Preparation and Application of Si@Al Adsorbents for Different Pollutants Removal from Aqueous Solution
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagent Consumables and Instruments
2.2. Experimental Methods
2.2.1. Si@Al Preparation of Adsorbent
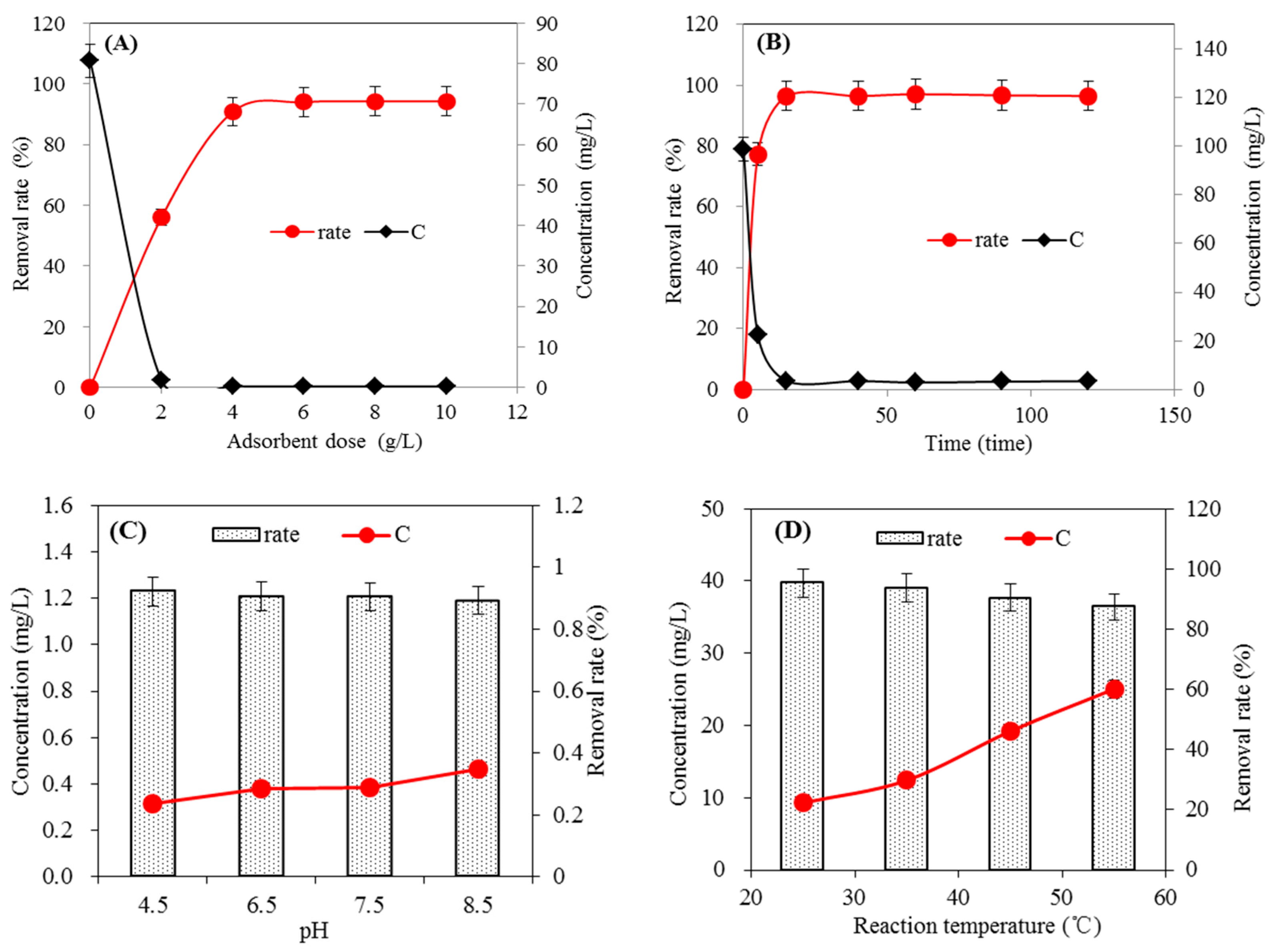
2.2.2. Optimization Experiment of Preparation Conditions
2.2.3. Adsorption Experiment
2.3. Analysis Method
3. Results and Discussion
3.1. Preparation and Characterization of Adsorbent
3.2. Adsorption Effect of Tetracycline
3.3. Adsorption Effect on Methylene Blue
3.4. Adsorption Effect on Metal Cu
3.5. Adsorption Model Simulation
3.5.1. Quasi-First-Order Kinetic Equation
3.5.2. Quasi-Second-Order Kinetic Equation
3.5.3. Adsorption Isotherm
3.5.4. Adsorption Thermodynamics
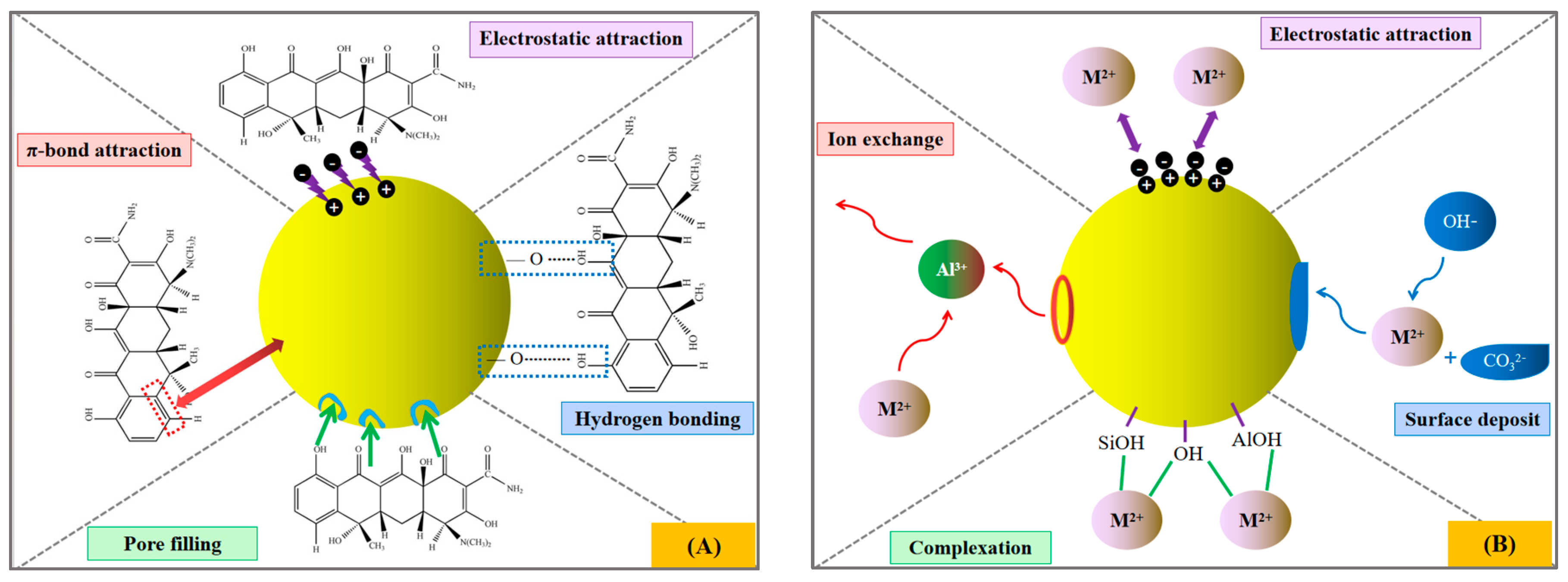
3.6. Adsorption Mechanism
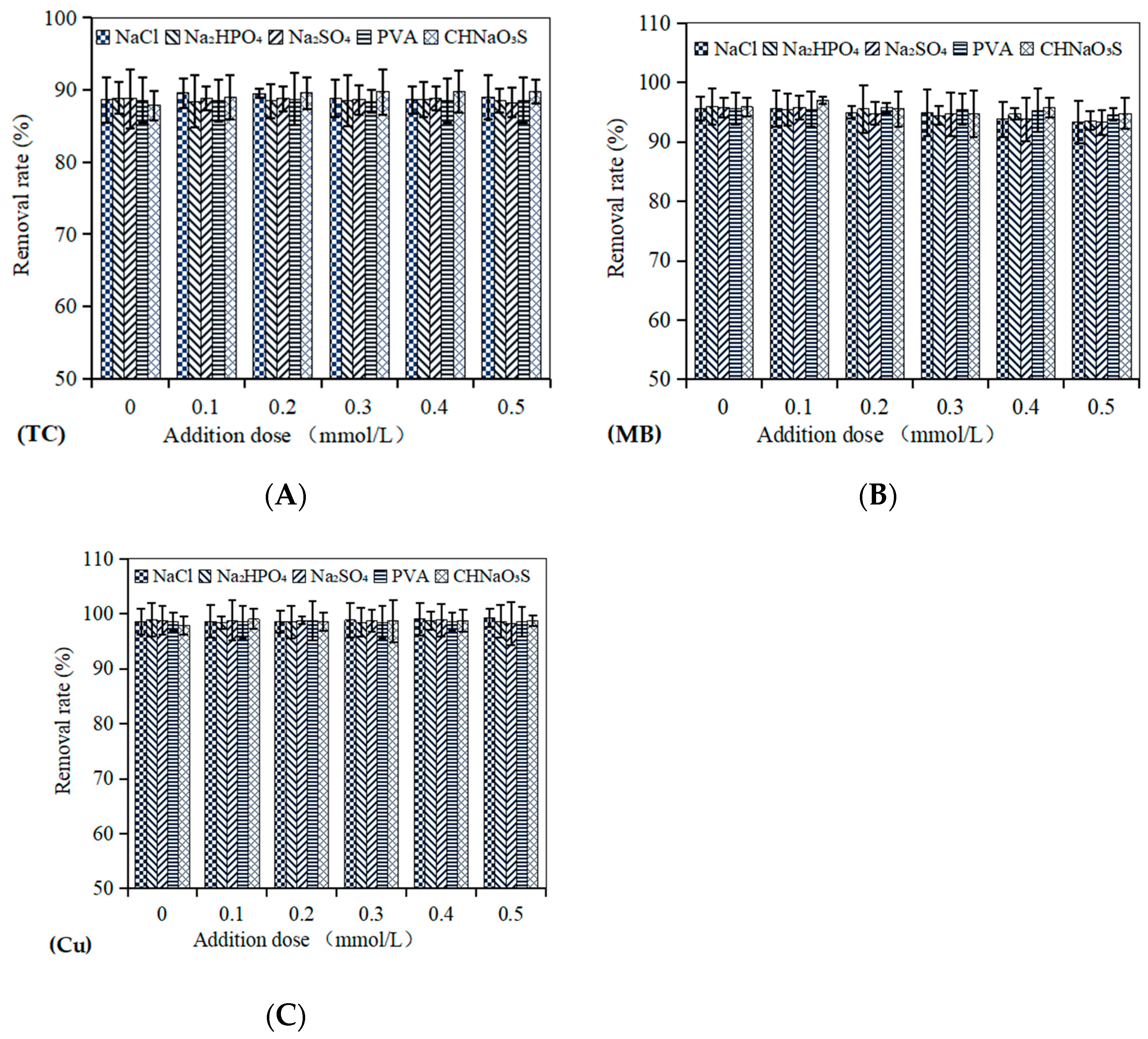
3.7. Effects of Co-Existing Substances
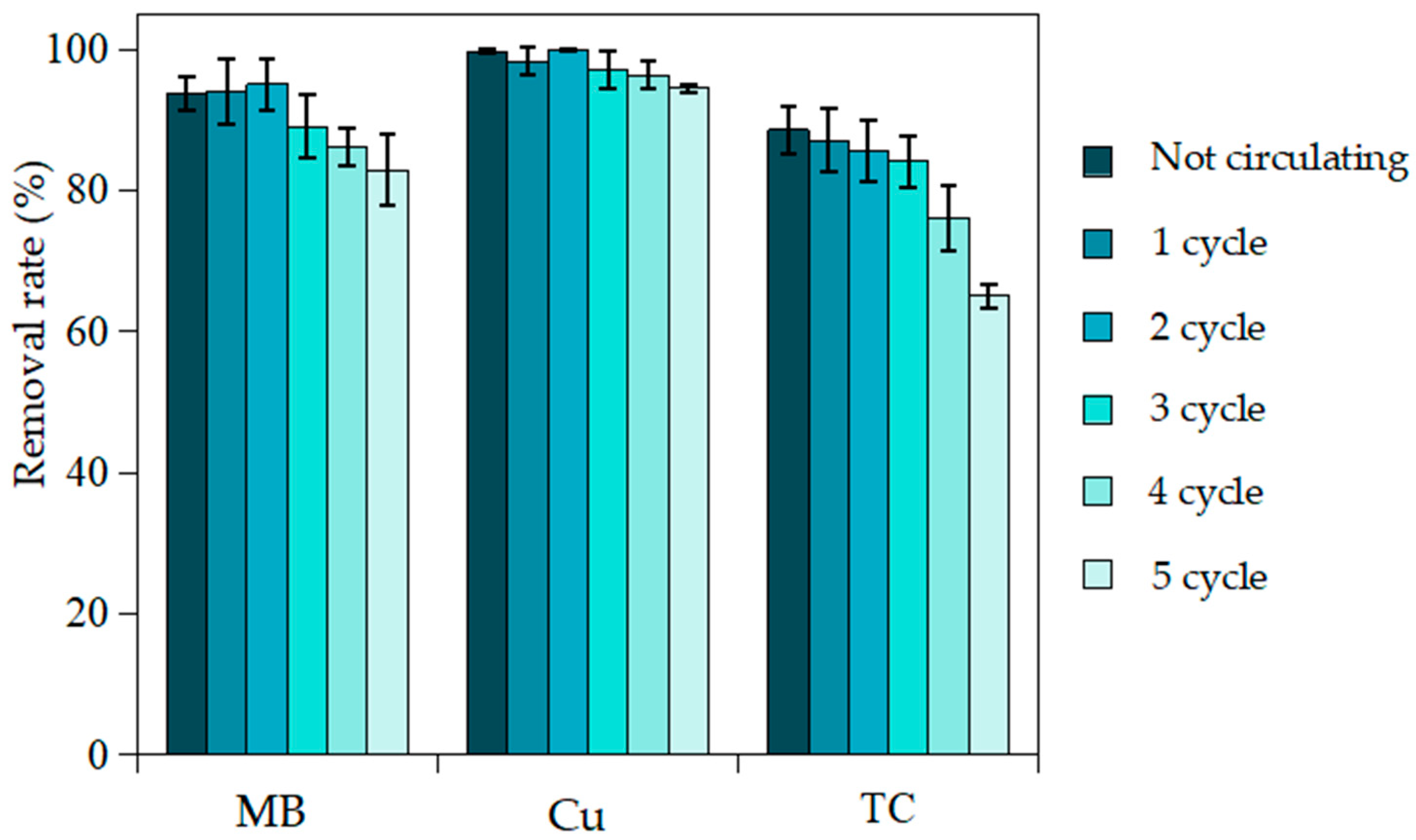
3.8. Recycling of Adsorbent and Ion Percolation
3.9. Comparison with Existing Literature
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Huang, D.; Wu, J.; Wang, L. Novel insight into adsorption and co-adsorption of heavy metal ions and an organic pollutant by magnetic graphene nanomaterials in water. Chem. Eng. J. 2019, 358, 1399–1409. [Google Scholar] [CrossRef]
- Guale, A.B.; Teshome, L.T.; Ahmed, O.Z.; Juyong, G.; Jaebeom, L.; Kedir, F.S. Microwave-assisted synthesis of rGO-ZnO/CuO nanocomposites for photocatalytic degradation of organic pollutants. Crystals 2023, 13, 133. [Google Scholar]
- Zhang, K.; Ruan, R.; Zhang, Z. An exhaustive investigation on antibiotics contamination from livestock farms within sensitive reservoir water area: Spatial density, source apportionment and risk assessment. Sci. Total Environ. 2022, 847, 157688. [Google Scholar] [CrossRef]
- Zhi, S.; Shen, S.; Zhou, J.; Ding, G.; Zhang, K. Systematic analysis of occurrence, density and ecological risks of 45 veterinary antibiotics: Focused on family livestock farms in Erhai Lake basin, Yunnan, China. Environ. Pollut. 2020, 267, 115539. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Xie, Q.; Zhang, S.; Li, Y.; Zhang, Y.; Xie, H. Photochemical behavior of antibiotics impacted by complexation effects of concomitant metals: A case for ciprofloxacin and Cu(II). Environ. Sci. Process. Impacts 2015, 17, 1220–1227. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Rong, F.; Hu, S.; Wang, G.; Liu, J.; Hou, G. Competitive adsorption and desorption of three antibiotics in distinct soil aggregate size fractions. Ecotoxicol. Environ. Saf. 2023, 259, 115002. [Google Scholar] [CrossRef]
- Phoon, B.L.; Ong, C.C.; Saheed, M.S.M.; Show, P.L.; Chang, J.S.; Ling, T.C.; Lam, S.S.; Juan, J.C. Conventional and emerging technologies for removal of antibiotics from wastewater. J. Hazard. Mater. 2020, 400, 122961. [Google Scholar] [CrossRef] [PubMed]
- Emin, M.K.; Iryna, M.; Teemu, K.; Antti, H. Simultaneous adsorption of Cu(II), Zn(II), Cd(II) and Pb(II) from synthetic wastewater using NaP and LTA zeolites prepared from biomass fly ash. Heliyon 2023, 9, e20253. [Google Scholar]
- Valentina, S.; Louros, V.L.; Patrícia, C.S.; Marta, T.; Marta, O.; Vânia, C. A solar flow photo-reactor for antibiotic removal from aquaculture effluents using TiO2/carbon quantum dots. Chemosphere 2023, 348, 140723. [Google Scholar]
- Mai, E.; Elsayed, E.; Emad, A. Ecofriendly nanoparticles derived from water industry byproducts for effective removal of Cu(II) from wastewater: Adsorption isotherms and kinetics. Inorg. Chem. Commun. 2022, 102, 110062. [Google Scholar]
- Li, M.; Liu, H.; Chen, T.; Dong, C.; Sun, Y. Synthesis of magnetic biochar composites for enhanced uranium(VI) adsorption. Sci. Total Environ. 2019, 651, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, B.M. Heavy metal ions removal from wastewater using various low-cost agricultural wastes as adsorbents: A survey, Zanco. Pure Appl. Sci. 2021, 33, 76–91. [Google Scholar]
- Khalfa, L.; Sdiri, A.; Bagane, M.; Cervera, M.L. A calcined clay fixed bed adsorption studies for the removal of heavy metals from aqueous solutions. J. Clean. Prod. 2021, 278, 123935. [Google Scholar] [CrossRef]
- Singh, E.; Kumar, A.; Mishra, R.; You, S.; Singh, L.; Kumar, S.; Kumar, R. Pyrolysis of waste biomass and plastics for production of biochar and its use for removal of heavy metals from aqueous solution. Bioresour. Technol. 2021, 320, 124278. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Lan, J.; Sun, H.; Miao, F.; Zhang, P.; Shao, G. Design and preparation of hierarchical porous carbon-based materials with bionic “ant nest” structure for high performance asymmetric supercapacitors. J. Alloys Compd. 2023, 968, 172029. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, M.; Li, Q.; Chen, X.; Chen, D. Subtle introduction of membrane polarization-catalyzed H2O dissociation actuates highly efficient electrocoagulation for hardness ion removal. Water Res. 2023, 242, 120240. [Google Scholar] [CrossRef]
- Raquel, P.; Szabolcs, F.; Natalia, N.; Davy, G. Practical study on the impact of injection conditions in gradient elution mode for the analysis of therpeutic proteins when using very short columns. J. Chromatogr. A 2023, 1709, 464359. [Google Scholar]
- Yin, H.; Xiong, Q.; Zhang, M.; Wang, B.; Zhang, F. Multi-principles analysis of Cu(II) adsorption in water on magnetic microspheres and modified Chitosan. J. Environ. Chem. Eng. 2023, 11, 111285. [Google Scholar] [CrossRef]
- Tang, L.; Yu, J.; Pang, Y.; Zeng, G.; Deng, Y.; Wang, J. Sustainable efficient adsorbent: Alkali-acid modified magnetic biochar derived from sewage sludge for aqueous organic contaminant removal. Chem. Eng. J. 2018, 336, 160–169. [Google Scholar] [CrossRef]
- Kim, J.E.; Bhatia, S.K.; Song, H.J.; Yoo, E. Adsorptive removal of tetracycline from aqueous solution by maple leaf-derived biochar. Bioresour. Technol. 2020, 306, 123092. [Google Scholar] [CrossRef]
- Wang, P.; Sun, D.; Zhang, S.; Huang, X.; Bi, Q.; Qian, M.; Zhao, W.; Huang, F. Constructing mesoporous phosphated titanium oxide for efficient Cr(III) removal. J. Hazard. Mater. 2020, 384, 121278. [Google Scholar]
- Gao, Z.; Cizdziel, J.V.; Wontor, K.; Olubusoye, B.S. Adsorption/desorption of mercury (II) by artificially weathered microplastics: Kinetics, isotherms, and influencing factors. Environ. Pollut. 2023, 337, 122621. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Li, W.; Ge, C.; Müller, K.; Yu, H.; Huang, P. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018, 263, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Xiao, G.; Meng, Q. Adsorption of sulfadiazine on novel bilayer porous resin: Synergism of micropore filling and anion exchange. Mater. Sci. Eng. B 2024, 299, 117039. [Google Scholar] [CrossRef]
- Ebrahimi, N.; Farahbod, B.; Sadeghi, R. Salting-in and salting-out effects of organic and inorganic ammonium salts on the aqueous polymer solutions. J. Chem. Thermodyn. 2018, 123, 12386–12398. [Google Scholar] [CrossRef]
- Chen, Z.; Tang, T.; Wen, Q.; Hu, H. Evaluation of Fe(VI)/Fe(II) combined with sludge adsorbents in secondary effluent organic matter removal. Environ. Res. 2022, 208, 112737. [Google Scholar] [CrossRef]
- Tang, J.; Ma, Y.; Zeng, C.; Yang, L.; Cui, S.; Zhi, S. Fe-Al bimetallic oxides functionalized-biochar via ball milling for enhanced adsorption of tetracycline in water. Bioresour. Technol. 2023, 369, 128385. [Google Scholar] [CrossRef]
- Mohammadhossein, S.; Faramarz, A.D.; Mohammad, N.M. Lignocellulosic biomass functionalized with EDTA dianhydride for removing Cu(II) and dye from wastewater: Batch and fixed-bed column adsorption. Miner. Eng. 2023, 204, 108423. [Google Scholar]
- Jian, N.; Dai, Y.; Wang, Y.; Qi, F.; Li, S.; Wu, Y. Preparation of polydopamine nanofibers mat as a recyclable and efficient adsorbent for simultaneous adsorption of multiple tetracyclines in water. J. Chem. Phys. 2021, 320, 128875. [Google Scholar] [CrossRef]
- Nikita, G.; Tanushree, P.; Vijay, H.K. A review on the fate of micro and nano plastics (MNPs) and their implication in regulating nutrient cycling in constructed wetland systems. J. Environ. Manag. 2023, 350, 119559. [Google Scholar]



| Pollutants | Initial Concentration (mg/L) | qe (mg/g) | k1 (min−1) | R2 |
|---|---|---|---|---|
| TC | 2 | 0.2973 ± 0.0044 | 0.9548 ± 0.0790 | 0.9960 |
| 5 | 0.9824 ± 0.0026 | 0.4269 ± 0.0058 | 0.9999 | |
| 10 | 1.9602 ± 0.0594 | 0.3849 ± 0.0578 | 0.9897 | |
| 20 | 4.0230 ± 0.2676 | 0.3198 ± 0.0968 | 0.9598 | |
| MB | 100 | 24.6638 ± 0.0240 | 0.9961 ± 0.0693 | 1.0000 |
| 200 | 36.1360 ± 0.7901 | 0.4572 ± 0.1036 | 0.9861 | |
| 300 | 64.1581 ± 0.5997 | 0.3975 ± 0.0326 | 0.9974 | |
| 400 | 69.4206 ± 0.6345 | 0.4048 ± 0.0331 | 0.9976 | |
| Cu | 5 | 1.1816 ± 0.0076 | 0.1909 ± 0.0060 | 0.9988 |
| 25 | 6.1260 ± 0.1482 | 0.1685 ± 0.0189 | 0.9844 | |
| 50 | 12.8957 ± 0.1235 | 0.1737 ± 0.0078 | 0.9979 | |
| 100 | 24.4614 ± 0.4886 | 0.2241 ± 0.0235 | 0.9903 |
| Pollutants | Initial Concentration (mg/L) | qe (mg/g) | k2 (g/(mg·min)) | R2 |
|---|---|---|---|---|
| TC | 2 | 0.3106 ± 0.0091 | 5.1408 ± 1.2410 | 0.9884 |
| 5 | 1.0582 ± 0.0452 | 0.5962 ± 0.1611 | 0.9831 | |
| 10 | 2.1240 ± 0.1617 | 0.2548 ± 0.1183 | 0.9538 | |
| 20 | 4.4192 ± 0.5103 | 0.0937 ± 0.0619 | 0.9166 | |
| MB | 100 | 24.6941 ± 0.0191 | 0.9700 ± 0.0278 | 1.0000 |
| 200 | 35.7059 ± 0.8401 | 0.4689 ± 0.3045 | 0.9788 | |
| 300 | 64.1018 ± 0.9179 | 0.0424 ± 0.0062 | 0.9929 | |
| 400 | 69.2090 ± 1.0490 | 0.0460 ± 0.0074 | 0.9918 | |
| Cu | 5 | 1.2443 ± 0.0190 | 0.2689 ± 0.0272 | 0.9960 |
| 25 | 6.5718 ± 0.2986 | 0.0369 ± 0.0103 | 0.9720 | |
| 50 | 13.7484 ± 0.3815 | 0.0194 ± 0.0034 | 0.9886 | |
| 100 | 20.1271 ± 3.1451 | 4 × 10−44 ± 5.23 × 10−45 | 0.6347 |
| Pollutants | Freundlich | Langmuir | ||||
|---|---|---|---|---|---|---|
| KF | 1/n | R2 | qm (mg/g) | KL | R2 | |
| TC | 1.0020 ± 0.2602 | 1.87854 ± 0.2566 | 0.9732 | 5.5160 ± 0.6685 | 0.1996 ± 0.0820 | 0.9444 |
| MB | 58.3113 ± 2.3331 | 2.7590 ± 0.1653 | 0.9933 | 157.4483 ± 12.3750 | 0.5283 ± 0.1543 | 0.9549 |
| Cu | 5.6356 ± 1.0684 | 1.6583 ± 0.5646 | 0.9569 | 50.5656 ± 2.9571 | 0.1525 ± 0.0263 | 0.9632 |
| Pollutants | Temperature | T | KL | CW | K | ∆G | lnK | ∆S | ∆H |
|---|---|---|---|---|---|---|---|---|---|
| 25 | 298 | 0.1996 | 1.732066 | 345.7204 | −14.483 | 5.84563 | |||
| TC | 35 | 308 | 0.1896 | 1.85241 | 351.2169 | −15.0094 | 5.861404 | −11.57 | −1.8135 |
| 45 | 318 | 0.1104 | 1.9652 | 216.9581 | −14.2231 | 5.379704 | |||
| 25 | 298 | 0.5432 | 9.302987 | 5053.382 | −21.1283 | 8.527813 | |||
| MB | 35 | 308 | 0.5283 | 12.42001 | 6561.493 | −22.506 | 8.788973 | 158.62 | 2.6212 |
| 45 | 318 | 0.5121 | 19.23032 | 9847.848 | −24.3102 | 9.195008 | |||
| 25 | 298 | 0.1506 | 0.88962 | 133.9768 | −12.1343 | 4.897666 | |||
| Cu | 35 | 308 | 0.1525 | 0.806123 | 122.9338 | −12.3212 | 4.811646 | 26.315 | −4.246 |
| 45 | 318 | 0.1586 | 0.758895 | 120.3607 | −12.6654 | 4.790493 |
| Reference | Adsorbent Type | Preparation Method | Targeted Pollutants | Removal Rate (%) |
|---|---|---|---|---|
| Tang et al., 2023 [27] | Fe-Al bimetallic oxides functionalized-biochar | The pretreated sugarcane bagasse was heated at 600 °C for 2 h under nitrogen condition. First, 40 g pretreated bagasse was immersed in 800 mL mixed aqueous solution of 10.81 g FeCl3•6H2O and 9.66 g AlCl3•6H2O, and then mixed uniformly for 2 h to acquire the admixture, and its pH was controlled to 10 by making use of NaOH solution. Next, the dispersion was continuously magnetically agitated for 4 h, naturally deposed for 18 h, then put into an oven at 80 °C to dry to constant weight. Ball milled-Fe-Al oxides-decorated BC was manufactured by a planetary ball mill machine. Briefly, the precursor material and the abrasive pellets were placed in a ball milling canister at the mass ratio of 1:100, and the machine was programmed to 700 rpm, then the product was obtained after ball milling for 2 h. | TC | - |
| Jian et al., 2021 [29] | polydopamine nanofibers mat | Briefly, 0.12 g of dopamine hydrochloride was dissolved into 200 mL water. Then, 0.2 g of polyacrylonitrile nanofibers mat was soaked entirely into the above solution. After that, 0.48 g of tris(hydroxymethyl) aminomethane was added and dissolved to initiate dopamine polymerization. After gentle oscillation for 2 h at room temperature, the obtained PDA-NFsM was washed with water and dried at 60 °C under vacuum for 24 h. | TC, OTC, CTC | 85.7–96.8% |
| Yin et al., 2023 [18] | magnetic microspheres and modified Chitosan | First, 3.0 g of SA was added to 50 mL of deionized water at 60 °C and stirred for 1 h until it completely dissolved into a uniform viscous solution. At the same time, 1.0 g of CS was added to a 4% PVA solution (50 mL) prepared from deionized water and stirred until the solution was well mixed. The above mixture was then slowly poured into the SA solution while adding and stirring 1.5 g of Fe3O4. The solution was then slowly dropped into 200 mL of CaCl2 (5%) using a 5 mL syringe, resulting in the formation of gel beads within a few seconds. After curing for 30 min, 2 mL of Epichlorohydrin was added, and the curing process was continued in a water bath at 60 °C for 2 h. Finally, the gel beads were rinsed repeatedly using deionized water and freeze-dried under a vacuum at −60 °C to obtain SCFP with an average particle size of 4.5 mm. | Cu2+ | 97.1% |
| This study | Si@Al adsorbent | Precipitated waste was calcined with sodium silicate at a mass ratio of 4:1 at 200 °C for 2 h. | TC, Cu, MB | 98.02–99.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, X.; Liu, J.; Cao, Y.; Wang, H.; Zhang, K.; Chang, C.-C.; Zhi, S. Preparation and Application of Si@Al Adsorbents for Different Pollutants Removal from Aqueous Solution. Separations 2024, 11, 29. https://doi.org/10.3390/separations11010029
Xu X, Liu J, Cao Y, Wang H, Zhang K, Chang C-C, Zhi S. Preparation and Application of Si@Al Adsorbents for Different Pollutants Removal from Aqueous Solution. Separations. 2024; 11(1):29. https://doi.org/10.3390/separations11010029
Chicago/Turabian StyleXu, Xiaoyu, Jiahua Liu, Yuang Cao, Han Wang, Keqiang Zhang, Chein-Chi Chang, and Suli Zhi. 2024. "Preparation and Application of Si@Al Adsorbents for Different Pollutants Removal from Aqueous Solution" Separations 11, no. 1: 29. https://doi.org/10.3390/separations11010029





