Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bat Feces Preparation
2.2. Preparation of Standards
2.3. Determination of Antioxidant Capacity of Fecal Samples
2.4. Vitamin Analysis
2.4.1. Water-Soluble Vitamins
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Place 1 g of sample powder in a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 min, and then ultrasonicate for 15 min;
- (2)
- Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C.Mobile phase: as shown in Table 1;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 1.00 | 100 | 0 |
| 6.80 | 0 | 100 |
| 8.80 | 0 | 100 |
| 9.00 | 100 | 0 |
| 12.00 | 100 | 0 |
- Flow rate: 0.4 mL/min;
- Injection volume: 15 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 2.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| B1 (thiamine) | 265.00→122.10 | 265.00→144.10 |
| B2 (riboflavin) | 377.20→243.10 | 377.20→198.10 |
| B3 (nicotinamide) | 123.00→80.10 | 123.00→96.10 |
| B3 (nicotinic acid) | 123.90→80.10 | 123.90→78.10 |
| B5 (pantothenic acid) | 220.20→90.10 | 220.20→202.10 |
| B6 (pyridoxine) | 170.00→152.10 | 170.00→134.10 |
| B8 (biotin) | 245.20→227.20 | 245.20→123.10 |
| B9 (folic acid) | 441.90→295.10 | 441.90→176.20 |
| B12 (cyanocobalamin) | 678.60→147.10 | 678.60→359.20 |
2.4.2. Vitamin C
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Place 1 g of sample powder in a 50 mL centrifuge tube, add 9 mL of 10 mM ammonium acetate aqueous solution, vortex for 1 min, and then ultrasonicate for 15 min;
- (2)
- Add another 10 mL of chloroform (chloroform) to the centrifuge tube and vortex for 1 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, take out the supernatant, filter it with a 0.22 μm filter membrane, and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:LC analysis conditions:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C:Mobile phase: as shown in Table 3;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 2.40 | 100 | 0 |
| 4.40 | 89 | 11 |
| 4.60 | 70 | 30 |
| 6.50 | 68 | 32 |
| 6.70 | 0 | 100 |
| 7.00 | 100 | 0 |
- Flow rate: 0.2 mL/min;
- Injection volume: 5 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 4.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| C | 177.10→95.10 | 177.10→141.20 |
2.4.3. Fat-Soluble Vitamins
- (A)
- Liquid chromatography/tandem mass spectrometry (LC/MS/MS) equipment:
- (1)
- Quaternary pump: Shimadzu LC-20AD;
- (2)
- Autosampler: Shimadzu SIL-20AC;
- (3)
- Photodiode array detector: Shimadzu SPD-M20;
- (4)
- Mass detector: Shimadzu LCMS-8040.
- (B)
- Sample preparation:
- (1)
- Take 0.25 g of sample powder and place it in a 15 mL centrifuge tube, add 1.5 mL of pure water and 1.5 mL of methanol (methanol), shake with a vortex mixer for 1 min, and then shake with ultrasonic for 20 min;
- (2)
- Add another 10 mL of n-Hexane to the centrifuge tube and vortex for 5 min;
- (3)
- Centrifuge at 3500 rpm for 10 min, and place 1 mL of supernatant into a glass centrifuge tube;
- (4)
- Blow dry with nitrogen in a 40 °C water bath and add 1 mL of methanol (methanol) to dissolve and mix evenly;
- (5)
- Filter with a 0.22 μm filter membrane and use the filtrate as the test solution.
- (C)
- LC/MS/MS analysis method:Chromatography column: Raptor Biphenyl (2.7 μm, 100 × 2.1 mm);Column temperature: 35 °C;Mobile phase: as shown in Table 5;A: 5 mM ammonium acetate, 0.1% formic acid in water;B: 5 mM ammonium acetate, 0.1% formic acid in methanol.
| Time (min) | A, % | B, % |
|---|---|---|
| Initial | 100 | 0 |
| 2.40 | 100 | 0 |
| 4.40 | 89 | 11 |
| 4.60 | 70 | 30 |
| 6.50 | 68 | 32 |
| 6.70 | 0 | 100 |
| 7.00 | 100 | 0 |
- Flow rate: 0.4 mL/min;
- Injection volume: 5 μL.
- Mass spectrometry conditions:
- Ion source: electrospray ionization (ESI+);
- Ion source interface voltage (probe voltage): 4.5 kV;
- Nebulizing gas flow: nitrogen, 3.0 mL/min;
- Drying gas flow: 15.00 L/min;
- Collision gas: argon, 230 kPa;
- Desolventization tube temperature (DL temp.): 250 °C;
- Heating module temperature (heat block temp.): 400 °C;
- Quantitative ion pair: as shown in Table 6.
| Vitamin | Quantitative Ion Pair | Qualitative Ion Pair |
|---|---|---|
| Precursor Ion (m/z)→Product Ion (m/z) | Precursor Ion (m/z)→Product Ion (m/z) | |
| A | 269.30→93.10 | 269.30→119.10 |
| D3 | 385.20→367.40 | 385.20→259.30 |
| E | 431.10→165.20 | 431.10→137.10 |
| K1 | 451.30→187.20 | 451.30→185.10 |
2.5. Heavy Metals Analysis
- (A)
- Inductively coupled plasma–mass spectrometry (ICP-MS) conditions:Agilent 7500a.
- (B)
- Sample preparation:
- (1)
- Take 0.4 g of the sample powder and place it in a microwave digestion bottle, add 8 mL of nitric acid, let it stand for about 10 min, and then digest it in a microwave digester. The operating conditions of microwave digestion are as shown in the table below;
Stage # Max Power (W) Ramp (min) Temperature (°C) Hold (min) 1 1200 15 175 05:00 2 1200 5 200 15:00 - (2)
- After the digestion is completed, cool to room temperature and transfer to a 100 mL quantitative flask. Wash the microwave digestion flask with pure water. Put the washing liquid into the quantitative flask, dilute it with pure water to a constant volume, mix evenly, and filter through a 0.45 μm filter membrane. The filtrate was the finished product sample solution, and this solution was used as the test solution.
- (C)
- ICP-MS analysis method.
- (1)
- Method settings:Acquisition mode: spectrum;Peak pattern: full quant;Every mass integration time: 0.33 s;Repetition: three times.
- (2)
- Peristaltic pump program:Uptake speed: 0.35 rps;Uptake time: 30 s;Stabilization time: 30 s.
- (3)
- Analysis conditions.Plasma Parameters:
- Plasma radio frequency power: 500~1600 W, normal setting 1200 W;
- Sampling depth: 3.0–23.0 mm, normal setting 10 mm;
- Carrier gas flow rate: 0.00–2.00 L/min, normal setting is 1 L/min;
- Auxiliary gas flow rate: 0.00–2.00 L/min, normal setting is 0.22 L/min;
- Nebulizer pump: 0.00–0.50 rps, normal setting is 0.1 rps;
- Premix chamber temperature (S/C temp): 2 °C.
Ion Lenses:- Extract 1: −200–10 V, normal setting −120 V;
- Extract 2: −200–0 V, normal setting −39 V;
- Einzel 1,3: −200–100 V, normal setting −80 V;
- Einzel 2: −200–100 V, normal setting 8 V;
- Omega bias: −200–100 V, normal setting −41 V;
- Omega (+): −200–100 V, normal setting 9 V;
- Omega (−): −200–100 V, normal setting 9 V;
- QF focus: −200–100 V, normal setting 9 V;
- Plate bias: −50–50 V, normal setting −10 V.
2.6. Statistical Analysis
3. Results
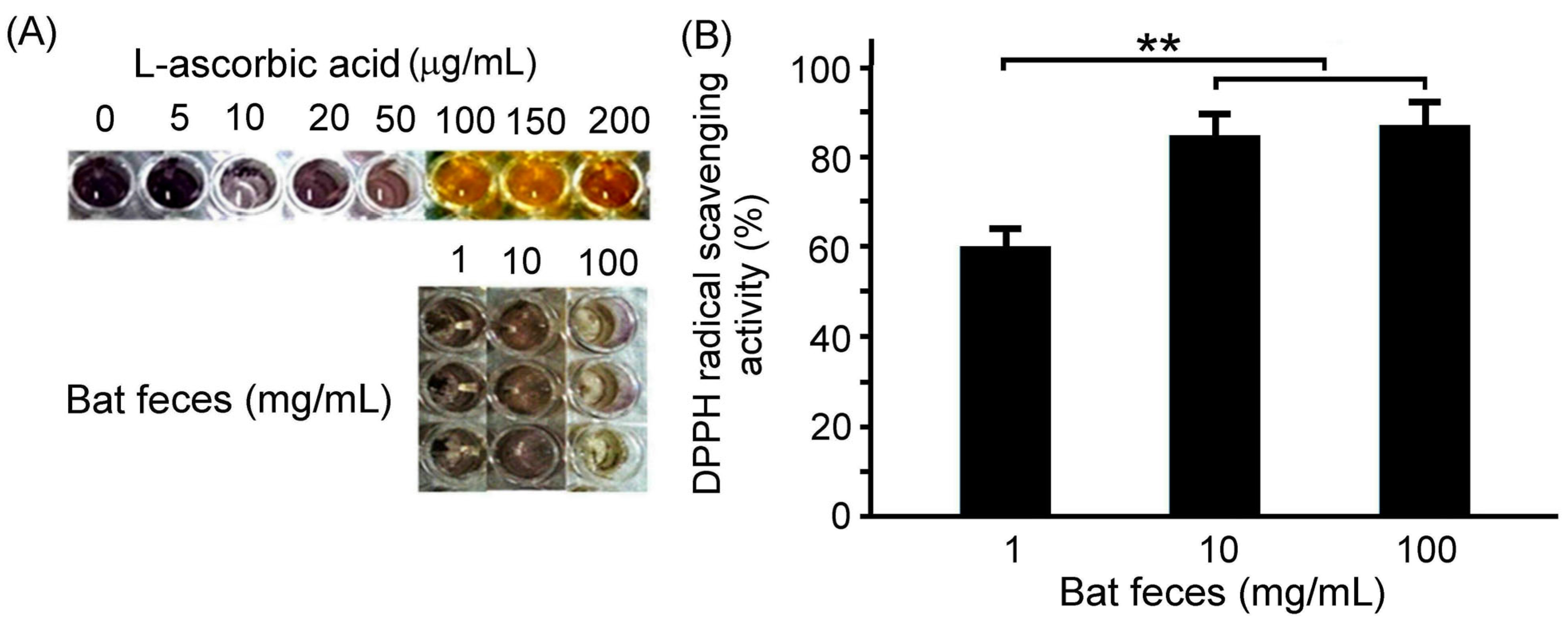
3.1. Antioxidant Capacity of Bat Feces Treatment
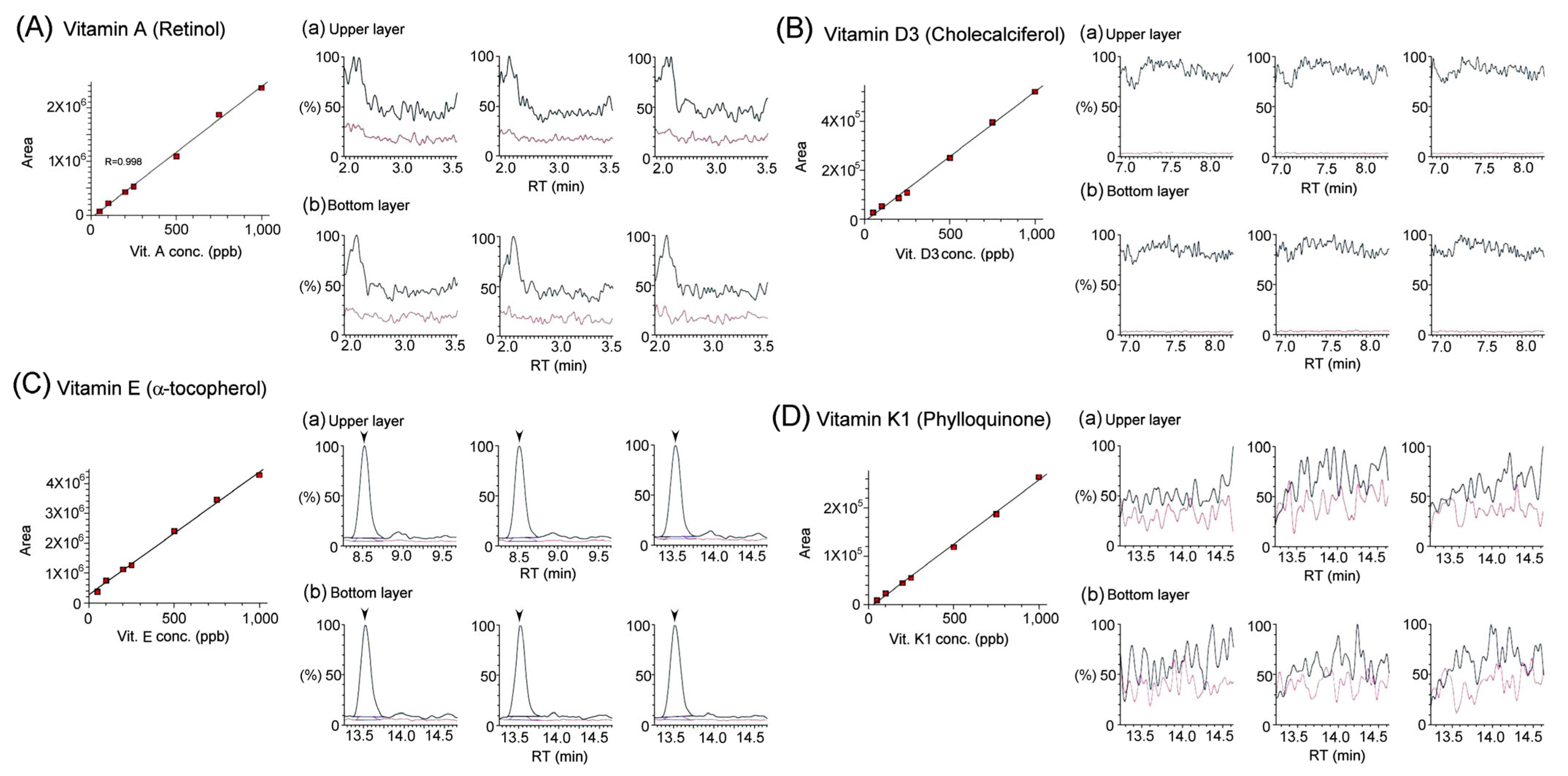
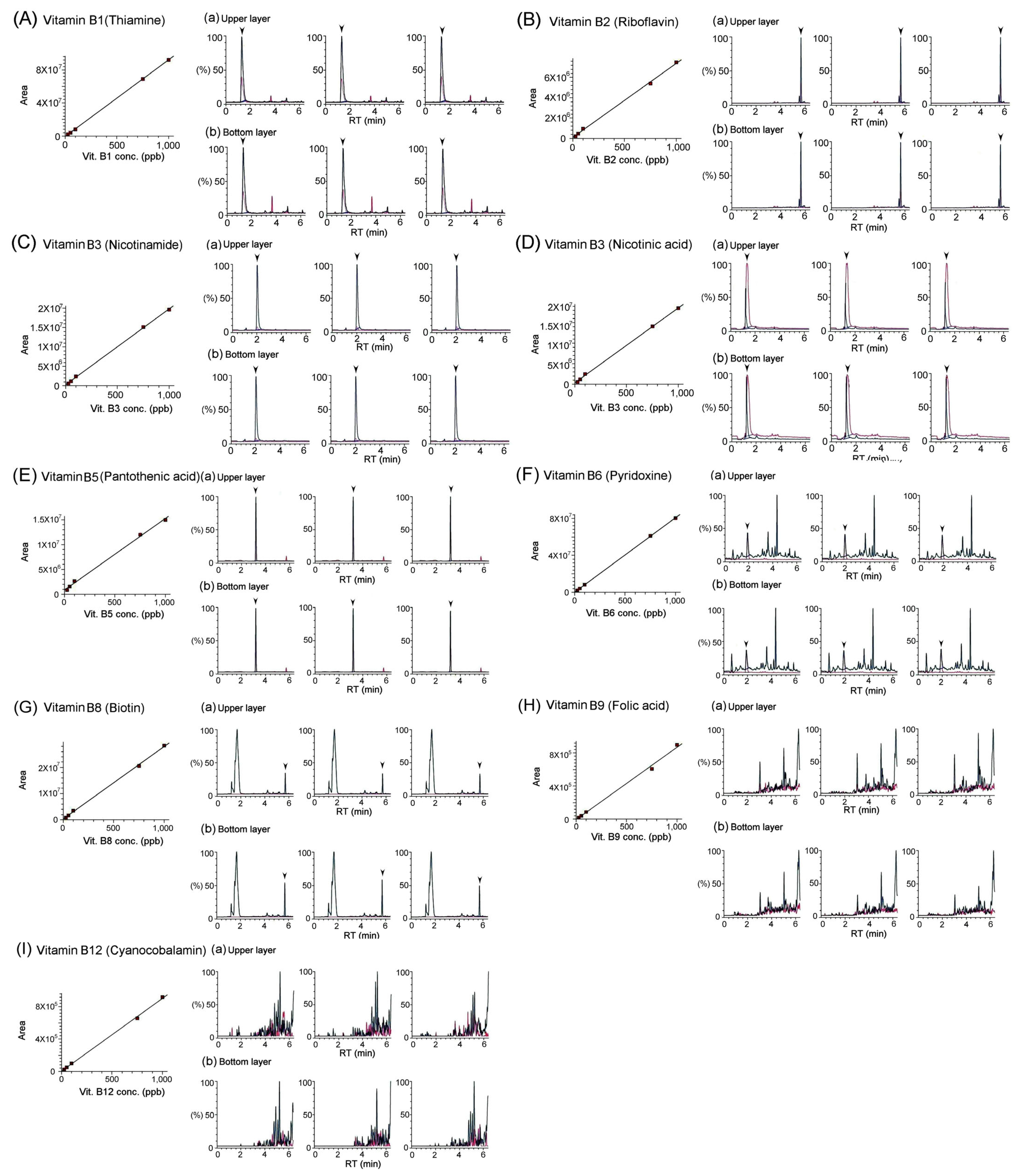
3.2. Quantification of Vitamins in Bat Feces Using LC/MS/MS Analysis
3.3. Quantification of Heavy Metals in Bat Feces Using ICP/MS Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jin, R.; Zhang, B.; Xue, C.M.; Liu, S.M.; Zhao, Q.; Li, K. [Classification of 365 Chinese medicines in Shennong’s Materia Medica Classic based on a semi-supervised incremental clustering method]. Zhong Xi Yi Jie He Xue Bao 2011, 9, 665–674. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Li, S.Z. Compendium of Materia Medica: Bencao Gangmu; Luo, X.W., Ed.; Beijing Foreign Languages Press: Beijing, China, 2003; ISBN 7119032607. [Google Scholar]
- Wu, Y. Rihuazi Materia Medica; Anhui Science and Technology Press: Hefei, China, 2005; ISBN 13:9787533732455. [Google Scholar]
- Dierenfeld, E.S.; Seyjagat, J. Plasma fat-soluble vitamin and mineral concentrations in relation to diet in captive pteropodid bats. J. Zoo Wildl. Med. 2000, 31, 315–321. [Google Scholar] [PubMed]
- Boyles, J.G.; Cryan, P.M.; McCracken, G.F.; Kunz, T.H. Conservation. Economic importance of bats in agriculture. Science 2011, 332, 41–42. [Google Scholar] [CrossRef] [PubMed]
- Russo, D.; Bosso, L.; Ancillotto, L. Novel perspectives on bat insectivory highlight the value of this ecosystem service in farmland: Research frontiers and management implications. Agric. Ecosyst. Environ. 2018, 266, 31–38. [Google Scholar] [CrossRef]
- Emerson, J.K.; Roark, A.M. Composition of guano produced by frugivorous, sanguivorous, and insectivorous bats. Acta Chiropterol. 2007, 9, 261–267. [Google Scholar] [CrossRef]
- Banskar, S.; Mourya, D.T.; Shouche, Y.S. Bacterial diversity indicates dietary overlap among bats of different feeding habits. Microb. Res. 2016, 182, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Kuang, T.T.; Qiu, S.; Xu, T.; Gang Huan, C.L.; Fan, G.; Zhang, Y. Fecal medicines used in traditional medical system of China: A systematic review of their names, original species, traditional uses, and modern investigations. Chin. Med. 2019, 14, 31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.M.; Fan, B.; Li, Y.L.; Zuo, Z.Y.; Li, G.Y. Oxidative Stress-Involved Mitophagy of Retinal Pigment Epithelium and Retinal Degenerative Diseases. Cell Mol. Neurobiol. 2023, 43, 3265–3276. [Google Scholar] [CrossRef] [PubMed]
- Gao, A.; Gong, J.; Zheng, H.; Jia, X.; Wang, M.; Wu, Y.; Zhang, X.; Chen, G.; Ni, S. Research on the traditional Chinese medicine bat feces. Jilin J. Tradit. Chin. Med. 2012, 32, 1047–1049. [Google Scholar]
- Steinert, R.E.; Lee, Y.K.; Sybesma, W. Vitamins for the Gut Microbiome. Trends Mol. Med. 2020, 26, 137–140. [Google Scholar] [CrossRef]
- Xia, Y.; Ji, C.; Li, M.; Zhang, W.; Cheng, X.; Qiu, Y.; Ge, W. Simultaneous quantification of seven B vitamins in human feces by stable isotope label-based high-performance liquid chromatography-tandem mass spectrometry. J. Pharm. Biomed. Anal. 2024, 237, 115784. [Google Scholar] [CrossRef] [PubMed]
- Magnúsdóttir, S.; Ravcheev, D.; de Crécy-Lagard, V.; Thiele, I. Systematic genome assessment of B-vitamin biosynthesis suggests co-operation among gut microbes. Front. Genet. 2015, 6, 148. [Google Scholar] [CrossRef] [PubMed]
- Alemanno, F.; Ghisi, D.; Westermann, B.; Bettoni, A.; Fanelli, A.; La Colla, L.; Danelli, G.; Cesana, B.M. The use of vitamin B1 as a perineural adjuvant to middle interscalene block for postoperative analgesia after shoulder surgery. Acta Biomed. 2016, 87, 22–27. [Google Scholar] [PubMed]
- Nebbioso, M.; Rusciano, D.; Pucci, B.; Zicari, A.M.; Grenga, R.; Pescocolido, N. Treat-ment of glaucomatous patients by means of food supplement to reduce the ocular dis-comfort: A double blind randomized trial. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1117–1122. [Google Scholar] [PubMed]
- Ren, X.; Chou, Y.; Wang, Y.; Jing, D.; Chen, Y.; Li, X. The Utility of Oral Vitamin B1 and Mecobalamin to Improve Corneal Nerves in Dry Eye Disease: An In Vivo Confocal Microscopy Study. Nutrients 2022, 14, 3750. [Google Scholar] [CrossRef]
- Pereira, A.; Adekunle, R.D.; Zaman, M.; Wan, M.J. Association Between Vitamin Deficiencies and Ophthalmological Conditions. Clin. Ophthalmol. 2023, 17, 2045–2062. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.A.; Harder, J.M.; Foxworth, N.E.; Cochran, K.E.; Philip, V.M.; Porciatti, V.; Smithies, O.; John, S.W. Vitamin B3 modulates mitochondrial vulnerability and prevents glaucoma in aged mice. Science 2017, 355, 756–760. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.M.J.; Barthes, S.; Féart, C.; Cougnard-Grégoire, A.; Korobelnik, J.F.; Rougier, M.B.; Delyfer, M.N.; Delcourt, C. B Vitamins and Incidence of Advanced Age-Related Macular Degeneration: The Alienor Study. Nutrients 2022, 14, 2821. [Google Scholar] [CrossRef]
- Das, T.; Sikdar, S.; Chowdhury, M.H.U.; Nyma, K.J.; Adnan, M. SARS-CoV-2 prevalence in domestic and wildlife animals: A genomic and docking based structural comprehensive review. Heliyon 2023, 9, e19345. [Google Scholar] [CrossRef] [PubMed]
- Gerbáčová, K.; Maliničová, L.; Kisková, J.; Maslišová, V.; Uhrin, M.; Pristaš, P. The Faecal Microbiome of Building-Dwelling Insectivorous Bats (Myotis myotis and Rhinolophus hipposideros) also Contains Antibiotic-Resistant Bacterial Representatives. Curr. Microbiol. 2020, 77, 2333–2344. [Google Scholar] [CrossRef]

| Vitamins | Bat Feces | ||
|---|---|---|---|
| Upper Layer | Bottom Layer | ||
| Water-Soluble Vitamins | B1(thiamine) | 3.44 ± 0.05 | 2.22 ± 0.02 |
| B2 (riboflavin) | 6.75 ± 0.34 | 2.37 ± 0.21 | |
| B3 (nicotinamide) | 52.53 ± 1.50 | 70.41 ± 1.46 | |
| B3 (nicotinic acid) | 19.67 ± 0.36 | 16.13 ± 0.49 | |
| B5 (pantothenic acid) | 62.63 ± 2.34 | 41.38 ± 0.33 | |
| B6 (pyridoxine) | 0.05 ± 0.02 | 0.04 ± 0.02 | |
| B8 (biotin) | N/A | N/A | |
| B9 (folic acid) | N/A | N/A | |
| B12 (cyanocobalamin) | N/A | N/A | |
| C (ascorbic acid) | N/A | N/A | |
| Fat-Soluble Vitamins | A (retinol) | N/A | N/A |
| D3 (cholecalciferol) | N/A | N/A | |
| E (α-tocopherol) | N/A | N/A | |
| K1 (phylloquinone) | N/A | N/A | |
| Types of Heavy Metals | Heavy Metals in Bat Feces (ppm) | Limitation Standards of Heavy Metals in TCM (ppm) |
|---|---|---|
| Chromium (Cr) | 2.87 ± 0.38 | -- |
| Manganese (Mn) | 55.53 ± 4.48 | -- |
| Copper (Cu) | 46.25 ± 3.51 | -- |
| Arsenic (As) | 5.57 ± 0.68 | 2.0 |
| Cadmium (Cd) | 0.39 ± 0.07 | 1.0 |
| Mercury (Hg) | 0.33 ± 0.07 | 0.2 |
| Lead (Pb) | 2.29 ± 0.37 | 5.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, K.-T.; Lin, C.-L.; Chuang, W.-C.; Lee, M.-C.; Chen, L.-W.; Wu, C.-H. Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine. Separations 2024, 11, 76. https://doi.org/10.3390/separations11030076
Chung K-T, Lin C-L, Chuang W-C, Lee M-C, Chen L-W, Wu C-H. Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine. Separations. 2024; 11(3):76. https://doi.org/10.3390/separations11030076
Chicago/Turabian StyleChung, Kou-Toung, Ching-Lung Lin, Wu-Chang Chuang, Ming-Chung Lee, Li-Wen Chen, and Chung-Hsin Wu. 2024. "Pharmaceutical Screening of Bat Feces and Their Applications and Risks in Traditional Chinese Medicine" Separations 11, no. 3: 76. https://doi.org/10.3390/separations11030076






