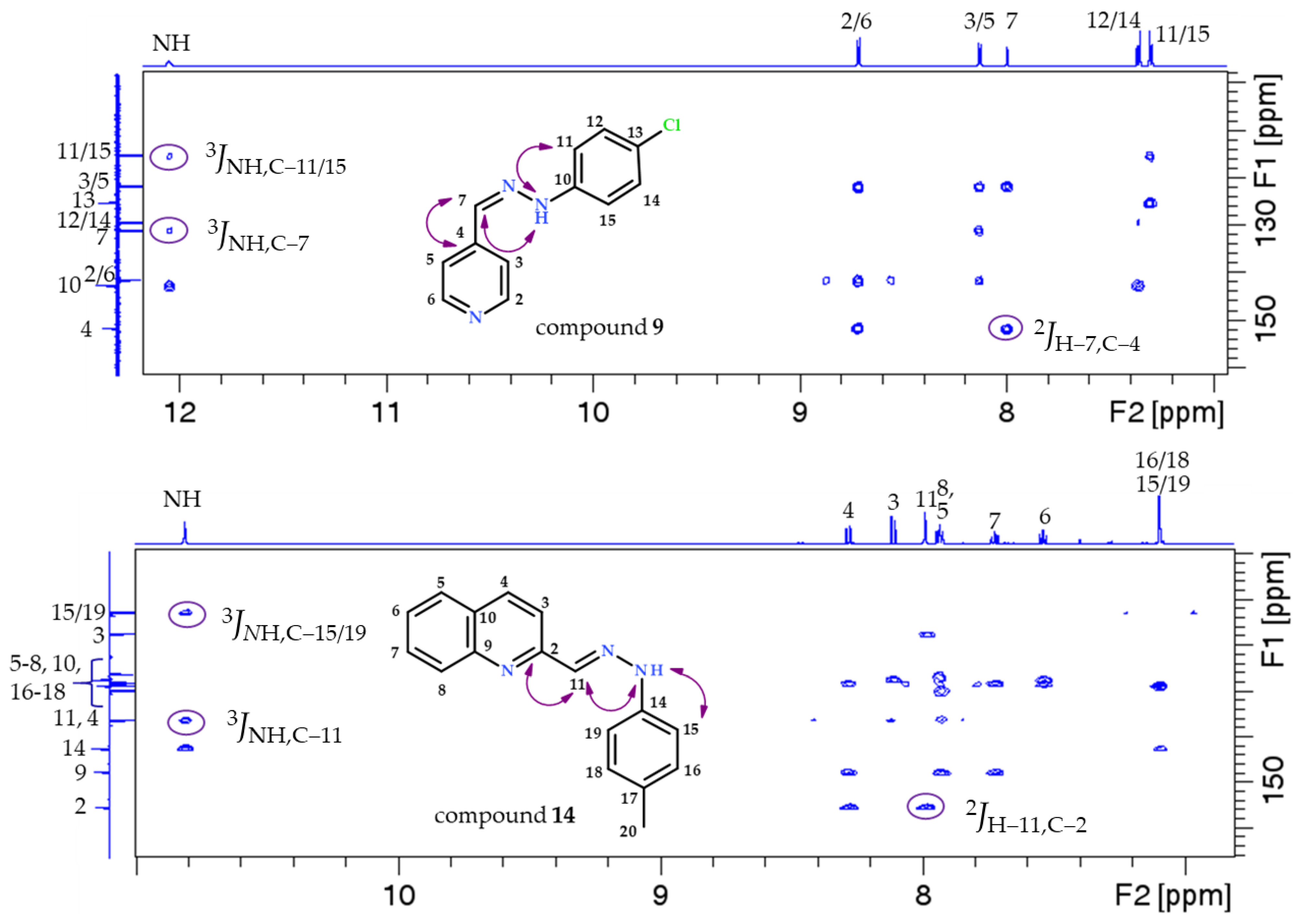
- 5-(hydroxymethyl)-2-methyl-4-((2-phenylhydrazineylidene)methyl)pyridin-3-ol (1)
- 4-((2-(2,4-dinitrophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (2)
- 4-((2-(4-fluorophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (3)
- 4-((2-(4-chlorophenyl)hydrazineylidene)methyl)-5-(hydroxymethyl)-2-methylpyridin-3-ol (4)
- 5-(hydroxymethyl)-2-methyl-4-((2-(p-tolyl)hydrazineylidene)methyl)pyridin-3-ol (5)
- Pyridine-4-carbaldehyde-phenylhydrazone (6)
- Pyridine-4-carbaldehyde-2,4-dinitrophenylhydrazone (7)
- Pyridine-4-carbaldehyde-4-fluorophenylhydrazone (8)
- Pyridine-4-carbaldehyde-4-chlorophenylhydrazone (9)
- Quinoline-2-carbaldehyde-phenylhydrazone (10)
- Quinoline-2-carbaldehyde-2,4-dinitrophenylhydrazone (11)
- Quinoline-2-carbaldehyde-4-fluorophenylhydrazone (12)
- Quinoline-2-carbaldehyde-4-chlorophenylhydrazone (13)
- Quinoline-2-carbaldehyde-4-methylphenylhydrazone (14)
3. Results and Discussion
3.1. Preparation of DESs and Water Content
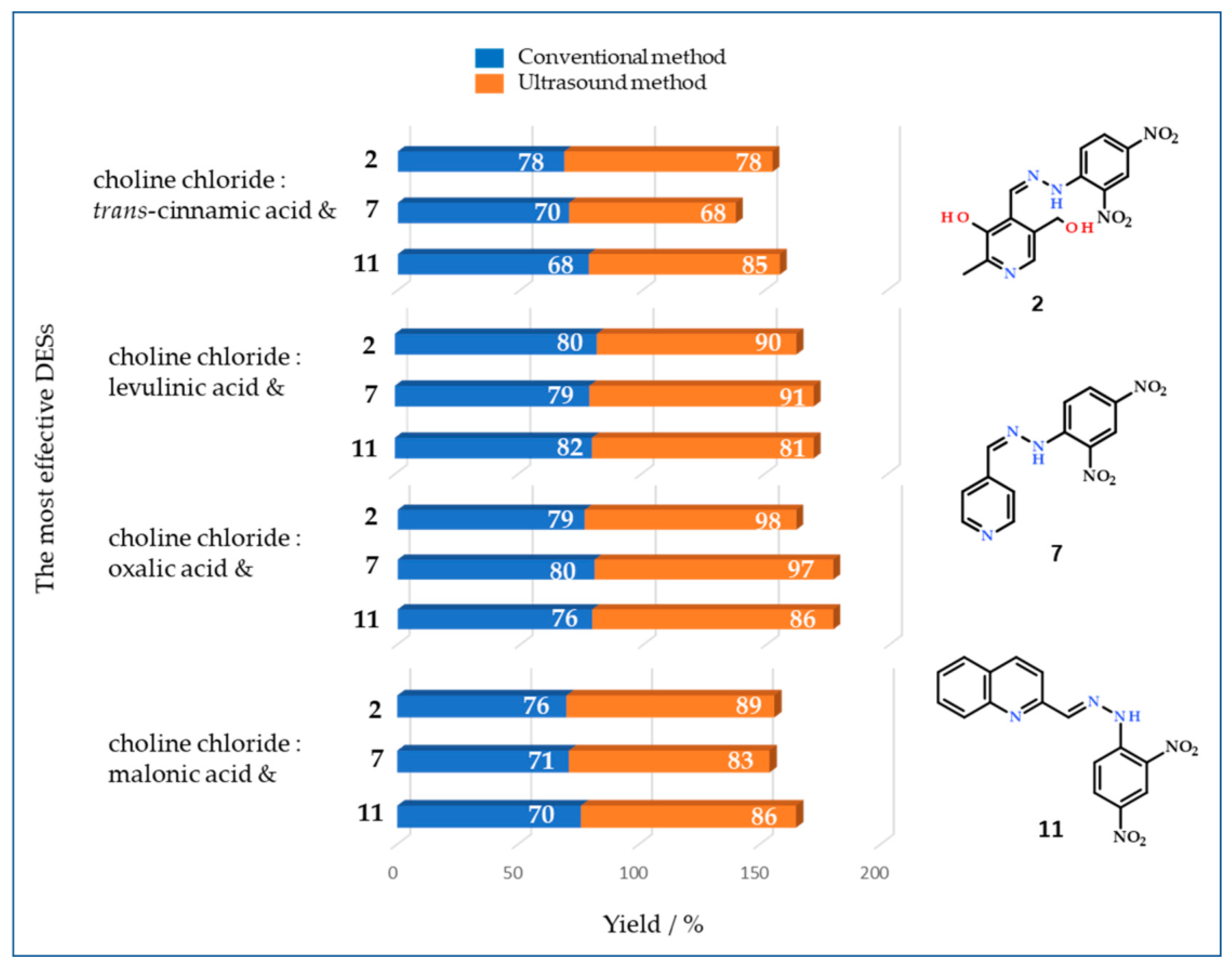
3.2. Conventional Synthesis
3.3. Ultrasound Synthesis
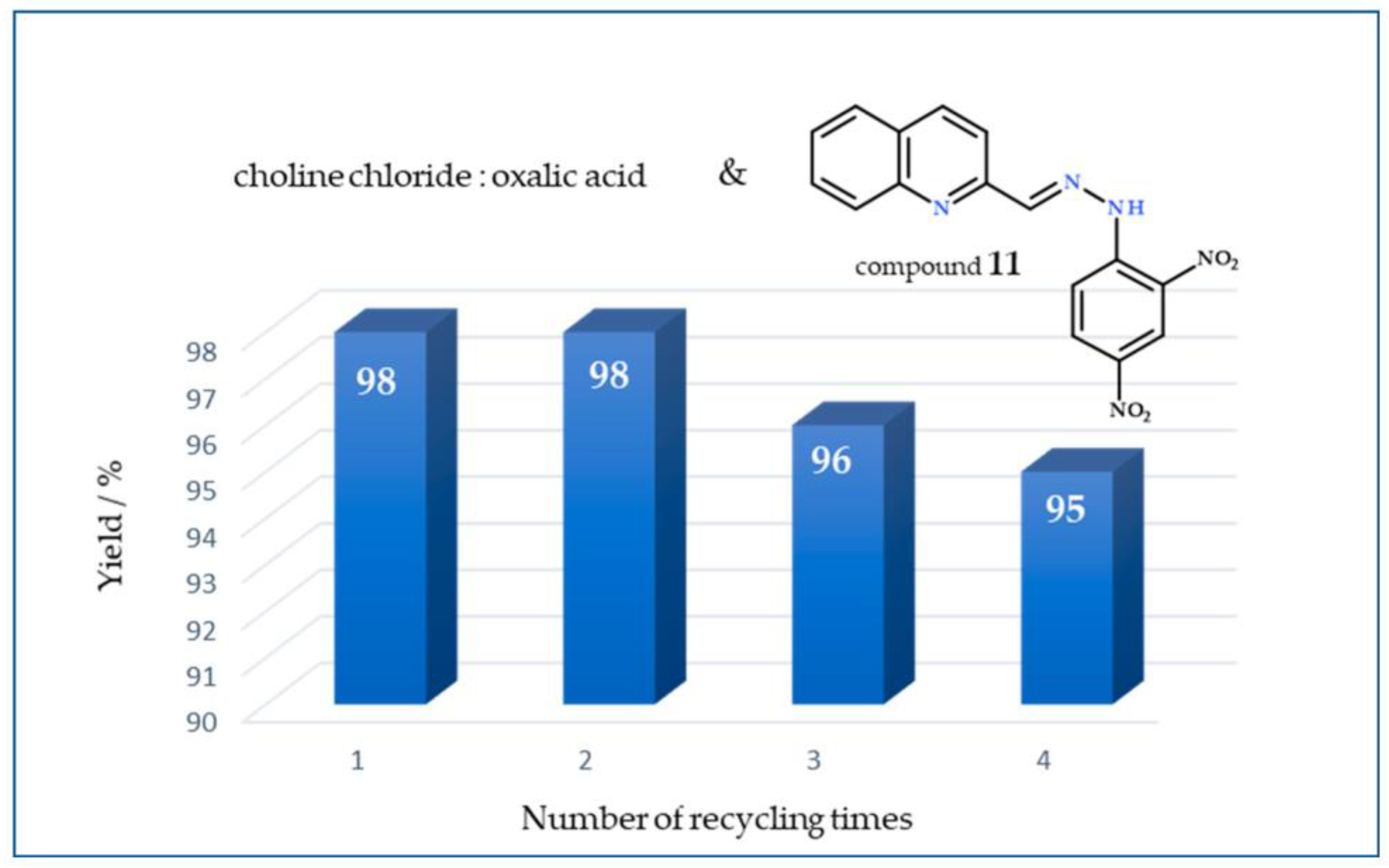
3.4. Recycling and Reuse of DESs Choline Chloride:Oxalic Acid
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sevim, R.; Küçükgüzel, G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Jain, J.; Kumar, Y.; Sinha, R.; Kumar, R.; Stables, J. Menthone aryl acid hydrazones: A new class of anticonvulsants. Med. Chem. 2011, 7, 56–61. [Google Scholar] [CrossRef]
- Tributino, J.L.; Duarte, C.D.; Corrêa, R.S.; Doriguetto, A.C.; Ellena, J.; Romeiro, N.C.; Castro, N.G.; Miranda, A.L.P.; Barreiro, E.J.; Fraga, C.A. Novel 6-methanesulfonamide-3, 4-methylenedioxyphenyl-N-acylhydrazones: Orally effective anti-inflammatory drug candidates. Bioorg. Med. Chem. 2009, 17, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Gil-Longo, J.; Laguna, M.D.L.R.; Verde, I.; Castro, M.E.; Orallo, F.; Fontenla, J.A.; Calleja, J.M.; Ravina, E.; Teran, C. Pyridazine derivatives. XI: Antihypertensive activity of 3-hydrazinocycloheptyl [1, 2-c] pyridazine and its hydrazone derivatives. J. Pharm. Sci. 1993, 82, 286–290. [Google Scholar] [CrossRef]
- Liu, W.Y.; Li, H.Y.; Zhao, B.X.; Shin, D.S.; Lian, S.; Miao, J.Y. Synthesis of novel ribavirin hydrazone derivatives and anti-proliferative activity against A549 lung cancer cells. Carbohydr. Res. 2009, 344, 1270–1275. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.; Pal, R.; Dwivedi, A.; Hajela, K. Synthesis of some new diaryl and triaryl hydrazone derivatives as possible estrogen receptor modulators. Arzneimittelforsch 2002, 52, 39–44. [Google Scholar] [CrossRef]
- Terzioğlu, N.; Gürsoy, A. Synthesis and anticancer evaluation of some new hydrazone derivatives of 2,6-dimethylimidazo[2,1-b]-[1,3,4]thiadiazole-5-carbohydrazide. Eur. J. Med. Chem. 2003, 38, 781–786. [Google Scholar] [CrossRef]
- Cocco, M.T.; Congiu, C.; Lilliu, V.; Onnis, V. Synthesis and in vitro antitumoral activity of new hydrazinopyrimidine-5-carbonitrile derivatives. Bioorg. Med. Chem. 2005, 14, 366–372. [Google Scholar] [CrossRef]
- Gürsoy, E.; Güzeldemirci-Ulusoy, N. Synthesis and primary cytotoxicity evaluation of new imidazo[2,1-b]thiazole derivatives. Eur. J. Med. Chem. 2007, 42, 320–326. [Google Scholar] [CrossRef]
- El-Hawash, S.A.M.; Wahab, A.E.; El-Dewellawy, M.A. Cyanoacetic acid hydrazones of 3-(and 4-) acetylpyridine and some derived ring systems as potential antitumor and anti-HCV agents. Arch. Der Pharm. Int. J. Pharm. Med. Chem. 2006, 339, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Rane, R.A.; Telvekar, V.N. Synthesis and evaluation of novel chloropyrrole molecules designed by molecular hybridization of common pharmacophores as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2010, 20, 5681–5685. [Google Scholar] [CrossRef] [PubMed]
- Turan-Zitouni, G.; Blache, Y.; Güven, K. Synthesis and antimicrobial activity of some imidazo-[1,2-a]pyridine-2-carboxylic acid arylidenehydrazide derivatives. Boll. Chim. Farm. 2001, 140, 397–400. [Google Scholar]
- Fattorusso, C.; Campiani, G.; Kukreja, G.; Persico, M.; Butini, S.; Romano, M.P.; Altarelli, M.; Ros, S.; Brindisi, M.; Savini, L. Design, synthesis, and structure–activity relationship studies of 4-quinolinyl-and 9-acrydinylhydrazones as potent antimalarial agents. J. Med. Chem. 2008, 51, 1333–1343. [Google Scholar] [CrossRef]
- Walcourt, A.; Loyevsky, M.; Lovejoy, D.B.; Gordeuk, V.R.; Richardson, D.R. Novel aroylhydrazone and thiosemicarbazone iron chelators with anti-malarial activity against chloroquine-resistant and -sensitive parasites. Int. J. Biochem. Cell Biol. 2004, 36, 401–407. [Google Scholar] [CrossRef]
- Gemma, S.; Kukreja, G.; Fattorusso, C.; Persico, M.; Romano, M.; Altarelli, M.; Savini, L.; Campiani, G.; Fattorusso, E.; Basilico, N. Synthesis of N1-arylidene-N2-quinolyl- and N2-acrydinylhydrazones as potent antimalarial agents active against CQ-resistant P. falciparum strains. Bioorg. Med. Chem. Let. 2006, 16, 5384–5388. [Google Scholar] [CrossRef]
- Ma, X.D.; Yang, S.Q.; Gu, S.X.; He, Q.Q.; Chen, F.E.; De Clercq, E.; Balzarini, J.; Pannecouque, C. Synthesis and anti-HIV activity of Aryl-2-[(4-cyanophenyl) amino]-4-pyrimidinone hydrazones as potent non-nucleoside reverse transcriptase inhibitors. Chem. Med. Chem. 2011, 6, 2225–2232. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, A.; Kremer, L.; Louw, S.; Guéradel, Y.; Chibale, K.; Biot, C. Synthesis and in vitro antitubercular activity of ferrocene-based hydrazones. Bioorg. Med. Chem. Lett. 2011, 21, 2866–2868. [Google Scholar] [CrossRef]
- Bukowski, L.; Janowiec, M. 1-Methyl-1H-2-imidazo[4,5-b]pyridinecarboxylic acid and some of derivatives with suspected antituberculotic activity. Pharmazie 1996, 51, 27–30. [Google Scholar]
- Bukowski, L.; Janowiec, M.; Zwolska-Kwiek, Z.; Andrzejczyk, Z. Synthesis and some reactions of 2- acetylimidazo[4,5-b]pyridine. Antituberculotic activity of the obtained compounds. Pharmazie 1999, 54, 651–654. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazole-2-ylthio]acetic acid arylidene-hydrazide derivatives. Farmaco 2001, 56, 587–592. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfi, E.; Scialino, G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazole-2-ylidene)hydrazide derivatives. Farmaco 2003, 58, 631–637. [Google Scholar] [CrossRef]
- Musad, E.A.; Mohamed, R.; Saeed, B.A.; Vishwanath, B.S.; Rai, K.L. Synthesis and evaluation of antioxidant and antibacterial activities of new substituted bis (1, 3, 4-oxadiazoles), 3, 5-bis (substituted) pyrazoles and isoxazoles. Bioorg. Med. Chem. Lett. 2011, 21, 3536–3540. [Google Scholar] [CrossRef]
- El-Sabbagh, O.; Shabaan, M.A.; Kadry, H.H.; Al-Din, E.S. New octahydroquinazoline derivatives: Synthesis and hypotensive activity. Eur. J. Med. Chem. 2010, 45, 5390–5396. [Google Scholar] [CrossRef]
- De Oliveira, K.N.; Costa, P.; Santin, J.R.; Mazzambani, L.; Bürger, C.; Mora, C.; Nunes, R.J.; De Souza, M.M. Synthesis and antidepressant-like activity evaluation of sulphonamides and sulphonyl-hydrazones. Bioorg. Med. Chem. 2011, 19, 4295–4306. [Google Scholar] [CrossRef] [PubMed]
- Ergenç, N.; Günay, N.S. Synthesis and antidepressant evaluation of new 3-phenyl-5-sulfonamidoindole derivatives. Eur. J. Med. Chem. 1998, 33, 143–148. [Google Scholar] [CrossRef]
- Shtyrlin, V.; Khaziev, R.M.; Shtyrlin, V.G.; Gilyazetdinov, E.M.; Agafonova, M.N.; Usachev, K.S.; Islamov, D.R.; Klimovitskii, A.E.; Vinogradova, T.I.; Dogonadze, M.Z.; et al. Isonicotinoyl hydrazones of pyridoxine derivatives: Synthesis and antimycobacterial activity. Med. Chem. Res. 2021, 30, 952–963. [Google Scholar] [CrossRef]
- Aljuhani, A.; Rezki, N.; Al-Sodies, S.; Messali, M.; ElShafei, G.M.S.; Hagar, M.; Aouad, M.R. Dicationic Bis-Pyridinium Hydrazone-Based Amphiphiles Encompassing Fluorinated Counteranions: Synthesis, Characterization, TGA-DSC, and DFT Investigations. Molecules 2022, 27, 2492. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Abid, S.; Tahir, N.M.; Ashfaq, M.; Kanwal, F.; Lu, C.; Rehman, M.F. Green Synthesis, SC-XRD, Non-Covalent Interactive Potential and Electronic Communication via DFT Exploration of Pyridine-Based Hydrazone. Crystals 2020, 10, 778. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Abid, S.; Iqbal, J.; Tahir, N.M.; Raza, A.R.; Zukerman-Schpector, J.; Paixão, M.W. Facile synthesis, crystal growth, characterization and computational study of new pyridine-based halogenated hydrazones: Unveiling the stabilization behavior in terms of noncovalent interactions. Appl. Organometal Chem. 2020, 34, e5399. [Google Scholar] [CrossRef]
- Nogueira, T.C.M.; Cruz, L.S.; Lourenço, M.C.; de Souza, M.V.N. Design, Synthesis and Anti-tuberculosis Activity of Hydrazones and N-acylhydrazones Containing Vitamin B6 and Different Heteroaromatic Nucleus. Lett. Drug Des. Discov. 2019, 16, 792–798. [Google Scholar] [CrossRef]
- Alptüzün, V.; Parlar, S.; Taşli, H.; Erciyas, E. Synthesis and antimicrobial activity of some pyridinium salts. Molecules 2009, 14, 5203–5215. [Google Scholar] [CrossRef]
- Parlar, S.; Erzurumlu, Y.; Ilhan, R.; Ballar, P.; Kırmızıbayrak, P.B.; Alptüzün, V.; Erciyas, E. Synthesis and evaluation of pyridinium-hydrazone derivatives as potential antitumoral agents. Chem. Biol. Drug Des. 2018, 92, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, T.; Milton, M.D. Logic gate based novel phenothiazine-pyridylhydrazones: Halochromism in solid and solution state. Dye. Pigment. 2019, 164, 305–318. [Google Scholar] [CrossRef]
- Mali, S.N.; Thorat, B.R.; Gupta, D.R.; Pandey, A. Mini-Review of the Importance of Hydrazides and Their Derivatives—Synthesis and Biological Activity. Eng. Proc. 2021, 11, 21. [Google Scholar]
- Mandewale, M.C.; Thorat, B.; Nivid, Y.; Jadhav, R.; Nagarsekar, A.; Yamgar, R. Synthesis, structural studies and antituberculosis evaluation of new hydrazone derivatives of quinolone and their Zn(II) complexes. J. Saudi Chem. Soc. 2018, 22, 218–228. [Google Scholar] [CrossRef]
- Pisk, J.; Đilović, I.; Hrenar, T.; Cvijanović, D.; Pavlović, G.; Vrdoljak, V. Effective methods for the synthesis of hydrazones, quinazolines, and Schiff bases: Reaction monitoring using a chemometric approach. RCS Adv. 2020, 10, 38566–38577. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 9, 70–71. [Google Scholar] [CrossRef]
- Hansen, B.B.; Spittle, S.; Chen, B.; Poe, D.; Zhang, Y.; Klein, J.M.; Horton, A.; Adhikari, L.; Zelovich, T.; Doherty, B.W.; et al. Deep Eutectic Solvents: A Review of Fundamentals and Applications. Chem. Rev. 2021, 121, 1232–1285. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- El Achkar, T.; Greige-Gerges, H.; Fourmentin, S. Basics and properties of deep eutectic solvents: A review. Environ. Chem. Lett. 2021, 19, 3397–3408. [Google Scholar] [CrossRef]
- Lomba, L.; Ribate, M.P.; Sangüesa, E.; Concha, J.; Garralaga, M.P.; Errazquin, D.; García, C.B.; Giner, B. Deep Eutectic Solvents: Are They Safe? Appl. Sci. 2021, 11, 10061. [Google Scholar] [CrossRef]
- Vieira Sanches, M.; Freitas, R.; Oliva, M.; Mero, A.; De Marchi, L.; Cuccaro, A.; Fumagalli, G.; Mezzetta, A.; Dugoni, G.C.; Ferro, M.; et al. Are natural deep eutectic solvents always a sustainable option? A bioassay-based study. Environ. Sci. Pollut. Res. 2023, 30, 17268–17279. [Google Scholar] [CrossRef]
- Lapeña, D.; Errazquin, D.; Lomba, L.; Lafuente, C.; Giner, B. Ecotoxicity and biodegradability of pure and aqueous mixtures of deep eutectic solvents: Glyceline, ethaline and reline. Environ. Sci. Pollut. Res. 2021, 28, 8812–8821. [Google Scholar] [CrossRef] [PubMed]
- Wen, Q.; Chen, J.X.; Tang, Y.-L.; Wang, J.; Yang, Z. Assessing the toxicity and biodegradability of deep eutectic solvents. Chemosphere 2015, 132, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kudlak, B.; Owczarek, K.; Namiesnik, J. Selected issues related to the toxicity of ionic liquids and deep eutectic solvents-a review. Environ. Sciand Pollut. Res. 2015, 22, 11975–11992. [Google Scholar] [CrossRef] [PubMed]
- Juneidi, I.; Hayyan, M.; Hashim, M.A. Intensification of biotransformations using deep eutectic solvents: Overview and outlook. Process Biochem. 2018, 66, 33–60. [Google Scholar] [CrossRef]
- Marchel, M.; Cieśliński, H.; Boczkaj, G. Thermal Instability of Choline Chloride-Based Deep Eutectic Solvents and Its Influence on Their Toxicity─Important Limitations of DESs as Sustainable Materials. Ind. Eng. Chem. Res. 2022, 61, 11288–11300. [Google Scholar] [CrossRef]
- Chen, W.J.; Xue, Z.M.; Xue, J.F.; Wang, J.Y.; Jiang, X.H.; Zhao, T.C.; Mu, T. Investigation on the Thermal Stability of Deep Eutectic Solvents. Acta Phys.-Chim. Sin. 2018, 34, 904–911. [Google Scholar] [CrossRef]
- García-Álvarez, J. Deep Eutectic Mixtures: Promising Sustainable Solvents for Metal-Catalysed and Metal-Mediated Organic Reactions. Eur. J. Inorg. Chem. 2015, 2015, 5147–5157. [Google Scholar] [CrossRef]
- Nolan, M.D.; Mezzetta, A.; Lorenzo Guazzelli, L.; Scanlan, E.M. Radical-mediated thiol–ene ‘click’ reactions in deep eutectic solvents for bioconjugation. Green. Chem. 2022, 24, 1456–1462. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.R.S.; Silvestre, A.J.D.; Freire, M.G. Deep Eutectic Solvents and Pharmaceuticals. Encyclopedia 2021, 1, 942–963. [Google Scholar] [CrossRef]
- Sheldon, R.A. Biocatalysis and Biomass Conversion in Alternative Reaction Media. Chem.—A Eur. J. 2016, 22, 12984–12999. [Google Scholar] [CrossRef] [PubMed]
- Cicco, L.; Ríos-Lombardía, N.; Rodríguez-Álvarez, M.J.; Morís, F.; Perna, F.M.; Capriati, V.; García-Álvarez, J.; González-Sabín, J. Programming cascade reactions interfacing biocatalysis with transition-metal catalysis in Deep Eutectic Solvents as biorenewable reaction media. Green. Chem. 2018, 20, 3468–3475. [Google Scholar] [CrossRef]
- Martínez, R.; Berbegal, L.; Guillena, G.; Ramón, D.J. Bio-renewable enantioselective aldol reaction in natural deep eutectic solvents. Green. Chem. 2016, 18, 1724–1730. [Google Scholar] [CrossRef]
- Massolo, E.; Palmieri, S.; Benaglia, M.; Capriati, V.; Perna, F.M. Stereoselective organocatalysed reactions in deep eutectic solvents: Highly tunable and biorenewable reaction media for sustainable organic synthesis. Green. Chem. 2016, 18, 792–797. [Google Scholar] [CrossRef]
- Millia, L.; Dall’Asta, V.; Ferrara, C.; Berbenni, V.; Quartarone, E.; Perna, F.M.; Capriati, V.; Mustarelli, P. Bio-inspired choline chloride-based deep eutectic solvents as electrolytes for lithium-ion batteries. Solid State Ion. 2018, 323, 44–48. [Google Scholar] [CrossRef]
- Nguyen, T.T.; Nguyen, C.T.; Tran, P.H. Synthesis of a new series of 2-hydroxy-5-iodo-N’-(1-arylethylidene)benzohydrazides using a deep eutectic solvent as solvent/catalyst under sonication. Heliyon 2019, 5, e02353. [Google Scholar] [CrossRef] [PubMed]
- Hammond, O.S.; Bowron, D.T.; Edler, K.J. The Effect of Water upon Deep Eutectic Solvent Nanostructure: An Unusual Transition from Ionic Mixture to Aqueous Solution. Angew. Chem. Int. Ed. Engl. 2017, 56, 9782–9785. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Bobrova, L.S.; Baskevich, A.S.; Korniy, S.A.; Danilov, F.I. Electrodeposition of chromium coatings from a choline chloride based ionic liquid with the addition of water. J. Chem. Technol. Metall. 2018, 53, 906–915. [Google Scholar]
- McCalman, D.C.; Sun, L.; Zhang, Y.; Brennecke, J.F.; Maginn, E.J.; Schneider, W.F. Speciation, conductivities, diffusivities, and electrochemical reduction as a function of water content in mixtures of hydrated chromium chloride/choline chloride. J. Phys. Chem. B 2015, 119, 6018–6023. [Google Scholar] [CrossRef] [PubMed]
- Bušić, V.; Gašo-Sokač, D. Menshutkin Reaction in Choline Chloride-based Deep Eutectic Solvents. Org. Prep. Proced. Int. 2022, 55, 160–166. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the Synthesis and Properties of Deep Eutectic Solvents Based on Cholinium Chloride and Carboxylic Acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- Nolasco, M.N.; Pedro, S.N.; Vilela, C.; Vaz, P.D.; Ribeiro-Claro, P.; Rudić, S.; Parker, S.F.; Freire, C.R.S.; Freire, M.G.; Silvestre, A.D.J. Water in Deep Eutectic Solvents: New Insights from Inelastic Neutron Scattering Spectroscopy. Front. Phys. 2022, 10, 834571. [Google Scholar] [CrossRef]
- Gabriele, F.; Chiarini, M.; Germani, R.; Tiecco, M.; Spreti, N. Effect of Water Addition on Choline Chloride/glycol Deep Eutectic Solvents: Characterization of Their Structural and Physicochemical Properties. J. Mol. Liq. 2019, 291, 111301. [Google Scholar] [CrossRef]
- Nayyar, A.; Malde, A.; Coutinho, E.; Jain, R. Synthesis, antituberculosis activity, and 3D-QSAR study of ring-substituted-2/4-quinolinecarbaldehyde derivatives. Bioorg. Med. Chem. 2006, 14, 7302–7310. [Google Scholar] [CrossRef] [PubMed]
- Puskullu, M.O.; Shirinzadeh, H.; Nenni, M.; Gurer-Orhan, H.; Suzen, S. Synthesis and evaluation of antioxidant activity of new quinoline-2-carbaldehyde hydrazone derivatives: Bioisosteric melatonin analogues. J. Enzym. Inhib. Med. Chem. 2016, 31, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez Rodriguez, N.; van den Bruinhorst, A.; Kollau, J.B.M.L.; Kroon, C.M.; Binnemans, K. Degradation of deep-eutectic solvents based on choline chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2019, 7, 11521–11528. [Google Scholar] [CrossRef]



| HBD in Prepared DES with Applied Molar Ratio | wt */% | Yields in DESs (ChCl:HBDs) per Compound/% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | ||
| urea (1:2) | 0.99 | 21 | 30 | 40 | 57 | 20 | 31 | 41 | 44 | 17 | 43 | 50 | 37 | 48 | 11 |
| N-methylurea (1:3) | 1.86 | 35 | 68 | 34 | 53 | 19 | 15 | 58 | 50 | 24 | 56 | 54 | 46 | 56 | 18 |
| thiourea (1:2) | 4.84 | 48 | 54 | 46 | 57 | 36 | 34 | 52 | 39 | 27 | 63 | 51 | 54 | 62 | 23 |
| glycerol (1:2) | 0.76 | 51 | 60 | 47 | 50 | 43 | 36 | 61 | 60 | 34 | 48 | 65 | 78 | 68 | 24 |
| acetamide (1:2) | 1.86 | 57 | 22 | 37 | 59 | 36 | 51 | 32 | 67 | 15 | 37 | 30 | 67 | 58 | 16 |
| malic acid (1:1) | 0.79 | 61 | 65 | 62 | 52 | 40 | 38 | 45 | 54 | 22 | 36 | 54 | 57 | 63 | 37 |
| citric acid (1:2) | 1.05 | 65 | 58 | 59 | 48 | 39 | 26 | 56 | 56 | 34 | 66 | 65 | 55 | 63 | 26 |
| malonic acid (1:1) | 1.07 | 58 | 70 | 60 | 37 | 36 | 54 | 71 | 45 | 33 | 72 | 76 | 70 | 69 | 31 |
| oxalic acid (1:1) | 1.92 | 65 | 76 | 72 | 64 | 42 | 44 | 80 | 60 | 32 | 75 | 79 | 72 | 75 | 42 |
| lactic acid (1:2) | 6.58 | 47 | 34 | 45 | 46 | 32 | 42 | 64 | 56 | 24 | 50 | 59 | 53 | 70 | 28 |
| levulinic acid (1:2) | 0.83 | 63 | 82 | 78 | 56 | 34 | 45 | 79 | 69 | 36 | 61 | 80 | 58 | 70 | 38 |
| trans-cinnamic acid (1: 1) | 0.92 | 62 | 68 | 72 | 62 | 58 | 50 | 70 | 45 | 45 | 47 | 78 | 60 | 57 | 56 |
| HBD in Prepared DES with Applied Molar Ratio | Yields in DESs (ChCl:HBDs) per Compound/% | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | |
| urea (1:2) | 70 | 74 | 60 | 69 | 60 | 70 | 89 | 67 | 73 | 64 | 69 | 82 | 70 | 45 |
| N-methylurea (1:3) | 57 | 71 | 70 | 65 | 59 | 35 | 64 | 66 | 79 | 80 | 71 | 81 | 73 | 67 |
| thiourea (1:2) | 60 | 78 | 66 | 62 | 54 | 64 | 75 | 73 | 64 | 78 | 67 | 67 | 70 | 63 |
| glycerol (1:2) | 68 | 75 | 57 | 78 | 49 | 46 | 76 | 82 | 59 | 58 | 73 | 87 | 65 | 55 |
| acetamide (1:2) | 70 | 34 | 45 | 49 | 76 | 62 | 78 | 70 | 32 | 56 | 80 | 71 | 68 | 34 |
| malic acid (1:1) | 71 | 83 | 80 | 66 | 58 | 59 | 60 | 76 | 40 | 38 | 72 | 67 | 74 | 42 |
| citric acid (1:2) | 68 | 80 | 75 | 72 | 66 | 47 | 48 | 57 | 46 | 71 | 43 | 56 | 73 | 45 |
| malonic acid (1:1) | 78 | 86 | 80 | 70 | 66 | 72 | 83 | 64 | 59 | 65 | 89 | 67 | 70 | 65 |
| oxalic acid (1:1) | 72 | 86 | 80 | 56 | 55 | 75 | 97 | 78 | 80 | 92 | 98 | 86 | 78 | 61 |
| lactic acid (1:2) | 54 | 80 | 64 | 65 | 46 | 72 | 78 | 89 | 60 | 80 | 77 | 67 | 59 | 51 |
| levulinic acid (1:2) | 70 | 81 | 88 | 67 | 70 | 82 | 91 | 95 | 56 | 79 | 90 | 47 | 85 | 40 |
| trans-cinnamic acid (1: 1) | 68 | 85 | 89 | 69 | 85 | 40 | 68 | 55 | 34 | 67 | 78 | 62 | 81 | 58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bušić, V.; Roca, S.; Gašo-Sokač, D. Application of Choline Chloride-Based Deep Eutectic Solvents in the Synthesis of Hydrazones. Separations 2023, 10, 551. https://doi.org/10.3390/separations10110551
Bušić V, Roca S, Gašo-Sokač D. Application of Choline Chloride-Based Deep Eutectic Solvents in the Synthesis of Hydrazones. Separations. 2023; 10(11):551. https://doi.org/10.3390/separations10110551
Chicago/Turabian StyleBušić, Valentina, Sunčica Roca, and Dajana Gašo-Sokač. 2023. "Application of Choline Chloride-Based Deep Eutectic Solvents in the Synthesis of Hydrazones" Separations 10, no. 11: 551. https://doi.org/10.3390/separations10110551








