Optimized Centrifugal Partition Chromatography (CPC) Protocol for Isolation of Urease Inhibitors: Magnoflorine and Berberine from Berberis vulgaris Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Extractions
2.2. Fractionation of the Extract by CPC
2.2.1. The Selection of the Biphasic Solvent Composition for the Fractionation of Berberis vulgaris Extracts
2.2.2. Centrifugal Partition Chromatography-Based Fractionation of Alkaloids from the Methanolic Extracts of Berberis vulgaris Root and Stem
2.3. Chromatographic Analysis of the Extract by HPLC-ESI-Q-TOF-MS
2.4. The Antibacterial Assay against H. pylori
2.5. Assay of Urease Inhibitory Activity
3. Results and Discussion
3.1. HPLC-ESI-QTOF-MS/MS Fingerprinting of the Extracts
3.2. Centrifugal Partition Chromatography as a Tool for the Fractionation of Extracts
3.3. Helicobacter pylori and Urease Inhibition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhardwaj, D.; Kaushik, N. Phytochemical and pharmacological studies in genus Berberis. Phytochem. Rev. 2012, 11, 523–542. [Google Scholar] [CrossRef]
- Rahimi-Madiseh, M.; Lorigoini, Z.; Zamani-Gharaghoshi, H.; Rafieian-Kopaei, M. Berberis vulgaris: Specifications and traditional uses. Iran. J. Basic Med. Sci. 2017, 20, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.-X.; Li, G.-Y.; Lü, S.-W.; Su, H.; Xu, D.; Guo, Y.-Y.; Kuang, H.-X.; Wang, Q.-H. Analysis of bioactive components and pharmacokinetics of Caulophyllum robustum in rat plasma after oral administration by UPLC-ESI-MS/MS. J. Asian Nat. Prod. Res. 2018, 23, 258–270. [Google Scholar] [CrossRef]
- Kukula-Koch, W. The elevation of LC-ESI-Q-TOF-MS response in the analysis of isoquinoline alkaloids from some Papaveraceae and Berberidaceae representatives. J. Anal. Methods Chem. 2017, 2017, 8384107. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, Z.; Zhang, Y.; Zhang, X.; Zhang, Z.; Liao, Y.; Zhang, B. A new method for simultaneous determination of phenolic acids, alkaloids and limonoids in Phellodendri amurensis cortex. Molecules 2019, 24, 709. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Tong, C.; Fu, Q.; Xu, J.; Shi, S.; Xiao, Y. Identification of minor lignans, alkaloids, and phenylpropanoid glycosides in Magnolia officinalis by HPLC-DAD-QTOF-MS/MS. J. Pharm. Biomed. Anal. 2019, 170, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-M.; Wang, L.-J.; Pang, H.-Q.; Guo, Y.; Xiao, P.T.; Chu, C.; Guo, L.; Liu, E.-H. Rapid profiling of alkaloid analogues in Sinomenii caulis by an integrated characterization strategy and quantitative analysis. J. Pharm. Biomed. Anal. 2019, 174, 376–385. [Google Scholar] [CrossRef]
- Hung, T.M.; Na, M.; Min, B.S.; Zhang, X.; Lee, I.; Ngoc, T.M.; Thuong, P.T.; Sok, D.E.; Bae, K. Protective effect of magnoflorine isolated from coptidis rhizoma on Cu2+-induced oxidation of human low density lipoprotein. Planta Med. 2007, 73, 1281–1284. [Google Scholar] [CrossRef]
- Ahmad, W.; Jantan, I.; Kumolosasi, E.; Haque, M.A.; Bukhari, S.N.A. Immunomodulatory effects of Tinospora crispa extract and its major compounds on the immune functions of RAW 264.7 macrophages. Int. Immunopharmacol. 2018, 60, 141–151. [Google Scholar] [CrossRef]
- Wang, L.J.; Jiang, Z.M.; Xiao, P.T.; Sun, J.B.; Bi, Z.M.; Liu, E.H. Identification of anti-inflammatory components in Sinomenii Caulis based on spectrum-effect relationship and chemometric methods. J. Pharm. Biomed. Anal. 2019, 167, 38–48. [Google Scholar] [CrossRef]
- Mohamed, S.M.; Hassan, E.M.; Ibrahim, N.A. Cytotoxic and antiviral activities of aporphine alkaloids of Magnolia grandiflora L. Nat. Product Res. 2010, 24, 1395–1402. [Google Scholar] [CrossRef]
- Chen, J.H.; Du, Z.Z.; Shen, Y.M.; Yang, Y.P. Aporphine alkaloids from Clematis parviloba and their antifungal activity. Arch. Pharmacal Res. 2009, 32, 3–5. [Google Scholar] [CrossRef]
- Jenkins, A.L.; Hedgepeth, W.A. Analysis of chiral pharmaceuticals using HPLC with CD detection. Chirality 2005, 17, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Xie, X.; Cao, P. Magnoflorine improves sensitivity to doxorubicin (DOX) of breast cancer cells via inducing apoptosis and autophagy through AKT/mTOR and p38 signaling pathways. Biomed. Pharmacother. 2020, 121, 109139. [Google Scholar] [CrossRef]
- Sun, X.L.; Zhang, X.W.; Zhai, H.J.; Zhang, D.; Ma, S.Y. Magnoflorine inhibits human gastric cancer progression by inducing autophagy, apoptosis and cell cycle arrest by JNK activation regulated by ROS. Biomed. Pharmacother. 2020, 125, 109118. [Google Scholar] [CrossRef] [PubMed]
- Grabarska, A.; Luszczki, J.J.; Gawel, K.; Kukula-Koch, W.; Juszczak, M.; Slawinska-Brych, A.; Adamczuk, G.; Dmoszynska-Graniczka, M.; Kosheva, N.; Rzeski, W.; et al. Heterogeneous Cellular Response of Primary and Metastatic Human Gastric Adenocarcinoma Cell Lines to Magnoflorine and Its Additive Interaction with Docetaxel. Int. J. Mol. Sci. 2023, 24, 15511. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Wang, J.; Liu, E.; Zhang, X.; Xiang, J.; Li, W.; Wei, P.; Zeng, J.; Zhang, Y.; Ma, X. Protective effect of berberine in diabetic nephropathy: A systematic review and meta-analysis revealing the mechanism of action. Pharmacol. Res. 2022, 185, 106481. [Google Scholar] [CrossRef] [PubMed]
- Chrysovergis, A.; Papanikolaou, V.; Tsiambas, E.; Stavraka, C.; Ragos, V.; Peschos, D.; Psyrri, A.; Mastronikolis, N.; Kyrodimos, E. P53/MDM2 Co-expression in laryngeal squamous cell carcinoma based on digital image analysis. Anticancer Res. 2019, 39, 4137–4142. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, H.; Huang, S.; Zheng, Y. p53, Bcl-2 and cox2 are involved in berberine hydrochloride-induced apoptosis of HeLa229 cells. Mol. Med. Rep. 2016, 14, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Sala, N.; Rokkas, T. Gastric cancer: Epidemiologic aspects. Helicobacter 2013, 1, 34–38. [Google Scholar] [CrossRef]
- Kuo, S.H.; Wu, M.S.; Yeh, K.H.; Lin, C.W.; Hsu, P.N.; Chen, L.T.; Cheng, A.L. Novel Insights of Lymphomagenesis of Helicobacter pylori-Dependent Gastric Mucosa-Associated Lymphoid Tissue Lymphoma. Cancers 2019, 11, 547. [Google Scholar] [CrossRef]
- De Francesco, V.; Manta, R.; Marmo, R.; Marmo, C.; Rago, A.; Antonelli, G.; Hassan, C.; Zullo, A. Efficacy of Helicobater pylori eradication in patients with diffuse large B-cell lymphoma of the stomach: A systematic review. Eur. J. Haematol. 2022, 109, 643–647. [Google Scholar] [CrossRef]
- Yang, T.; Wang, R.; Liu, H.; Wang, L.; Li, J.; Wu, S.; Chen, X.; Yang, X.; Zhao, Y. Berberine regulates macrophage polarization through IL-4-STAT6 signaling pathway in Helicobacter pylori-induced chronic atrophic gastritis. Life Sci. 2021, 266, 118903. [Google Scholar] [CrossRef]
- Kukula-Koch, W.; Koch, W.; Angelis, A.; Halabalaki, M.; Aligiannis, N. Application of pH-zone refining hydrostatic countercurrent chromatography (hCCC) for the recovery of antioxidant phenolics and the isolation of alkaloids from Siberian barberry herb. Food Chem. 2016, 203, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Szalak, R.; Matysek, M.; Koval, M.; Dziedzic, M.; Kowalczuk-Vasilev, E.; Kruk-Slomka, M.; Koch, W.; Arciszewski, M.B.; Kukula-Koch, W. Magnoflorine from Berberis vulgaris Roots-Impact on Hippocampal Neurons in Mice after Short-Term Exposure. Int. J. Mol. Sci. 2023, 24, 7166. [Google Scholar] [CrossRef] [PubMed]
- Haselgrübler, R.; Lanzerstorfer, P.; Röhrl, C.; Stübl, F.; Schurr, J.; Schwarzinger, B.; Schwarzinger, C.; Brameshuber, M.; Wieser, S.; Winkler, S.M.; et al. Hypolipidemic effects of herbal extracts by reduction of adipocyte differentiation, intracellular neutral lipid content, lipolysis, fatty acid exchange and lipid droplet motility. Sci. Rep. 2019, 9, 10492. [Google Scholar] [CrossRef] [PubMed]
- Dąbrowski, D.; Lech, K.; Jarosz, M. Capillary-HPLC with tandem mass spectrometry in analysis of alkaloid dyestuffs—A new approach. Electrophoresis 2017, 39, 1276–1283. [Google Scholar] [CrossRef]
- Lu, J.Z.; Ye, D.; Ma, B.L. Constituents, Pharmacokinetics, and Pharmacology of Gegen-Qinlian Decoction. Front. Pharmacol. 2021, 12, 668418. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Arya, K.R.; Sharma, K.R.; Kumar, B. Quantitative determination of isoquinoline alkaloids and chlorogenic acid in Berberis species using ultra high performance liquid chromatography with hybrid triple quadrupole linear ion trap mass spectrometry. J. Sep. Sci. 2015, 38, 2007–2013. [Google Scholar] [CrossRef] [PubMed]
- Tian, P.P.; Zhang, X.X.; Wang, H.P.; Li, P.L.; Liu, Y.X.; Li, S.J. Rapid Analysis of Components in Coptis chinensis Franch by Ultra-Performance Liquid Chromatography with Quadrupole Time-of-Flight Mass Spectrometry. Pharmacogn. Mag. 2017, 13, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Naman, C.B.; Gupta, G.; Varikuti, S.; Chai, H.; Doskotch, R.W.; Satoskar, A.R.; Kinghorn, A.D. Northalrugosidine is a bisbenzyltetrahydroisoquinoline alkaloid from Thalictrum alpinum with in vivo antileishmanial activity. J. Nat. Prod. 2015, 78, 552–556. [Google Scholar] [CrossRef]
- Hostalkova, A.; Marikova, J.; Opletal, L.; Korabecny, J.; Hulcova, D.; Kunes, J.; Novakova, L.; Perez, D.I.; Jun, D.; Kucera, T.; et al. Isoquinoline Alkaloids from Berberis vulgaris as Potential Lead Compounds for the Treatment of Alzheimer’s Disease. J. Nat. Prod. 2019, 82, 239–248. [Google Scholar] [CrossRef]
- Qing, Z.; Xu, Y.; Yu, L.; Liu, J.; Huang, X.; Tang, Z.; Cheng, P.; Zeng, J. Investigation of fragmentation behaviours of isoquinoline alkaloids by mass spectrometry combined with computational chemistry. Sci. Rep. 2020, 10, 733. [Google Scholar] [CrossRef]
- Tian, X.; Li, Z.; Lin, Y.; Chen, M.; Pan, G.; Huang, C. Study on the PK profiles of magnoflorine and its potential interaction in Cortex phellodendri decoction by LC-MS/MS. Anal. Bioanal. Chem. 2013, 406, 841–849. [Google Scholar] [CrossRef]
- Mittal, J.; Sharma, M.M. Enhanced production of berberine in In vitro regenerated cell of Tinospora cordifolia and its analysis through LCMS QToF. 3 Biotech 2017, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Kotland, A.; Chollet, S.; Diard, C.; Autret, J.-M.; Meucci, J.; Renault, J.-H.; Marchal, L. Industrial case study on alkaloids purification by pH-zone refining centrifugal partition chromatography. J. Chromatogr. A 2016, 1474, 59–70. [Google Scholar] [CrossRef]
- Berthod, A.; Hassoun, M.; Ruiz-Angel, M.J. Alkane effect in the Arizona liquid systems used in countercurrent chromatography. Anal. Bioanal. Chem. 2005, 383, 327–340. [Google Scholar] [CrossRef]
- Correa, D.I.; Pastene-Navarrete, E.; Bustamante, L.; Baeza, M.; Alarcón-Enos, J. Isolation of Three Lycorine Type Alkaloids from Rhodolirium speciosum (Herb.) Ravenna Using pH-Zone-Refinement Centrifugal Partition Chromatography and Their Acetylcholinesterase Inhibitory Activities. Metabolites 2020, 10, 309. [Google Scholar] [CrossRef]
- Sun, C.; Duan, W.; Wang, X.; Geng, Y.; Li, J.; Wang, D. Combinative Application of pH-Zone-Refining Counter-Current Chromatography and Preparative HPLC for the Separation of Alkaloids from Lycoris radiata. J. Liq. Chromatogr. Relat. Technol. 2016, 38, 1031–1036. [Google Scholar] [CrossRef]
- Wu, H.; Sun, Q.; Dong, H.; Qiao, J.; Lin, Y.; Yu, C.; Li, Y. Gastroprotective action of the extract of Corydalis yanhusuo in Helicobacter pylori infection and its bioactive component, dehydrocorydaline. J. Ethnopharmacol. 2023, 307, 116173. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ke, L.; Ni, Z.; Chen, Y.; Zhang, L.H.; Zhu, S.H.; Li, C.J.; Shang, L.; Liang, J.; Shi, Y.Q. Berberine containing quadruple therapy for initial Helicobacter pylori eradication: An open-label randomized phase IV trial. Medicine 2017, 96, e7697. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Jiang, C.; Huang, C.; Chen, G.; Jiang, K.; Huang, B.; Liu, F. Berberine combined with triple therapy versus triple therapy for Helicobacter pylori eradication: A meta-analysis of randomized controlled trials. Evid.-Based Complement. Altern. Med. 2018, 2018, 8716910. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Huang, P.; Wong, K.; Xu, Y.; Tan, L.; Chen, H.; Lu, Q.; Luo, C.; Tam, C.; Zhu, L.; et al. Coptisine-induced inhibition of Helicobacter pylori: Elucidation of specific mechanisms by probing urease active site and its maturation process. J. Enzym. Inhib. Med. Chem. 2018, 33, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, S.; Aziz, M.; Saeed, A.; Ejaz, S.A.; Channar, P.A.; Zargar, S.; Abbas, Q.; Alanazi, H.; Hussain, M.; Alharbi, M.; et al. Analysis of 1-Aroyl-3-[3-chloro-2-methylphenyl] Thiourea Hybrids as Potent Urease Inhibitors: Synthesis, Biochemical Evaluation and Computational Approach. Int. J. Mol. Sci. 2022, 23, 11646. [Google Scholar] [CrossRef]
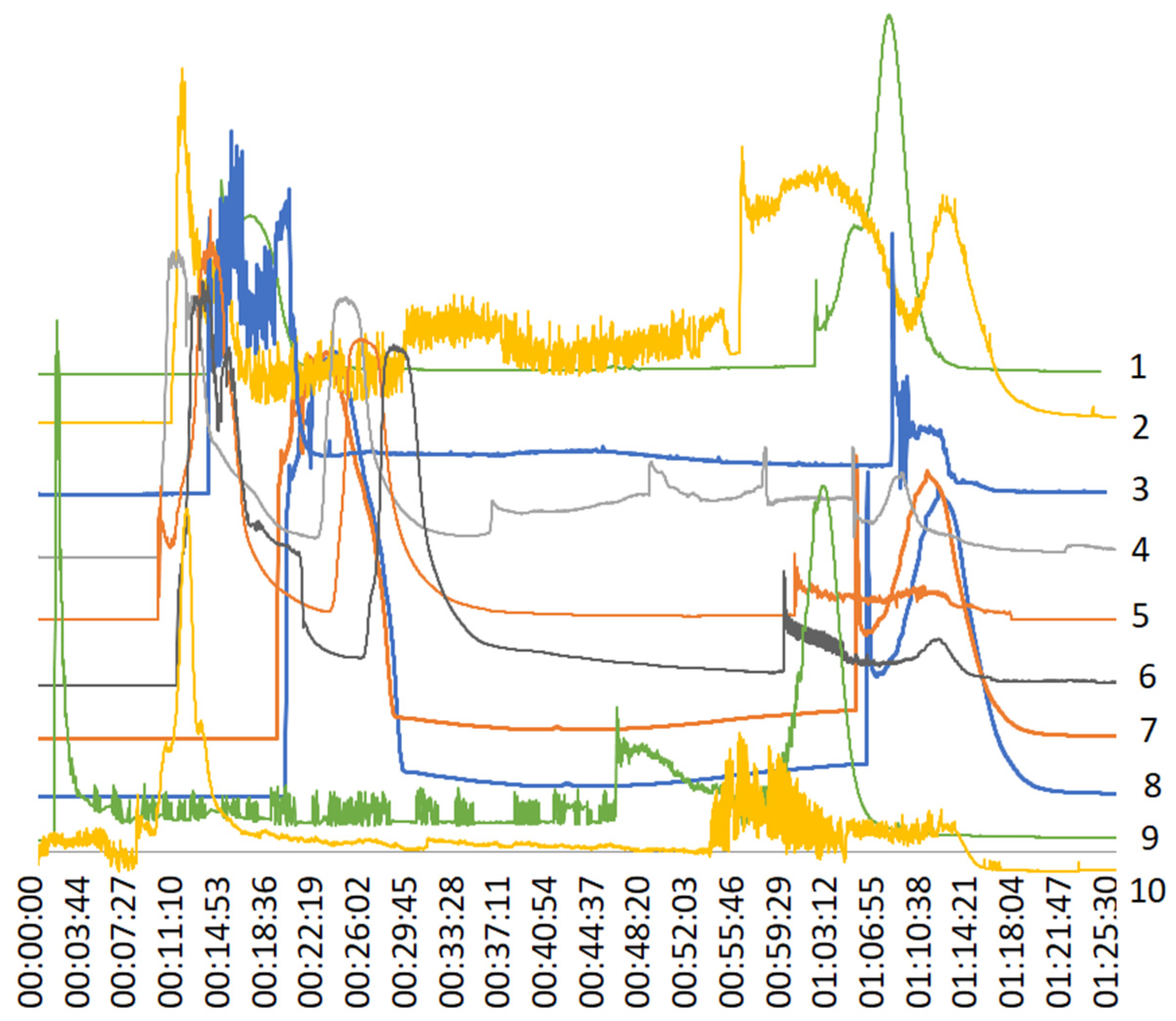
| Trial No | Plant Material | Rotor Spped [rpm] | Flow Rate [mL/min] | UV Absorbance [nm] | Stationary Phase | Injected Volume [mL] | Solvent System [v/v/v/v] |
|---|---|---|---|---|---|---|---|
| 1 | Root B. vulgaris | 800 | 8 | 290/320 | lower | 3 | n-hex:BuOH:EtOH:H2O 3:12:4:16 |
| 2 | Root B. vulgaris | 1600 | 8 | 290/320 | lower | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 4 | Root B. vulgaris | 1600 | 8 | 290/320 | upper | 6 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 3 | Root B. vulgaris | 1600 | 8 | 290/320 | upper | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 5 | Root B. vulgaris | 1600 | 8 | 290/320 | upper | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 6 | Root B. vulgaris | 1600 | 8 | 290/320 | upper | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 7 | Stem B. vulgaris | 800 | 4 | 290/320 | lower | 3 | n-hex:BuOH:EtOH: H2O 4:12:8:12 |
| 8 | Stem B. vulgaris | 1300 | 5 | 290/320 | lower | 3 | n-hex:BuOH:EtOH: H2O 4:12:8:12 |
| 9 | Stem B. vulgaris | 1600 | 8 | 290/320 | lower | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
| 10 | Stem B. vulgaris | 1600 | 8 | 290/320 | upper | 3 | n-hex:BuOH:EtOH: H2O 3:12:4:16 |
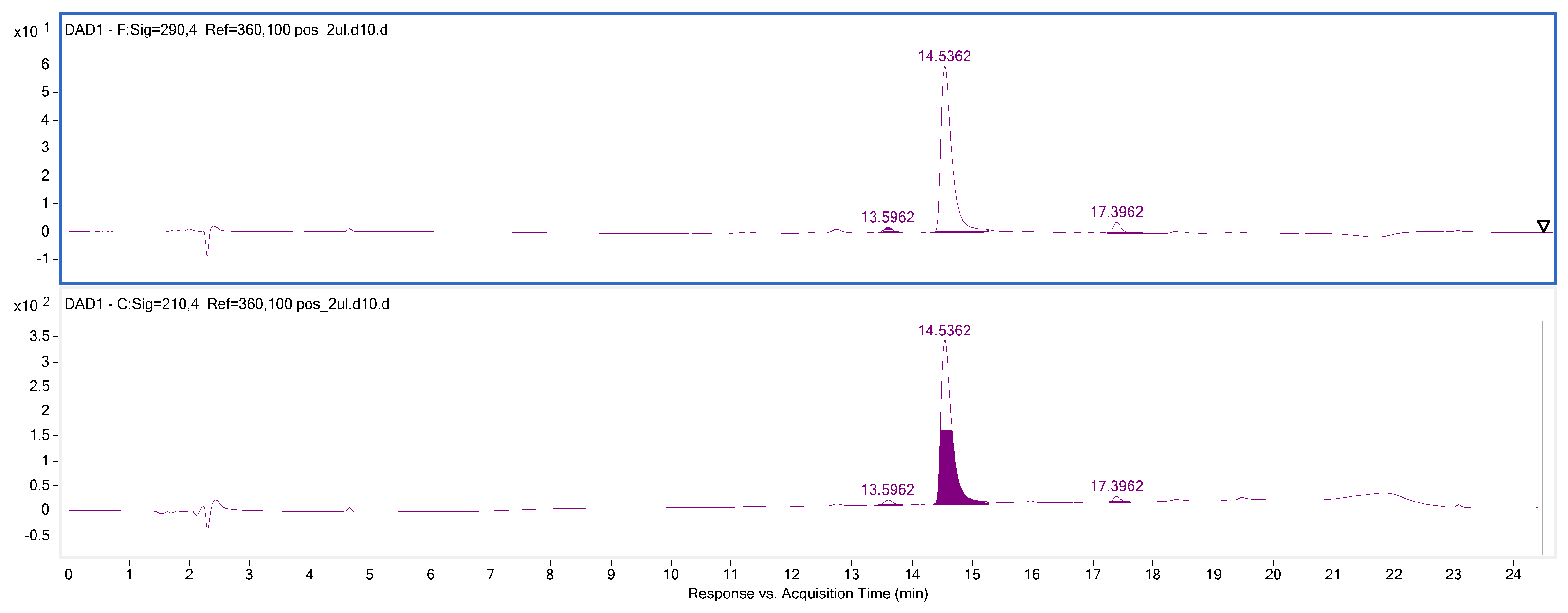
| Ion (+/−) | Rt (min) | Molecular Formula | m/z Calculated | m/z Experimental | Error (ppm) | DBE | MS/MS Fragments | Proposed Compound | References |
|---|---|---|---|---|---|---|---|---|---|
| [M+H]+ | 15.3 | C20H24NO4 | 342.1700 | 342.1704 | −1.22 | 10 | 297.1104 265.0890 | Magnoflorine | [26] |
| [M+H]+ | 15.4 | C19H23NO3 | 314.1751 | 314.1776 | −8.08 | 9 | 300.1377 269.1052 192.0853 | 4-O-Methyl-N-methylcoclaurine | [27] |
| [M+H]+ | 16.4 | C20H21NO4 | 340.1543 | 340.1554 | −3.14 | 11 | 295.1899 263.0634 235.0677 | Tetrahydroberberine (canadine) | [28,29] |
| [M+H]+ | 16.9 | C19H23NO3 | 609.2959 | 609.2954 | −0.84 | 19 | 579.2569, 567.2530 | Oxyacanthine | [27] |
| [M+H]+ | 17.4 | C20H20NO5 | 354.1336 | 354.1325 | 3.11 | 12 | 339.1105 324.0905 280.0899 | Protopine | [30] |
| [M+H]+ | 17.5 | C37H40O7N2 | 625.2908 | 625.2926 | −2.84 | 19 | 608.3629, 581.2192 381.2131 | Northalrugosine | [31] |
| [M+H]+ | 17.9 | C19H17NO4 | 324.1230 | 324.1229 | −6.7 | 12 | 280.0935 175.0565 | Demethyleneberberine/isomer | [27,30] |
| [M+H]+ | 19.1 | C20H19NO4 | 338.1387 | 338.1382 | 1.44 | 12 | 294.1114 208.0594 | Jatrorrhizine | [26,32] |
| [M+H]+ | 19.6 | C19H16NO4 | 322.1074 | 322.1085 | 5.56 | 13 | 250.0954 151.9525 | Thalifendine | [26] |
| [M+H]+ | 19.8 | C20H18NO4 | 336.1230 | 336.1231 | −0.2 | 13 | 304.084, 292.0859 278.0710 | Epiberberine | [26] |
| [M+H]+ | 20.2 | C21H21NO4 | 352.1543 | 352.1549 | −0.47 | 12 | 308.1281 248.0918 | Palmatine | [30] |
| [M+H]+ | 20.5 | C20H17NO4 | 336.1230 | 336.1231 | −1.99 | 13 | 292.0983 220.1163 | Berberine | [30] |
| Alkaloid | B. vulgaris Root | B. vulgaris Stem |
|---|---|---|
| Tetrahydroberberine (canadine) | tr | tr |
| 4-O-Methyl-N-methylcoclaurine | + | - |
| Magnoflorine | ++ | + |
| Demethyleneberberine/isomer | + | tr |
| Thalifendine | + | tr |
| Jatrorrhizine | +++ | ++ |
| Epiberberine | ++ | + |
| Palmatine | ++ | ++ |
| Berberine | +++ | +++ |
| Solvent System v/v/v/v | 3:12:6:15 | 1:14:6:15 | 4:12:8:12 | 3:12:4:16 | ||||
|---|---|---|---|---|---|---|---|---|
| B. vulgaris | Root | Stem | Root | Stem | Root | Stem | Root | Stem |
| Berberine | 1.42 | 0.98 | 1.12 | 0.97 | 0.88 | 4.94 | 0.97 | 1.08 |
| Magnoflorine | 0.14 | 0.17 | 0.19 | 1.42 | 1.58 | 0.87 | 0.18 | 0.16 |
| Palmatine | 0.22 | 1.78 | 1.45 | 0.98 | 0.92 | 0.44 | 0.58 | 1.32 |
| Jatrorhizine | 0.35 | 0.81 | 0.42 | 0.97 | 1.08 | 0.82 | 0.71 | 0.86 |
| Compound | MIC (mg/L) | MBC (mg/L) | MBC/MIC | Urease Inhibition IC50 (mg/L) |
|---|---|---|---|---|
| Magnoflorine | 320 | 640 | 2 | 25.5 |
| Berberine | 32 | 32 | 1 | 729.5 |
| Thiourea | 92.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakonieczna, S.; Susniak, K.; Bozhadze, A.; Grabarska, A.; Głowniak-Lipa, A.; Głowniak, K.; Kukula-Koch, W. Optimized Centrifugal Partition Chromatography (CPC) Protocol for Isolation of Urease Inhibitors: Magnoflorine and Berberine from Berberis vulgaris Extracts. Separations 2024, 11, 94. https://doi.org/10.3390/separations11040094
Nakonieczna S, Susniak K, Bozhadze A, Grabarska A, Głowniak-Lipa A, Głowniak K, Kukula-Koch W. Optimized Centrifugal Partition Chromatography (CPC) Protocol for Isolation of Urease Inhibitors: Magnoflorine and Berberine from Berberis vulgaris Extracts. Separations. 2024; 11(4):94. https://doi.org/10.3390/separations11040094
Chicago/Turabian StyleNakonieczna, Sylwia, Katarzyna Susniak, Anna Bozhadze, Aneta Grabarska, Anna Głowniak-Lipa, Kazimierz Głowniak, and Wirginia Kukula-Koch. 2024. "Optimized Centrifugal Partition Chromatography (CPC) Protocol for Isolation of Urease Inhibitors: Magnoflorine and Berberine from Berberis vulgaris Extracts" Separations 11, no. 4: 94. https://doi.org/10.3390/separations11040094






