Construction of High-Activity Nano-NiTiO3/g-C3N4 Composite Catalysts for Enhanced Photodegradation Activities under Visible Light
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials and Reagents
2.2. Synthesis of Nano-NiTiO3
2.3. Synthesis of Nanostructures g-C3N4 and Bulk g-C3N4
2.4. Synthesis of Nano-NiTiO3/g-C3N4 and Bulk-NiTiO3/g-C3N4 Composite Catalysts
2.5. Characterization
2.6. Photocatalytic Activity Evaluation
2.7. Photocatalytic Mechanism Evaluation
3. Results and Discussion
3.1. Structure and Morphology of Photocatalysts
3.1.1. XRD Analysis
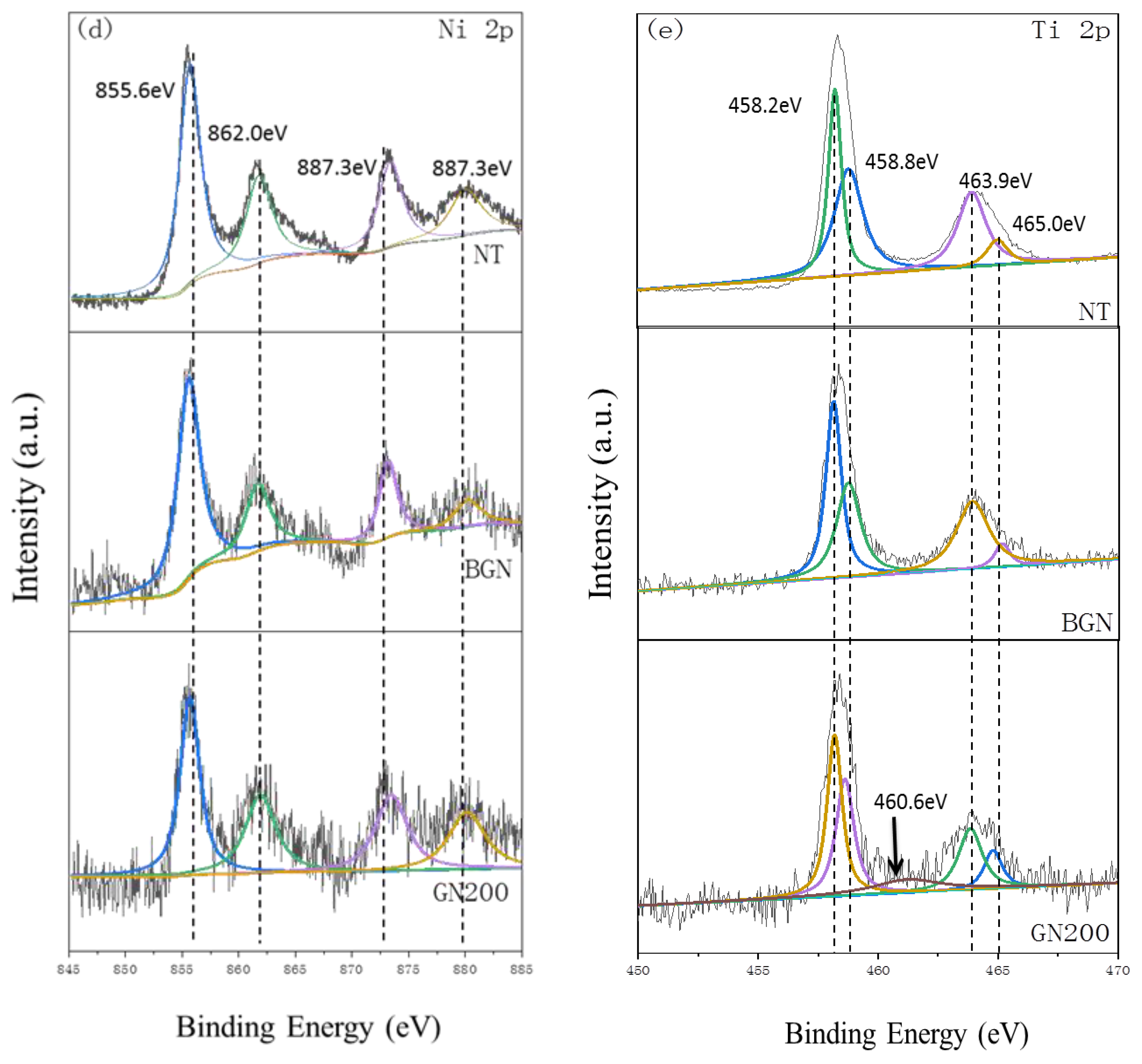
3.1.2. XPS Analysis
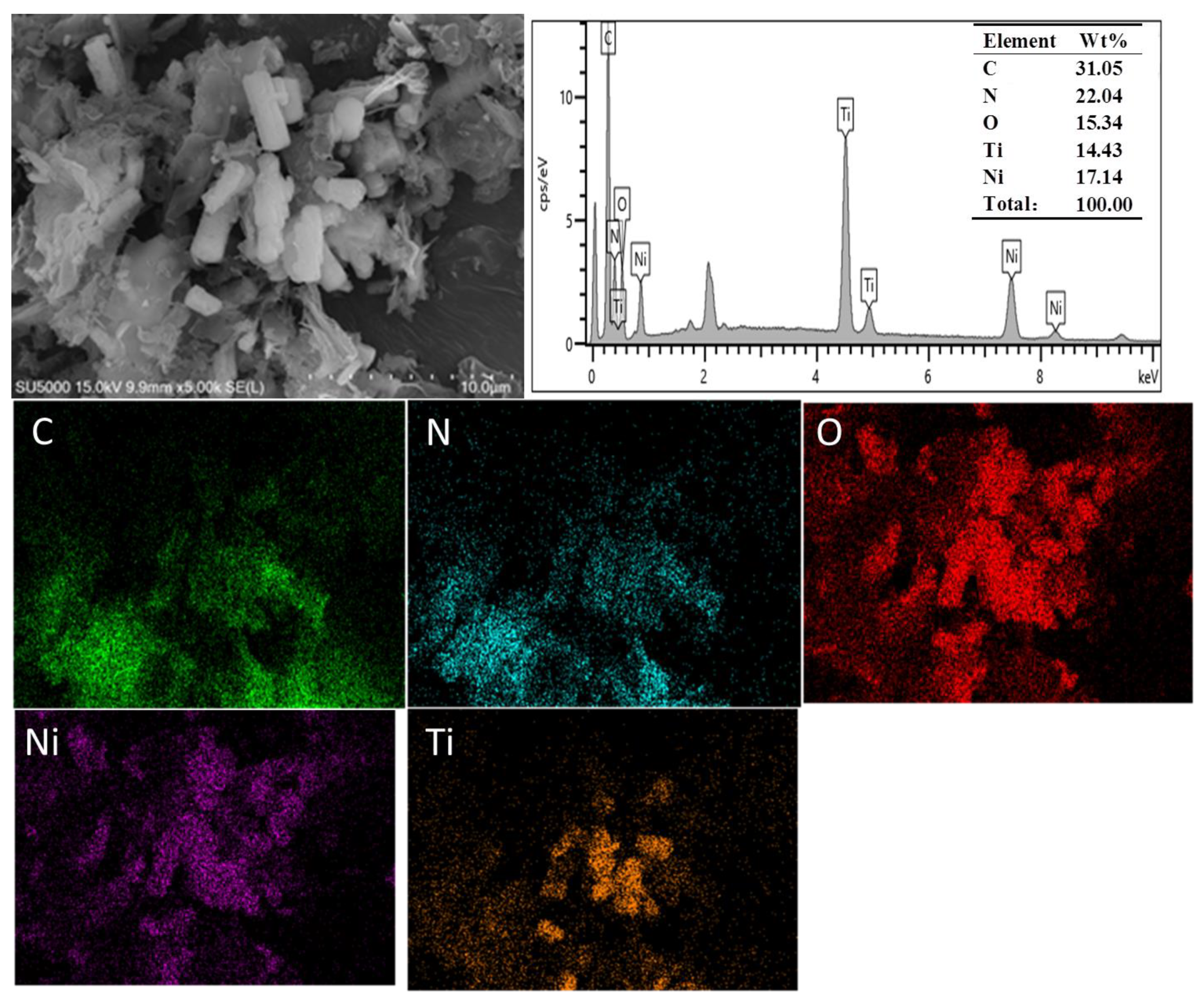
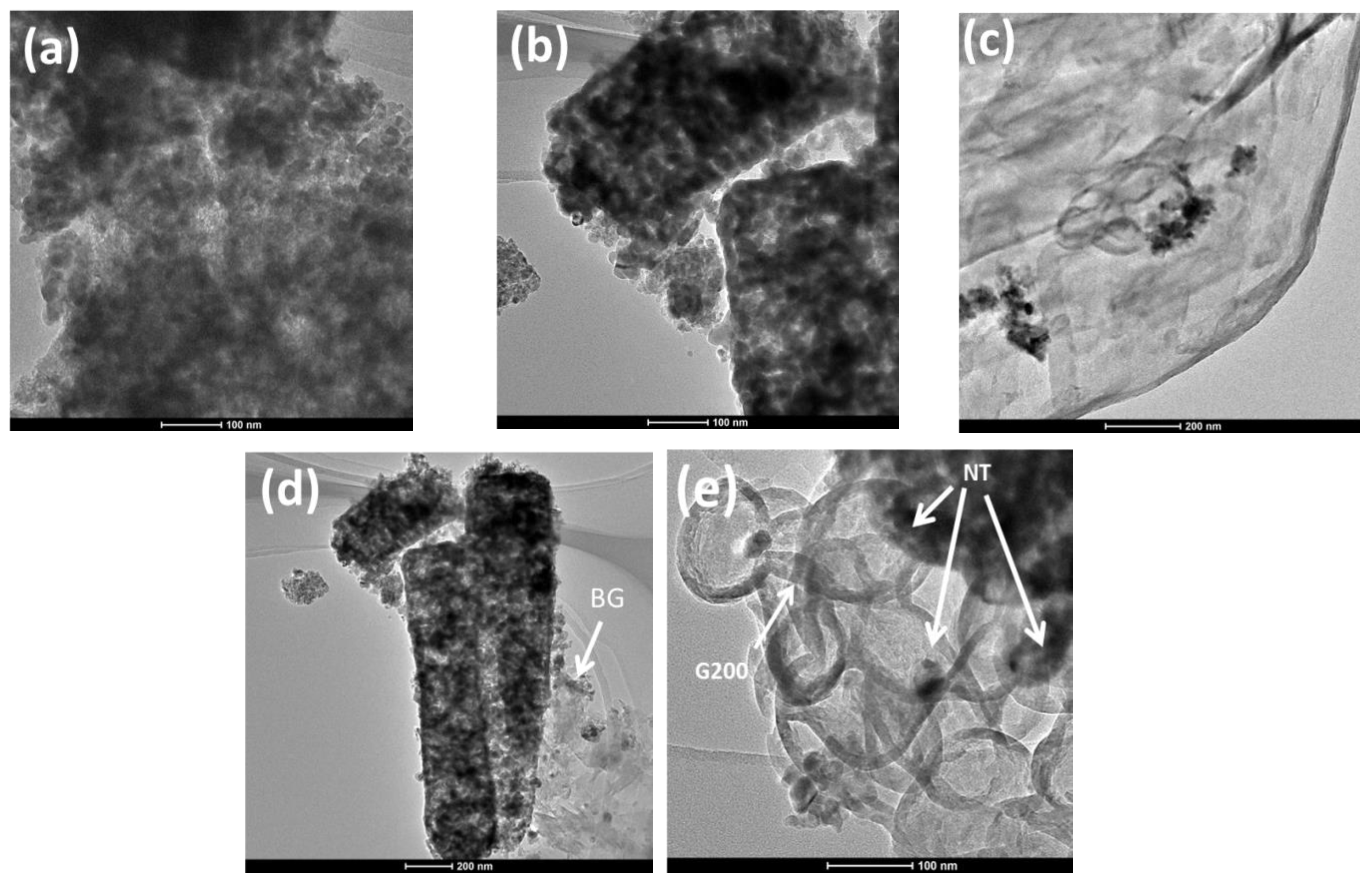
3.1.3. Morphology
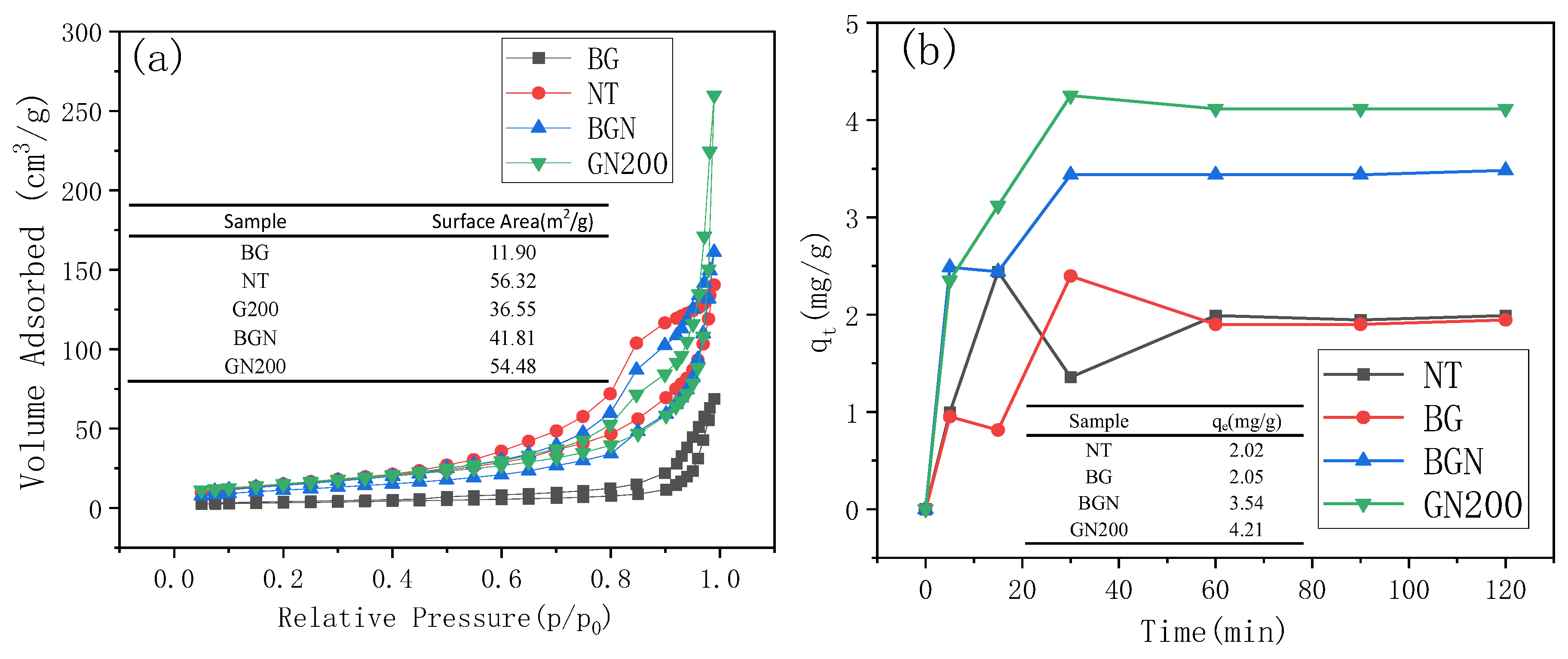
3.1.4. Brunauer–Emmett–Teller and Adsorption Capacities
3.2. Photoelectric Property
3.2.1. Uv–Vis Absorption Spectra
3.2.2. Photoluminescence Spectra
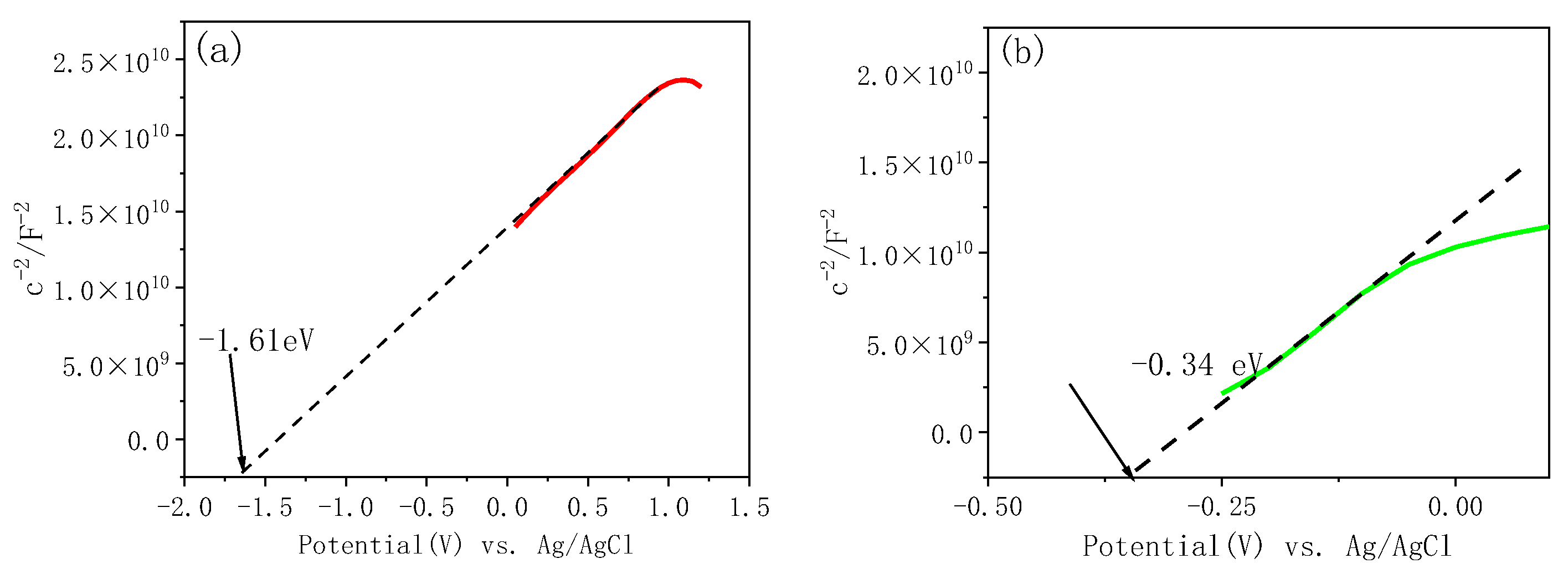
3.2.3. Mott–Schottky Analysis
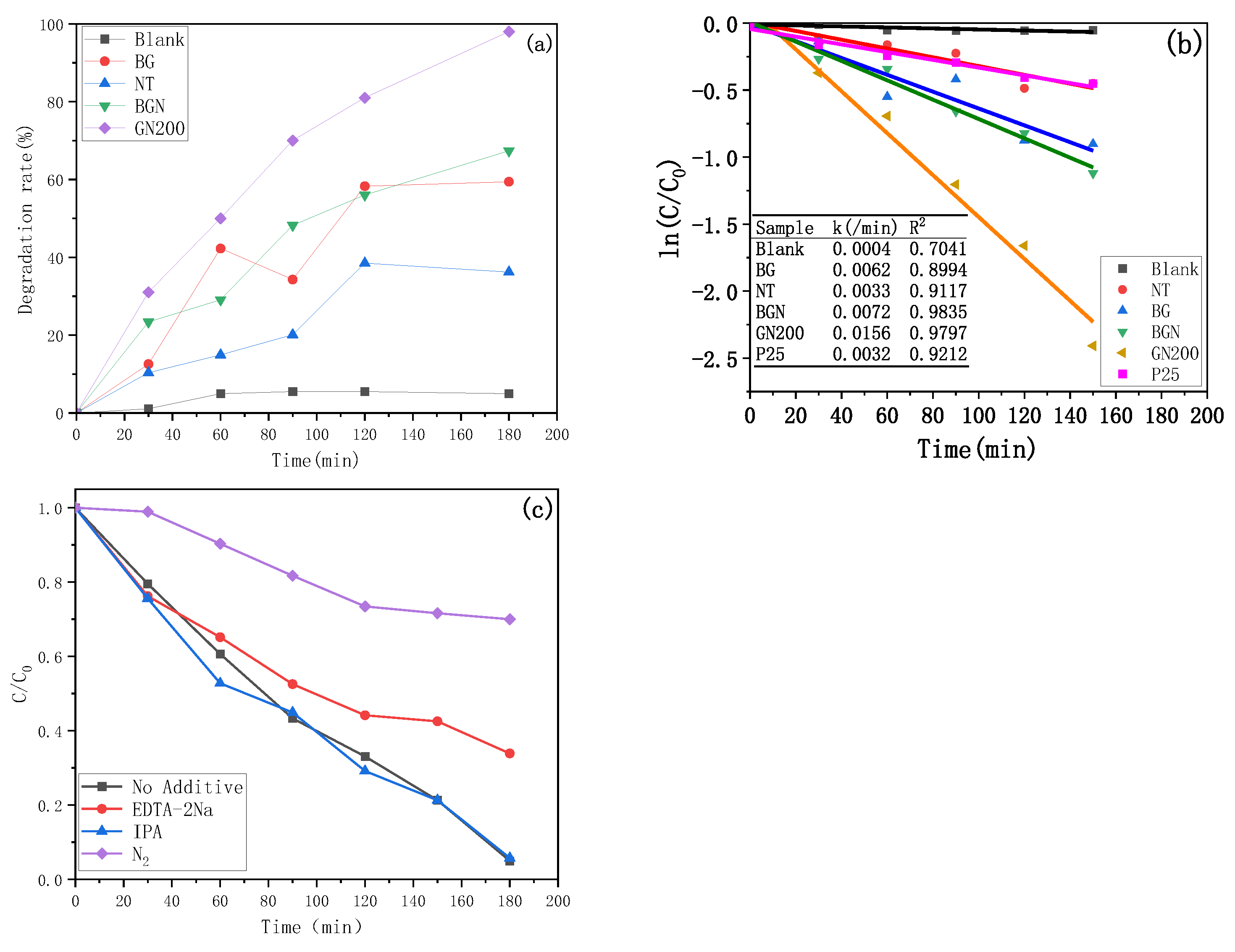
3.3. Photocatalytic Evaluation
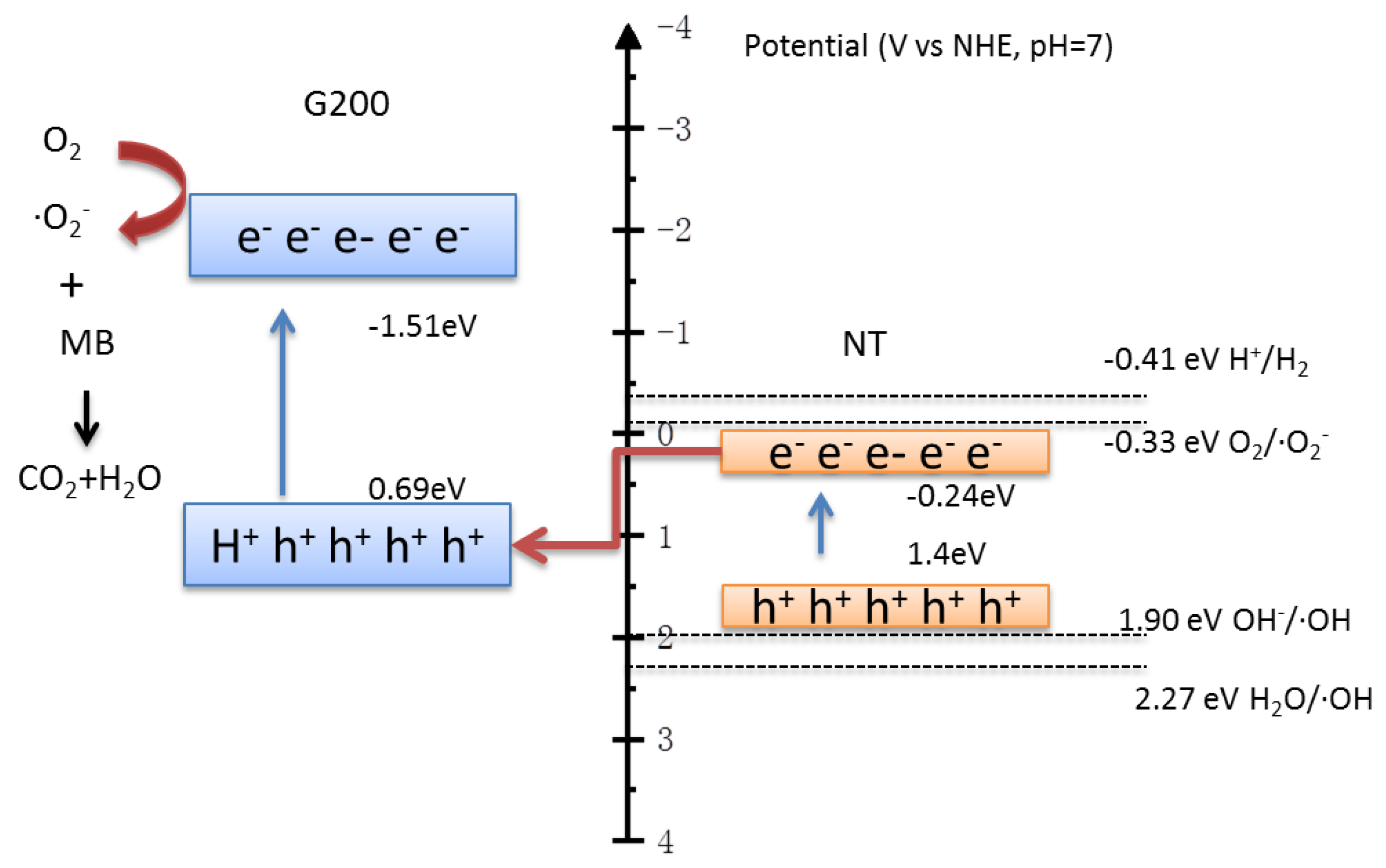
3.4. Photocatalytic Mechanisms
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, S.; Han, X.; Zhang, Y.; Tian, N.; Ma, T.; Huang, H. Inside-and-out Semiconductor Engineering for CO2 Photoreduction: From Recent Advances to New Trends. Small Struct. 2020, 2, 2000061. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Ong, W.J.; Tan, L.L.; Ng, Y.H.; Yong, S.T.; Chai, S.P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer to Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, H.; Antonietti, M. Graphitic nitride “reloaded”: Emerging applications beyond (photo)catalysis. Chem. Soc. Rev. 2016, 45, 2308. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Sun, Y.; Dong, F. Graphitic carbon nitride based nanocomposites: A review. Nanoscale 2014, 7, 15–37. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.P.; Wang, Y.; Chen, J.W.; Jiang, Y.W.; Kiani, M.; Li, B.Q.; Wang, R.L. Fabrication of High-activity Hybrid NiTiO3/g-C3N4 Heterostructured Photocatalysts for Water Splitting to Enhanced Hydrogen Production. Ceram. Int. 2016, 42, 12297–12305. [Google Scholar] [CrossRef]
- Ye, R.Q.; Fang, H.B.; Zheng, Y.Z.; Li, N.; Wang, Y.; Tao, X. Fabrication of CoTiO3/g-C3N4 Hybrid Photocatalysts with Enhanced H2 Evolution: Z-Scheme Photocatalytic Mechanism Insight. ACS Appl. Mater. Interfaces 2016, 8, 13879–13889. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Wang, B.; Zhao, Y.; Hu, C.G.; Qu, L.T. A Graphitic-C3N4 “Seaweed” Architecture for Enhanced Hydrogen Evolution. Angew. Chem. 2015, 54, 11595–11599. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, L.; Shi, R.; Zhu, Y. Chemical exfoliation of graphitic carbon nitride for efficient heterogeneous photocatalysis. J. Mater. Chem. A 2013, 1, 14766–14772. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, X.Q.; Xie, C.F.; Long, J.Q.; Wang, Y.Q.; Wei, L.; Yang, X.D. Preparation of g-C3N4 with high specific surface area and photocatalytic stability. J. Electron. Mater. 2021, 55, 1067–1074. [Google Scholar] [CrossRef]
- Groenewolt, M.; Antonietti, M. Synthesis of g-C3N4 Nanoparticles in Mesoporous Silica Host Matrices. Adv. Mater. 2005, 17, 1789–1792. [Google Scholar] [CrossRef]
- Shi, W.; Song, S.; Zhang, H. Hydrothermal synthetic strategies of inorganic semiconducting nanostructures. Chem. Soc. Rev. 2013, 42, 5714–5743. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.; Li, C.; Fang, Z.; Luo, B.; Shi, W. Rational synthesis of ultrathin graphitic carbon nitride nanosheets for efficient photocatalytic hydrogen evolution. Carbon 2017, 121, 463–471. [Google Scholar] [CrossRef]
- Jung, H.; Pham, T.T.; Shin, E.W. Interactions between ZnO nanoparticles and amorphous g-C3N4 nanosheets in thermal formation of g-C3N4/ZnO composite materials: The annealing temperature effect. Appl. Surf. Sci. 2018, 458, 369–381. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Zou, Z.; Han, X. Graphitic-C3N4-Hybridized TiO2 Nanosheets with Reactive {001} Facets to Enhance the UV-And Visible-Light Photocatalytic Activity. J. Hazard. Mater. 2014, 268, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Chang, B.; Tian, Y.; Xi, F.; Dong, X. Novel C3N4–CdS Composite Photocatalysts with Organic Inorganic Heterojunctions: In Situ Synthesis, Exceptional Activity, High Stability and Photocatalytic Mechanism. J. Mater. Chem. A 2013, 1, 3083–3090. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Ren, Z.R.; Du, S.; Meng, X.; Tian, G.; Pan, K.; Wang, G.; Fu, H. Facile preparation of porous NiTiO3 nanorods with enhanced visible-light-driven photocatalytic performance. J. Mater. Chem. 2012, 22, 16471–16476. [Google Scholar] [CrossRef]
- Inceesungvorn, B.; Teeranunpong, T.; Nunkaew, J.; Suntalelat, S.; Tantraviwat, D. Novel NiTiO3/Ag3VO4 composite with enhanced photocatalytic performance under visible light. Catal. Commun. 2014, 54, 35–38. [Google Scholar] [CrossRef]
- Wang, X.; Maeda, K.; Thomas, A.; Takanabe, K.; Xin, G.; Carlsson, J.M.; Domen, K.; Antonietti, M. A metal-free polymeric photocatalyst for hydrogen production from water under visible light. Nat. Mater. 2009, 8, 76–80. [Google Scholar] [CrossRef]
- Qu, Y.; Zhou, W.; Jiang, L.; Fu, H. Novel heterogeneous CdS nanoparticles/NiTiO3 nanorods with enhanced visible-light-driven photocatalytic activity. RSC Adv. 2013, 3, 18305–18310. [Google Scholar] [CrossRef]
- Jiang, L.; Yuan, X.; Pan, Y.; Liang, J.; Zeng, G.; Wu, Z.; Wang, H. Doping of graphitic carbon nitride for photocatalysis: A reveiw. Appl. Catal. B Environ. 2017, 217, 388–406. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, X.; Wang, H.; Chen, X.; Wu, Z.; Jiang, L.; Xiong, W.; Zhang, Y.; Zeng, G. One-step calcination method for synthesis of mesoporous g-C3N4/NiTiO3 heterostructure photocatalyst with improved visible light photoactivity. RSC Adv. 2015, 5, 95643–95648. [Google Scholar] [CrossRef]
- Yuan, P.-H.; Fan, C.-M.; Ding, G.-Y.; Wang, Y.-F.; Zhang, X.-C. Preparation and photocatalytic properties of ilmenite NiTiO3 powders for degradation of humic acid in water. Int. J. Miner. Met. Mater. 2012, 19, 372–376. [Google Scholar] [CrossRef]
- Huang, Z.; Zeng, X.; Li, K.; Gao, S.; Wang, Q.; Lu, J. Z-scheme NiTiO3/g-C3N4 heterojunctions with enhanced photoelectrochemical and photocatalytic performances under visible LED light irradiation. ACS Appl. Mater. Interfaces 2017, 9, 41120–41125. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, B.; Dou, M.; Huang, X.; Ma, Z. A porous g-C3N4 nanosheets containing nitrogen defects for enhanced photocatalytic removal meropenem: Mechanism, degradation pathway and DFT calculation. Environ. Res. 2020, 184, 109339. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Jin, J.; Liu, C.; Xu, S.; Yao, C.; Chen, Z.; Li, Z. Hydrothermal fabrication of alpha-SnWO4/C3N4 heterostructure with enhanced visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2017, 28, 11279–11283. [Google Scholar] [CrossRef]
- Huang, T.; Pan, S.; Shi, L.; Yu, A.; Wang, X.; Fu, Y. Hollow porous prismatic graphitic carbon nitride with nitrogen vacancies and oxygen doping: A high-performance visible light-driven catalyst for nitrogen fixation. Nanoscale 2019, 12, 1833–1841. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.Y.; Xu, J.H.; He, M.Q.; Zhang, X.Q. Preparation of 2D graphite phase carbon nitride nanosheets and their photocatalytic performance. Fine Chem. 2021, 1, 83–90. [Google Scholar]
- Pham, T.-T.; Nguyen-Huy, C.; Lee, H.-J.; Nguyen-Phan, T.-D.; Son, T.-H.; Kim, C.-K.; Shin, E.-W. Cu-doped TiO2/reduced graphene oxide thin-film photocatalysts: Effect of Cu content upon methylene blue removal in water. Ceram. Int. 2015, 41, 11184–11193. [Google Scholar] [CrossRef]
- Zang, Y.P.; Li, L.P.; Li, X.G.; Lin, R.; Li, G.S. Synergistic Collaboration of g-C3N4/SnO2 Composites for Enhanced Visible-light Photocatalytic Activity. Chem. Eng. J. 2014, 246, 277–286. [Google Scholar] [CrossRef]
- Lin, Q.; Li, L.; Liang, S.; Liu, M.; Bi, J.; Wu, L. Efficient synthesis of monolayer carbon nitride 2D nanosheet with tunable concentration and enhanced visible-light photocatalytic activities. Appl. Catal. B Environ. 2015, 163, 135–142. [Google Scholar] [CrossRef]
- Ding, W.; Liu, S.; He, Z. One-step synthesis of graphitic carbon nitride nanosheets for efficient catalysis of phenol removal under visible light. Chin. J. Catal. 2017, 38, 1711–1718. [Google Scholar] [CrossRef]
- Nguyen, T.K.A.; Pham, T.-T.; Nguyen-Phu, H.; Shin, E.W. The effect of graphitic carbon nitride precursors on the photocatalytic dye degradation of water-dispersible graphitic carbon nitride photocatalysts. Appl. Surf. Sci. 2020, 537, 148027. [Google Scholar] [CrossRef]
- Pham, T.T.; Shin, E.W. Influence of g-C3N4 Precursors in g-C3N4/NiTiO3 Composites on Photocatalytic Behavior and the Interconnection between g-C3N4 and NiTiO3. Langmuir 2018, 34, 13144–13154. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-R.; Jo, W.-K. Application of a photostable silver-assisted Z-scheme NiTiO3 nanorod/g-C3N4 nanocomposite for efficient hydrogen generation. Int. J. Hydrogen Energy 2018, 44, 801–808. [Google Scholar] [CrossRef]
- Wang, X.J.; Yang, W.Y.; Li, F.T.; Xue, Y.B.; Liu, R.H.; Hao, Y.J. In Situ Microwave-assisted Synthesis of Porous NiTiO3/g-C3N4 Heterojunctions with Enhanced Visible-light Photocatalytic Properties. Ind. Eng. Chem. Res. 2013, 52, 17140–17150. [Google Scholar] [CrossRef]
- Oktay, S.; Kahraman, Z.; Urgen, M.; Kazmanli, K. XPS investigations of tribolayers formed on Ti-N and (Ti,Re)N coatings. Appl. Surf. Sci. 2015, 328, 255–261. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Iwuozor, K.O.; Igwegbe, C.A.; Adeniyi, A.G. Verification of pore size effect on aqueous-phase adsorption kinetics: A case study of methylene blue. Colloids Surf. A Physicochem. Eng. Asp. 2021, 626, 127119. [Google Scholar] [CrossRef]
- Zheng, J.; Lei, Z. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B Environ. 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Yang, B.; Wang, Z.; Zhao, J.; Sun, X.; Wang, R.; Liao, G.; Jia, X. 1D/2D carbon-doped nanowire/ultra-thin nanosheet g-C3N4 isotype heterojunction for effective and durable photocatalytic H2 evolution. Int. J. Hydrogen Energy 2021, 46, 25436–25447. [Google Scholar] [CrossRef]
- Luo, J.; Steier, L.; Son, M.K.; Schreier, M.; Mayer, M.T.; Grätzel, M. Cu2O nanowire photocathodes for efficient and durable solar water splitting. Nano Lett. 2016, 16, 1848–1857. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhou, G.; Wang, Y.; Suvorova, A.; Wang, S. A New Metal-Free Carbon Hybrid for Enhanced Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 16745–16754. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.J.; Xie, F.Z.; Zhu, S.F.; Liu, J.; Zhang, J.; Mei, S.F.; Zhao, W. A Novel Photofunctional g-C3N4/Ag3PO4 Bulk Heterojunction for Decolorization of RhB. Chem. Eng. J. 2013, 228, 435–441. [Google Scholar] [CrossRef]
- Dao, D.Q.; Nguyen, T.K.A.; Pham, T.T.; Shin, E.W. Synergistic effect on photocatalytic activity of co-doped NiTiO3/g-C3N4 composites under visible light irradiation. Catalysts 2020, 10, 1332. [Google Scholar] [CrossRef]
- Zheng, D.; Pang, C.; Liu, Y.; Wang, X. Shell-engineering of hollow g-C3N4 nanospheres via copolymerization for photocatalytic hydrogen evolution. Chem. Commun. 2015, 51, 9706–9709. [Google Scholar] [CrossRef]
- Yu, W.; Xu, D.; Peng, T. Enhanced photocatalytic activity of g-C3N4 for selective CO2 reduction to CH3OH via facile coupling of ZnO: A direct Z-scheme mechanism. J. Mater. Chem. A 2015, 3, 19936–19947. [Google Scholar] [CrossRef]
- Zhang, W. Studies on the Photo-Electrocatalytic Water Splitting Performance of BiVO4-Cu2O Photoelectrochemical Cell. Ph.D Thesis, Northwest University, Xi’an, China, 2021. [Google Scholar] [CrossRef]





| Photocatalyst | Precursor | Synthesis Method | Specific Surface Area | Reference |
|---|---|---|---|---|
| g-C3N4 | melamine | Calcining method | 11.5 m2/g | [25] |
| melamine | Calcining method | 24.35 m2/g | [26] | |
| dicyandiamidine | Calcining method | 7.24 m2/g | [27] | |
| melamine | Hydrothermal methods | 28.2 m2/g | [28] | |
| NiTiO3 | nickel acetate, etra-n-butyltitanate | Facile ethylene glycol-mediated method | 23.1 m2/g | [17] |
| nickel acetate, etra-n-butyltitanate | Sol-gel method | 11.4 m2/g | [24] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Yang, Z.; Wang, K.; Zhang, L.; Shi, L.; Qadeer, A.; Dong, J.; Ren, H. Construction of High-Activity Nano-NiTiO3/g-C3N4 Composite Catalysts for Enhanced Photodegradation Activities under Visible Light. Separations 2024, 11, 77. https://doi.org/10.3390/separations11030077
Li D, Yang Z, Wang K, Zhang L, Shi L, Qadeer A, Dong J, Ren H. Construction of High-Activity Nano-NiTiO3/g-C3N4 Composite Catalysts for Enhanced Photodegradation Activities under Visible Light. Separations. 2024; 11(3):77. https://doi.org/10.3390/separations11030077
Chicago/Turabian StyleLi, Da, Zhan Yang, Kun Wang, Lan Zhang, Linglong Shi, Abdul Qadeer, Jiao Dong, and Haoyu Ren. 2024. "Construction of High-Activity Nano-NiTiO3/g-C3N4 Composite Catalysts for Enhanced Photodegradation Activities under Visible Light" Separations 11, no. 3: 77. https://doi.org/10.3390/separations11030077





