An Evaluation of the Relationship between Membrane Properties and the Fouling Mechanism Based on a Blocking Filtration Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Prepration of Solutions
2.2. Experimental Apparatus and Technique
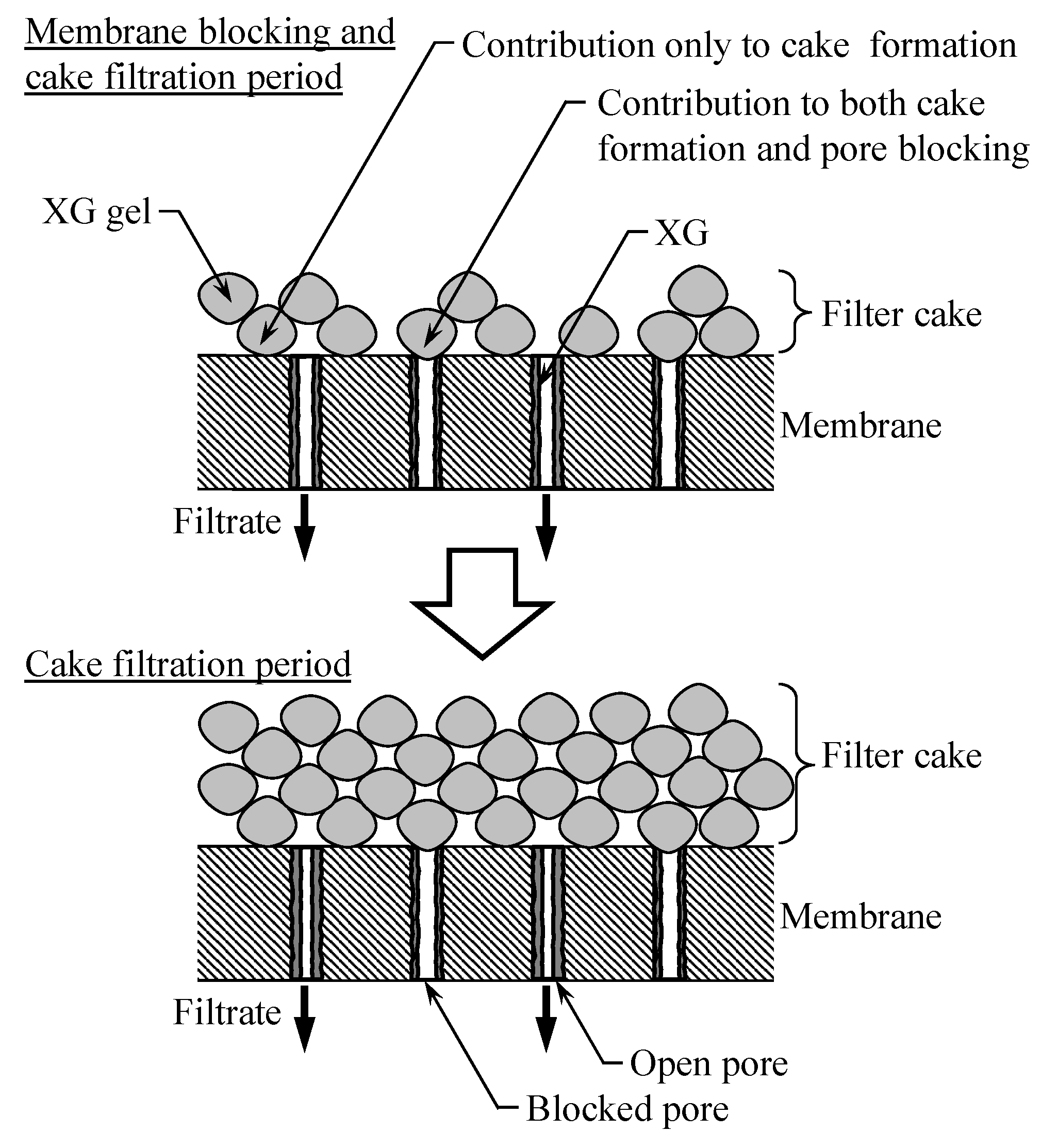
3. Blocking Filtration Model
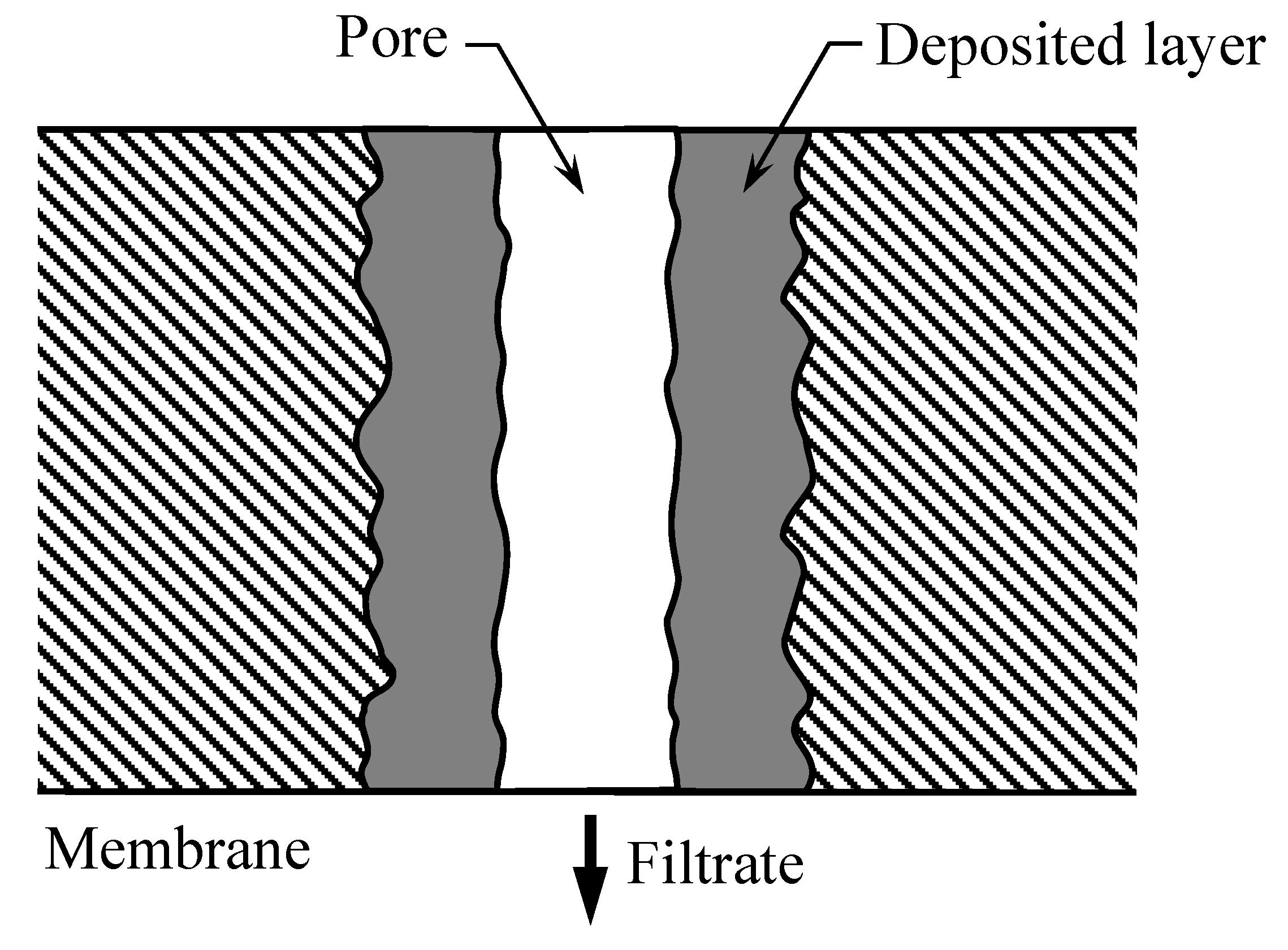
4. Results and Discussion
4.1. Membrane Filtration Properties of Sodium Alginate
4.2. Relationship between Membrane Properties and Fouling Mechanism
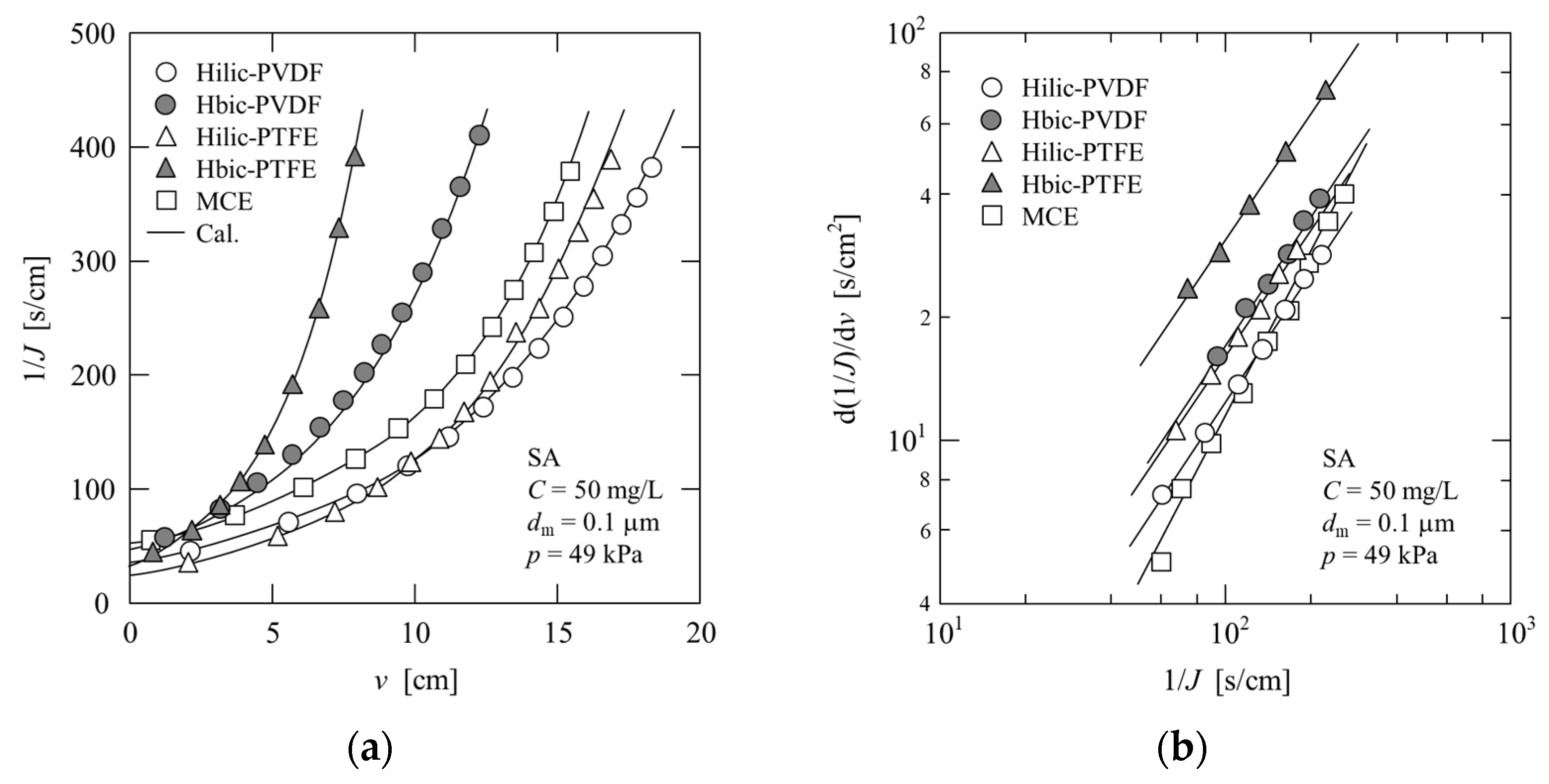
4.2.1. Sodium Alginate (SA)
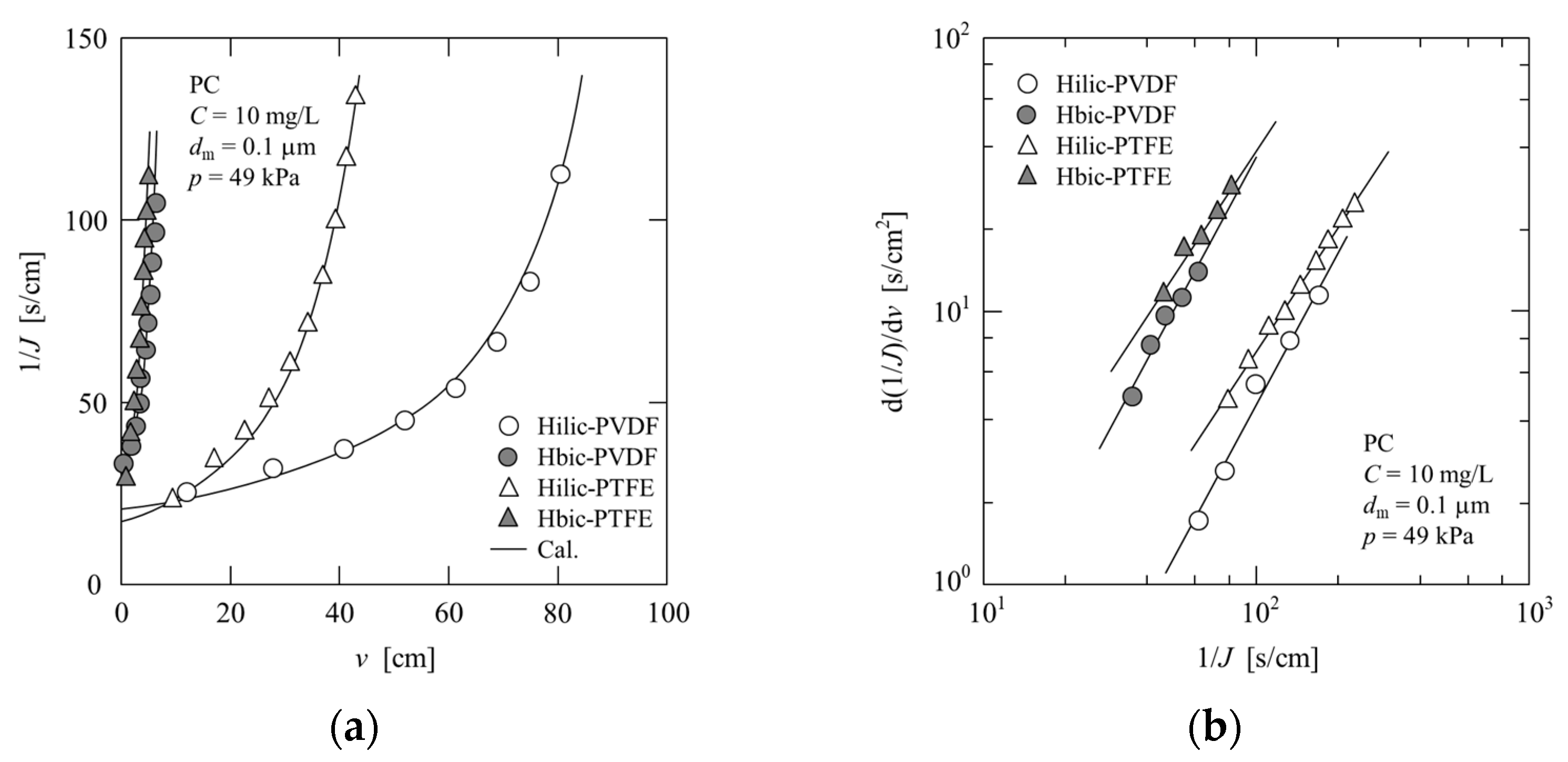
4.2.2. Pectin (PC)
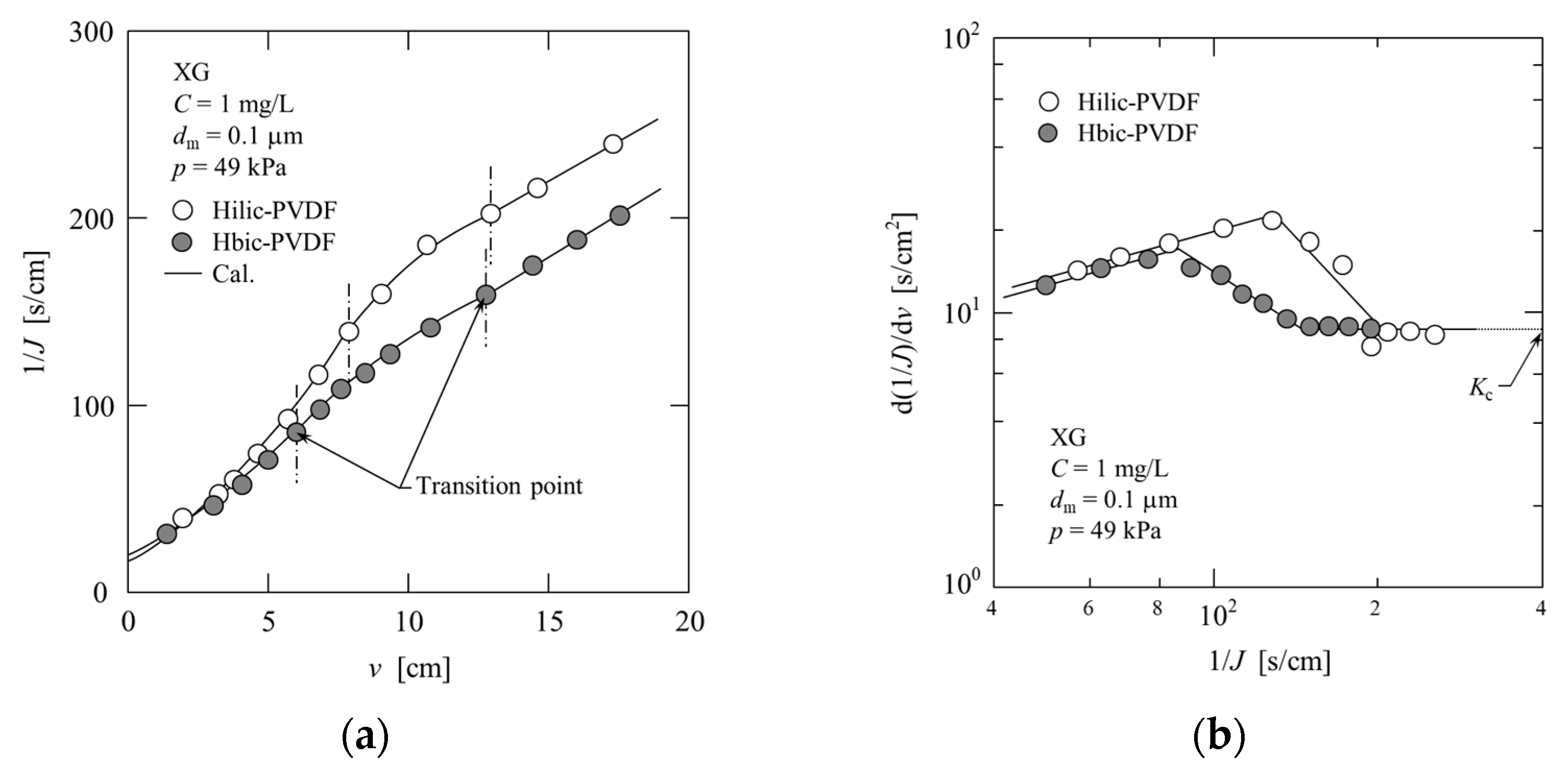
4.2.3. Xanthan Gum (XG)
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shah, S.S.A.; Sohail, M.; Murtza, G.; Waseem, A.; Rehman, A.; Hussain, I.; Bashir, M.S.; Alarfaji, S.S.; Hassan, A.M.; Nazir, M.A.; et al. Recent trends in wastewater treatment by using metal-organic frameworks (MOFs) and their composites: A critical view-point. Chemosphere 2024, 349, 140729. [Google Scholar] [CrossRef] [PubMed]
- Iritani, E.; Katagiri, N. Developments of blocking filtration model in membrane filtration. KONA Powder Part. J. 2016, 33, 179–202. [Google Scholar] [CrossRef]
- Hermans, P.H.; Bredée, H.L. Principles of the mathematical treatment of constant-pressure filtration. J. Soc. Chem. Ind. 1936, 55, 1–4. [Google Scholar]
- Grace, H.P. Structure and performance of filter media. II. Performance of filter media in liquid service. AIChE J. 1956, 2, 316–336. [Google Scholar] [CrossRef]
- Hermia, J. Constant pressure blocking filtration laws—Application to power-law non-Newtonian fluids. Trans. IChemE 1982, 60, 183–187. [Google Scholar]
- Blanpain-Avet, P.; Fillaudeau, L.; Lalande, M. Investigation of machanisms governing membrane fouling and protein rejection in the sterile microfiltration of beer with an organic membrane. Food Bioprod. Process. 1999, 77, 75–89. [Google Scholar] [CrossRef]
- Ho, C.C.; Zydney, A.L. A combined pore blockage and cake filtration model for protein fouling during microfiltration. J. Colloid Interface Sci. 2000, 232, 389–399. [Google Scholar] [CrossRef]
- Hwang, K.J.; Chou, F.Y.; Tung, K.L. Effects of operating conditions on the performance of cross-flow microfiltration of fine particle/protein binary suspension. J. Membr. Sci. 2006, 274, 183–191. [Google Scholar] [CrossRef]
- Bolton, G.; LaCasse, D.; Kuriyel, R. Combined models of membrane fouling: Development and application to microfiltration and ultrafiltration of biological fluids. J. Membr. Sci. 2006, 277, 75–84. [Google Scholar] [CrossRef]
- Iritani, E.; Katagiri, N.; Sugiyama, Y.; Yagishita, K. Analysis of flux decline behaviors in filtration of very dilute suspensions. AIChE J. 2007, 53, 2275–2283. [Google Scholar] [CrossRef]
- Iritani, E.; Katagiri, N.; Yamashita, Y. Effect of membrane morphology on rising properties of filtration resistance in microfiltration of dilute colloids. AIChE J. 2017, 63, 3511–3522. [Google Scholar] [CrossRef]
- Broeckmann, A.; Busch, J.; Wintgens, T.; Marquardt, W. Modeling of pore blocking and cake layer formation in membrane filtration for wastewater treatment. Desalination 2006, 189, 97–109. [Google Scholar] [CrossRef]
- Ho, C.C.; Zydney, A.L. Effect of membrane morphology on the initial rate of protein fouling during microfiltration. J. Membr. Sci. 1999, 155, 261–275. [Google Scholar] [CrossRef]
- Hwang, K.J.; Lin, T.T. Effect of morphology of polymeric membrane on the performance of cross-flow microfiltration. J. Membr. Sci. 2002, 199, 41–52. [Google Scholar] [CrossRef]
- Fu, X.; Maruyama, T.; Sotani, T.; Matsuyama, H. Effect of surface morphology on membrane fouling by humic acid with the use of cellulose acetate butyrate hollow fiber membranes. J. Membr. Sci. 2008, 320, 483–491. [Google Scholar] [CrossRef]
- Tanaka, T.; Mimura, T.; Koga, M.; Yoshida, M.; Taniguchi, M.; Nakanishi, K. Microfiltration of incompressible particle suspension with an asymmetric depth filter. Kagaku Kogaku Ronbunshu 2009, 35, 81–86. [Google Scholar] [CrossRef]
- Yoshida, M.; Taniguchi, M.; Tanaka, T. Microfiltration of bacterial cell suspensions with an asymmetric depth filter. Kagaku Kogaku Ronbunshu 2010, 36, 494–500. [Google Scholar] [CrossRef]
- Tung, K.L.; Li, Y.L.; Wang, S.; Nanda, D.; Hu, C.C.; Li, C.L.; Lai, J.Y.; Huang, J. Performance and effects of polymeric membranes on the dead-end microfiltration of protein solution during filtration cycles. J. Membr. Sci. 2010, 352, 143–152. [Google Scholar] [CrossRef]
- Hashino, M.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of membrane surface morphology on membrane fouling with sodium alginate. J. Membr. Sci. 2011, 366, 258–265. [Google Scholar] [CrossRef]
- Hashino, M.; Katagiri, T.; Kubota, N.; Ohmukai, Y.; Maruyama, T.; Matsuyama, H. Effect of surface roughness of hollow fiber membranes with gear-shaped structure on membrane fouling by sodium alginate. J. Membr. Sci. 2011, 366, 389–397. [Google Scholar] [CrossRef]
- Hwang, K.J.; Liao, C.Y. Effects of membrane morphology and operating conditions on microfiltration particle fouling. J. Taiwan Inst. Chem. Eng. 2012, 43, 46–52. [Google Scholar] [CrossRef]
- Xiao, K.; Sun, J.; Mo, Y.; Fang, Z.; Liang, P.; Huang, X.; Ma, J.; Ma, B. Effect of membrane pore morphology on microfiltration organic fouling: PTFE/PVDF blend membranes compared with PVDF membranes. Desalination 2014, 343, 217–225. [Google Scholar] [CrossRef]
- Wu, S.E.; Lin, N.J.; Chou, C.Y.; Hu, C.C.; Tung, K.L. Biofouling mechanism of polysaccharide-protein-humic acid mixtures on polyvinylidene fluoride microfiltration membranes. J. Taiwan Inst. Chem. Eng. 2019, 94, 2–9. [Google Scholar] [CrossRef]
- Lin, Y.C.; Liu, K.M.; Chao, C.M.; Wang, D.K.; Tung, K.L.; Tseng, H.H. Enhanced anti-protein fouling of PVDF membrane via hydrophobic-hydrophobic adsorption of styrene-terminated amphiphilic linker. Chem. Eng. Res. Des. 2020, 156, 273–280. [Google Scholar] [CrossRef]
- Jørgensen, M.K.; Nierychlo, M.; Nielsen, A.H.; Larsen, P.; Christensen, M.L.; Nielsen, P.H. Unified understanding of physico-chemical properties of activated sludge and fouling propensity. Water Res. 2017, 120, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.L.; Jørgensen, M.K.; Van De Staey, G.; De Cock, L.; Smets, I. Hydraulic resistance and osmotic pressure effects in fouling layers during MBR operations. J. Membr. Sci. 2021, 627, 119213. [Google Scholar] [CrossRef]
- Krzeminski, P.; Leverette, L.; Malamis, S.; Katsou, E. Membrane bioreactors—A review on recent developments in energy reduction, fouling control, novel configurations, LCA and market prospects. J. Membr. Sci. 2017, 527, 207–227. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, S.; Oh, Y.; Zhou, Z.; Shin, H.S.; Chae, S.R. Fouling in membrane bioreactors: An updated review. Water Res. 2017, 114, 151–180. [Google Scholar] [CrossRef]
- Ye, Y.; Le Clech, P.; Chen, V.; Fane, A.G. Evolution of fouling during crossflow filtration of model EPS solutions. J. Membr. Sci. 2005, 264, 190–199. [Google Scholar] [CrossRef]
- Ye, Y.; Le Clech, P.; Chen, V.; Fane, A.G.; Jefferson, B. Fouling mechanisms of alginate solutions as model extracellular polymeric substances. Desalination 2005, 175, 7–20. [Google Scholar] [CrossRef]
- Ye, Y.; Chen, V.; Fane, A.G. Modeling long-term subcritical filtration of model EPS solutions. Desalination 2006, 191, 318–327. [Google Scholar] [CrossRef]
- Katsoufidou, K.; Yiantsios, S.G.; Karabelas, A.J. Experimental study of ultrafiltration membrane fouling by sodium alginate and flux recovery by backwashing. J. Membr. Sci. 2007, 300, 137–146. [Google Scholar] [CrossRef]
- Listiarini, K.; Chun, W.; Sun, D.D.; Leckie, J.O. Fouling mechanism and resistance analyses of systems containing sodium alginate, calcium, alum and their combination in dead-end fouling of nanofiltration membranes. J. Membr. Sci. 2009, 344, 244–251. [Google Scholar] [CrossRef]
- Hwang, K.J.; Tsai, P.C.; Iritani, E.; Katagiri, N. Effect of polysaccharide concentration on the membrane filtration of microbial cells. J. Appl. Sci. Eng. 2012, 15, 323–332. [Google Scholar] [CrossRef]
- Karabelas, A.J.; Karanasiou, A.; Sioutopoulos, D.C. Experimental study on the effect of polysaccharides on incipient membrane scaling during desalination. Desalination 2017, 416, 106–121. [Google Scholar] [CrossRef]
- Meng, S.; Fan, W.; Li, X.; Liu, Y.; Liang, D.; Liu, X. Intermolecular interactions of polysaccharides in membrane fouling during microfiltration. Water Res. 2018, 143, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Meng, S.; Liu, H.; Zhao, Q.; Shen, N.; Zhang, M. Filtration performances of different polysaccharides in microfiltration process. Processes 2019, 7, 897. [Google Scholar] [CrossRef]
- Meng, X.; Wang, F.; Meng, S.; Wang, R.; Mao, Z.; Li, Y.; Yu, M.; Wang, X.; Zhao, Q.; Yang, L. Novel surrogates for membrane fouling and the application of support vector machine in analyzing fouling mechanism. Membranes 2021, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zhao, X.H.; Wu, Y.H.; Bai, Y.; Ikuno, N.; Ishii, K.; Hu, H.Y. The molecular structures of polysaccharides affect their reverse osmosis membrane fouling behaviors. J. Membr. Sci. 2021, 625, 118984. [Google Scholar] [CrossRef]
- Ruth, B.F. Studies in filtration. III. Derivation of general filtration equations. Ind. Eng. Chem. 1935, 27, 708–723. [Google Scholar] [CrossRef]
- Katagiri, N.; Tomimatsu, K.; Date, K.; Iritani, E. Yeast cell cake characterization in alcohol solution for efficient microfiltration. Membranes 2021, 11, 89. [Google Scholar] [CrossRef]
- Katagiri, N.; Kuwajima, Y.; Kawahara, H.; Yamashita, R.; Iritani, E. Special features of microbial cake under high pressure conditions in microfiltration. Sep. Purif. Technol. 2022, 303, 122234. [Google Scholar] [CrossRef]
- Katagiri, N.; Shinoda, T.; Yamashita, R.; Fukuchi, S. Estimation of filtration behavior based on simplified evaluation of membrane fouling characteristics. J. Jpn. Soc. Water Environ. 2021, 44, 149–155. [Google Scholar] [CrossRef]
- Hwang, K.J.; Liao, C.Y.; Tung, K.L. Analysis of particle fouling during microfiltration by use of blocking models. J. Membr. Sci. 2007, 287, 287–293. [Google Scholar] [CrossRef]



| Membrane | Hilic- PVDF | Hbic- PVDF | Hilic- PTFE | Hbic- PTFE | MCE |
|---|---|---|---|---|---|
| n [-] | 1.05 | 1.05 | 1.02 | 1.03 | 1.32 |
| k [s1−n/cm2−n] | 0.0949 | 0.134 | 0.144 | 0.258 | 0.0246 |
| Membrane | Hilic- PVDF | Hbic- PVDF | Hilic- PTFE | Hbic- PTFE | MCE |
|---|---|---|---|---|---|
| n [-] | 1.86 | 1.85 | 1.50 | 1.50 | 1.98 |
| k [s1−n/cm2−n] | 0.00084 | 0.00725 | 0.00714 | 0.0384 | 0.00213 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katagiri, N.; Uchida, T.; Takahashi, H.; Iritani, E. An Evaluation of the Relationship between Membrane Properties and the Fouling Mechanism Based on a Blocking Filtration Model. Separations 2024, 11, 70. https://doi.org/10.3390/separations11030070
Katagiri N, Uchida T, Takahashi H, Iritani E. An Evaluation of the Relationship between Membrane Properties and the Fouling Mechanism Based on a Blocking Filtration Model. Separations. 2024; 11(3):70. https://doi.org/10.3390/separations11030070
Chicago/Turabian StyleKatagiri, Nobuyuki, Takehiro Uchida, Hironori Takahashi, and Eiji Iritani. 2024. "An Evaluation of the Relationship between Membrane Properties and the Fouling Mechanism Based on a Blocking Filtration Model" Separations 11, no. 3: 70. https://doi.org/10.3390/separations11030070





