Isolation and Structural Elucidation of Unreported Prenylhydroquinone Glycoside from Sedum kamtschaticum Leaves and Its Effect on Hyperphosphorylated Tau Production in Aβ1–42-Treated SH-SY5Y Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
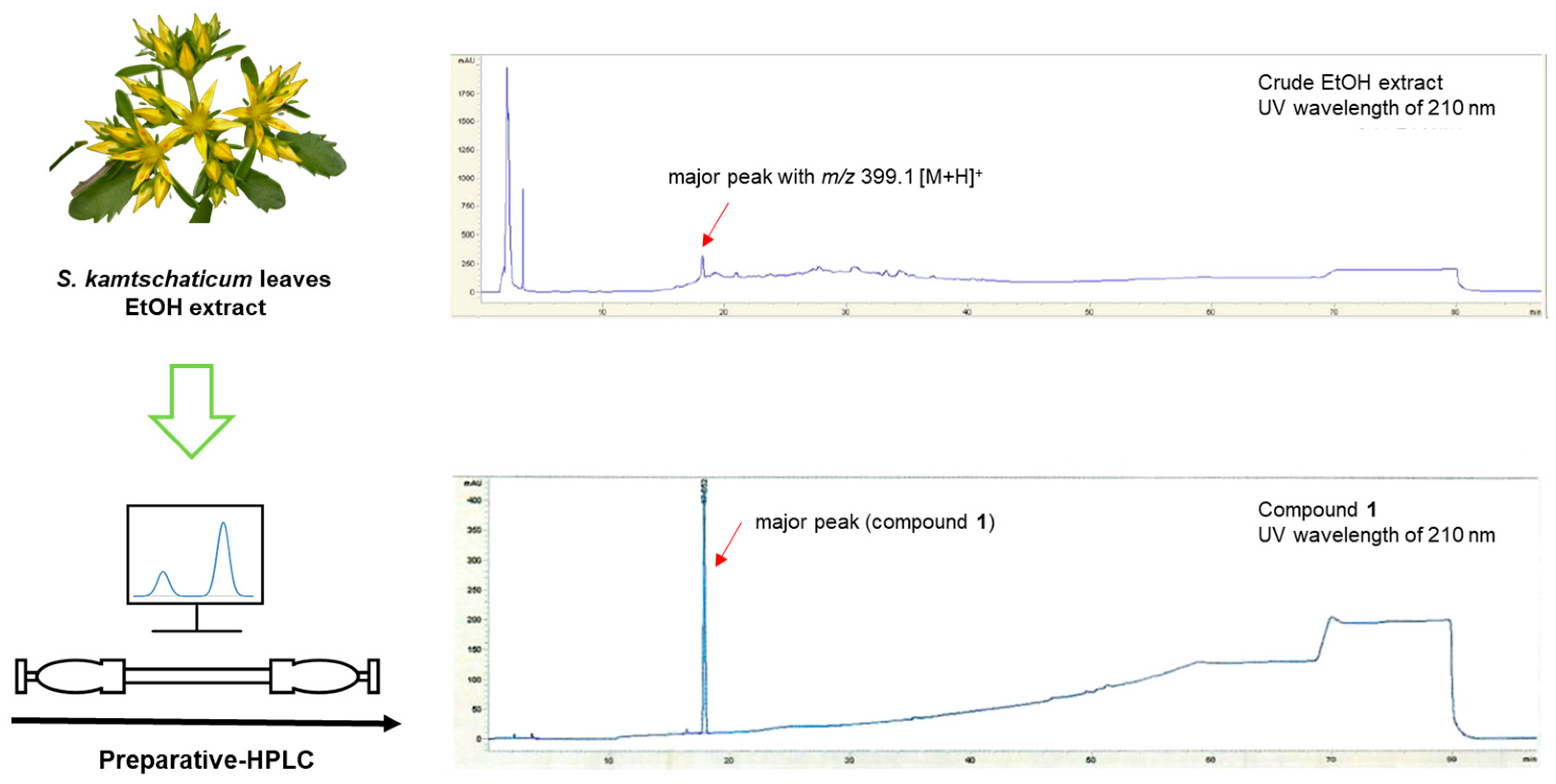
2.3. Extraction and Separation of 1
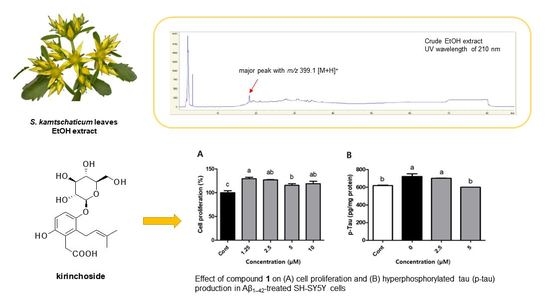
Kirinchoside (1)
2.4. Enzymatic Hydrolysis and Absolute Configuration Determination of the Sugar Moiety of Compound 1
2.5. Cell Culture
2.6. Analysis of Cell Proliferation in SH-SY5Y Cells
2.7. Analysis of p-Tau Production in SH-SY5Y Cells
2.8. Statistical Analysis
3. Results and Discussion
3.1. Isolation of Compound 1
3.2. Structural Determination of Compound 1
3.3. Evaluation of Effects of Compound 1 on p-Tau Accumulation in Aβ1–42-Treated SH-SY5Y Cell
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Blennow, K.; de Leon, M.J.; Zetterberg, H. Alzheimer’s disease. Lancet 2006, 368, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, K.; Alonso Adel, C.; Chen, S.; Chohan, M.O.; El-Akkad, E.; Gong, C.X.; Khatoon, S.; Li, B.; Liu, F.; Rahman, A.; et al. Tau pathology in Alzheimer disease and other tauopathies. Biochim. Biophys. Acta 2005, 1739, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jadhav, S.; Avila, J.; Scholl, M.; Kovacs, G.G.; Kovari, E.; Skrabana, R.; Evans, L.D.; Kontsekova, E.; Malawska, B.; de Silva, R.; et al. A walk through tau therapeutic strategies. Acta Neuropathol. Commun. 2019, 7, 22. [Google Scholar] [CrossRef]
- Bae, K. The Medicinal Plants of Korea; Kyohaksa Publishing Co., Ltd.: Seoul, Republic of Korea, 2000; Volume 260. [Google Scholar]
- Kim, S.-H.; Kwon, C.-S.; Lee, J.-S.; Son, K.-H.; Lim, J.-K.; Kim, J.-S. Inhibition of carbohydrate-digesting enzymes and amelioration of glucose tolerance by Korean medicinal herbs. Prev. Nutr. Food Sci. 2002, 7, 62–66. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, Y.S.; Lee, Y.; Kim, J.; Sun, H.; Kim, J.H.; Kim, J.S. Inhibitory activities of pancreatic lipase and phosphodiesterase from Korean medicinal plant extracts. Phytother. Res. 2012, 26, 778–782. [Google Scholar] [CrossRef]
- Kim, D.W.; Son, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of Sedum kamtschaticum. J. Ethnopharmacol. 2004, 90, 409–414. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Bae, J.-H.; Jung, H.-Y.; Kim, J.-H.; Park, D.-S. Antioxidant activity in water and methanol extracts from Korean edible wild plants. J. Korean Soc. Food Sci. Nutr. 2011, 40, 29–36. [Google Scholar] [CrossRef]
- Korul’kin, D.Y. Chemical composition of certain Sedum species of Kazakhstan. Chem. Nat. Compd. 2001, 37, 219–223. [Google Scholar] [CrossRef]
- Lee, B.S.; So, H.M.; Kim, S.; Kim, J.K.; Kim, J.C.; Kang, D.M.; Ahn, M.J.; Ko, Y.J.; Kim, K.H. Comparative evaluation of bioactive phytochemicals in Spinacia oleracea cultivated under greenhouse and open field conditions. Arch. Pharm. Res. 2022, 45, 795–805. [Google Scholar] [CrossRef]
- Cho, H.; Kim, K.H.; Han, S.H.; Kim, H.-J.; Cho, I.-H.; Lee, S. Structure determination of heishuixiecaoline A from Valeriana fauriei and its content from different cultivated regions by HPLC/PDA Analysis. Nat. Prod. Sci. 2022, 28, 181–186. [Google Scholar] [CrossRef]
- Yu, J.S.; Jeong, S.Y.; Li, C.S.; Oh, T.; Kwon, M.; Ahn, J.S.; Ko, S.K.; Ko, Y.J.; Cao, S.G.; Kim, K.H. New phenalenone derivatives from the Hawaiian volcanic soil-associated fungus Penicillium herquei FT729 and their inhibitory effects on indoleamine 2,3-dioxygenase 1 (IDO1). Arch. Pharm. Res. 2022, 45, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.R.; Lee, B.S.; Yu, J.S.; Kang, H.; Yoo, M.J.; Yi, S.A.; Han, J.W.; Kim, S.; Kim, J.K.; Kim, J.C.; et al. Identification of anti-adipogenic withanolides from the roots of Indian ginseng (Withania somnifera). J. Ginseng. Res. 2022, 46, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Kim, J.K.; Yu, J.S.; Jeong, S.Y.; Choi, J.H.; Kim, J.C.; Ko, Y.J.; Kim, S.H.; Kim, K.H. Ginkwanghols A and B, osteogenic coumaric acid-aliphatic alcohol hybrids from the leaves of Ginkgo biloba. Arch. Pharm. Res. 2021, 44, 514–524. [Google Scholar] [CrossRef]
- Ha, J.W.; Yu, J.S.; Lee, B.S.; Kang, D.-M.; Ahn, M.-J.; Kim, J.K.; Kim, K.H. Structural characterization of withanolide glycosides from the roots of Withania somnifera and their potential biological activities. Plants 2022, 11, 767. [Google Scholar] [CrossRef]
- Lee, S.; Jang, G.; Jung, J.; Park, S.; Lee, J.; Lee, Y.; Lee, J.; Ji, Y.; Choi, J.; Kim, G. Memory-Improving activity of the flower extract from Chrysanthemum boreale (Makino) Maskino in scopolamine-treated rodents. Processes 2023, 11, 159. [Google Scholar] [CrossRef]
- Akhter, H.; Katre, A.; Li, L.; Liu, X.; Liu, R.M. Therapeutic potential and anti-amyloidosis mechanisms of tert-butylhydroquinone for Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 26, 767–778. [Google Scholar] [CrossRef]
- Alishir, A.; Yu, J.S.; Park, M.; Kim, J.C.; Pang, C.; Kim, J.K.; Jang, T.S.; Jung, W.H.; Kim, K.H. Ulmusakidian, a new coumarin glycoside and antifungal phenolic compounds from the root bark of Ulmus davidiana var japonica. Bioorg. Med. Chem. Lett. 2021, 36, 127828. [Google Scholar] [CrossRef]
- David, J.M.; Chávez, J.P.; Chai, H.-B.; Pezzuto, J.M.; Cordell, G.A. Two new cytotoxic compounds from Tapirira guianensis. J. Nat. Prod. 1998, 61, 287–289. [Google Scholar] [CrossRef]
- Cherchar, H.; Lehbili, M.; Berrehal, D.; Morjani, H.; Alabdul Magid, A.; Voutquenne-Nazabadioko, L.; Kabouche, A.; Kabouche, Z. A new 2-alkylhydroquinone glucoside from Phagnalon saxatile (L.) Cass. Nat. Prod. Res. 2018, 32, 1010–1016. [Google Scholar] [CrossRef]
- Gongora, L.; Giner, R.M.; Manez, S.; del Carmen Recio, M.; Rios, J.L. New prenylhydroquinone glycosides from Phagnalon rupestre. J. Nat. Prod. 2001, 64, 1111–1113. [Google Scholar] [CrossRef] [PubMed]
- Alishir, A.; Kim, K.H. Antioxidant phenylpropanoid glycosides from Ginkgo biloba fruit and identification of a new phenylpropanoid glycoside, ginkgopanoside. Plants 2021, 10, 2702. [Google Scholar] [CrossRef]
- Coxon, B. Two-dimensional J-resolved proton nuclear magnetic resonance spectrometry of hydroxyl-coupled. α-and. β-D-glucose. Anal. Chem. 1983, 55, 2361–2366. [Google Scholar]
- Ren, F.X.; Zhao, Y.M.; Zhang, A.J.; Yang, Y.; Zhang, Y. Extraction Method of Effective Components from Chinese Medicine Pyrola and Its Application for Treating Hemorrhage and/or Pain-Related Disease. China CN102204937A, 5 October 2011. [Google Scholar]
- Kwan, K.K.L.; Yun, H.; Dong, T.T.X.; Tsim, K.W.K. Ginsenosides attenuate bioenergetics and morphology of mitochondria in cultured PC12 cells under the insult of amyloid beta-peptide. J. Ginseng Res. 2021, 45, 473–481. [Google Scholar] [CrossRef]
- Zhang, H.; Su, Y.; Sun, Z.; Chen, M.; Han, Y.; Li, Y.; Dong, X.; Ding, S.; Fang, Z.; Li, W.; et al. Ginsenoside Rg1 alleviates Aβ deposition by inhibiting NADPH oxidase 2 activation in APP/PS1 mice. J. Ginseng Res. 2021, 45, 665–675. [Google Scholar] [CrossRef]
- Giovinazzo, D.; Bursac, B.; Sbodio, J.I.; Nalluru, S.; Vignane, T.; Snowman, A.M.; Albacarys, L.M.; Sedlak, T.W.; Torregrossa, R.; Matthew Whiteman, M.; et al. Hydrogen sulfide is neuroprotective in Alzheimer’s disease by sulfhydrating GSK3β and inhibiting Tau hyperphosphorylation. Proc. Natl. Acad. Sci. USA 2021, 118, e2017225118. [Google Scholar] [CrossRef]
- Pooler, A.M.; Hanger, D.P. Functional implications of the association of tau with the plasma membrane. Biochem. Soc. Trans. 2010, 38, 1012–1015. [Google Scholar] [CrossRef] [Green Version]
- Jamwal, V.S.; Mishra, S.; Singh, A.; Kumar, R. Free radical scavenging and radioprotective activities of hydroquinone in vitro. J. Radioprot. Res. 2014, 2, 37–45. [Google Scholar]
- Conforti, F.; Rigano, D.; Formisano, C.; Bruno, M.; Loizzo, M.R.; Menichini, F.; Senatore, F. Metabolite profile and in vitro activities of Phagnalon saxatile (L.) Cass. relevant to treatment of Alzheimer’s disease. J. Enzyme Inhib. Med. Chem. 2010, 25, 97–104. [Google Scholar] [CrossRef]
- Dastan, Z.; Pouramir, M.; Ghasemi-Kasman, M.; Ghasemzadeh, Z.; Dadgar, M.; Gol, M.; Ashrafpour, M.; Pourghasem, M.; Moghadamnia, A.A.; Khafri, S. Arbutin reduces cognitive deficit and oxidative stress in animal model of Alzheimer’s disease. Int. J. Neurosci. 2019, 129, 1145–1153. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, X.; Huang, L.; Luo, Z.; Su, T.; Ding, K.; Li, X. Benzenediol-berberine hybrids: Multifunctional agents for Alzheimer’s disease. Bioorg. Med. Chem. 2011, 19, 7228–7235. [Google Scholar] [CrossRef]
- Tommonaro, G.; García-Font, N.; Vitale, R.M.; Pejin, B.; Iodice, C.; Canadas, S.; Marco-Contelles, J.; Oset-Gasque, M.J. Avarol derivatives as competitive AChE inhibitors, non hepatotoxic and neuroprotective agents for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 122, 326–338. [Google Scholar] [CrossRef]
- Bradford, M.M.A. Rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Han, K.M.; Hwang, J.; Lee, S.H.; Park, B.; Kim, H.; Baek, S.Y. Quantitative Analysis and Enantiomeric Separation of Ephedra Alkaloids in Ma Huang Related Products by HPLC-DAD and UPLC-MS/MS. Nat. Prod. Sci. 2022, 28, 168–180. [Google Scholar] [CrossRef]
- Kang, K.B.; Lee, D.Y.; Sung, S.H. LC-MS/MS-Based Comparative Investigation on Chemical Constituents of Six Aster Species Occurring in Korea. Nat. Prod. Sci. 2021, 27, 257–263. [Google Scholar] [CrossRef]

 ) and HMBC (
) and HMBC ( ) correlations for 1.
) correlations for 1.

| Position | 1 | |
|---|---|---|
| δH (J in Hz) | δC | |
| 1 | 127.7 s | |
| 2 | 129.0 s | |
| 3 | 149.2 s | |
| 4 | 6.83 d (9.0) | 114.4 d |
| 5 | 7.00 d (9.0) | 114.8 d |
| 6 | 160.9 s | |
| 7 | 3.65 s | 34.9 t |
| 8 | 180.4 s | |
| 1′ | 3.37 overlap | 25.3 t |
| 2′ | 5.10 t (6.5) | 121.6 d |
| 3′ | 133.7 s | |
| 4′ | 1.81 s | 17.1 q |
| 5′ | 1.71 s | 24.8 q |
| 1″ | 4.93 d (7.5) | 102.0 d |
| 2″ | 3.60 overlap | 73.0 d |
| 3″ | 3.60 overlap | 75.5 d |
| 4″ | 3.52 overlap | 69.4 d |
| 5″ | 3.54 overlap | 76.0 d |
| 6″ | 3.94 dd (12.0, 2.0); 3.77 dd (12.0, 5.5) | 60.6 t |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-E.; Jeong, S.Y.; Jang, Y.S.; Cho, K.-J.; Lee, J.; Lee, Y.; Kim, K.H. Isolation and Structural Elucidation of Unreported Prenylhydroquinone Glycoside from Sedum kamtschaticum Leaves and Its Effect on Hyperphosphorylated Tau Production in Aβ1–42-Treated SH-SY5Y Cells. Separations 2023, 10, 428. https://doi.org/10.3390/separations10080428
Lee S-E, Jeong SY, Jang YS, Cho K-J, Lee J, Lee Y, Kim KH. Isolation and Structural Elucidation of Unreported Prenylhydroquinone Glycoside from Sedum kamtschaticum Leaves and Its Effect on Hyperphosphorylated Tau Production in Aβ1–42-Treated SH-SY5Y Cells. Separations. 2023; 10(8):428. https://doi.org/10.3390/separations10080428
Chicago/Turabian StyleLee, Seung-Eun, Se Yun Jeong, Yoon Seo Jang, Kwang-Jin Cho, Jeonghoon Lee, Yunji Lee, and Ki Hyun Kim. 2023. "Isolation and Structural Elucidation of Unreported Prenylhydroquinone Glycoside from Sedum kamtschaticum Leaves and Its Effect on Hyperphosphorylated Tau Production in Aβ1–42-Treated SH-SY5Y Cells" Separations 10, no. 8: 428. https://doi.org/10.3390/separations10080428