Fabric Sol–gel Phase Sorptive Extraction Technique: A Review
Abstract
:1. Introduction
2. Sol–gel Technology in Developing Microextraction Sorbents
3. Preparation of FPSE Media
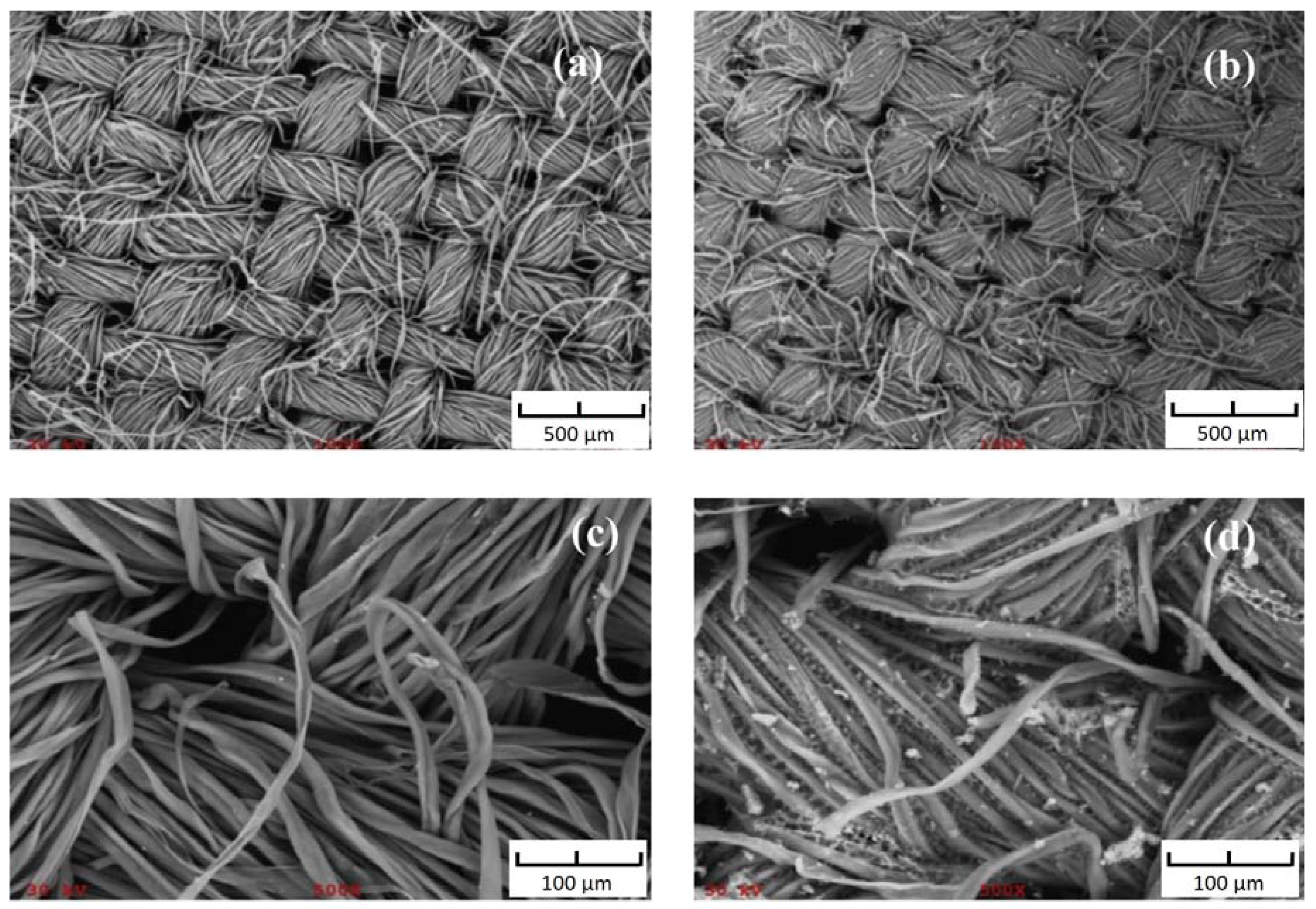
3.1. Pretreatment of Fabric Substrates
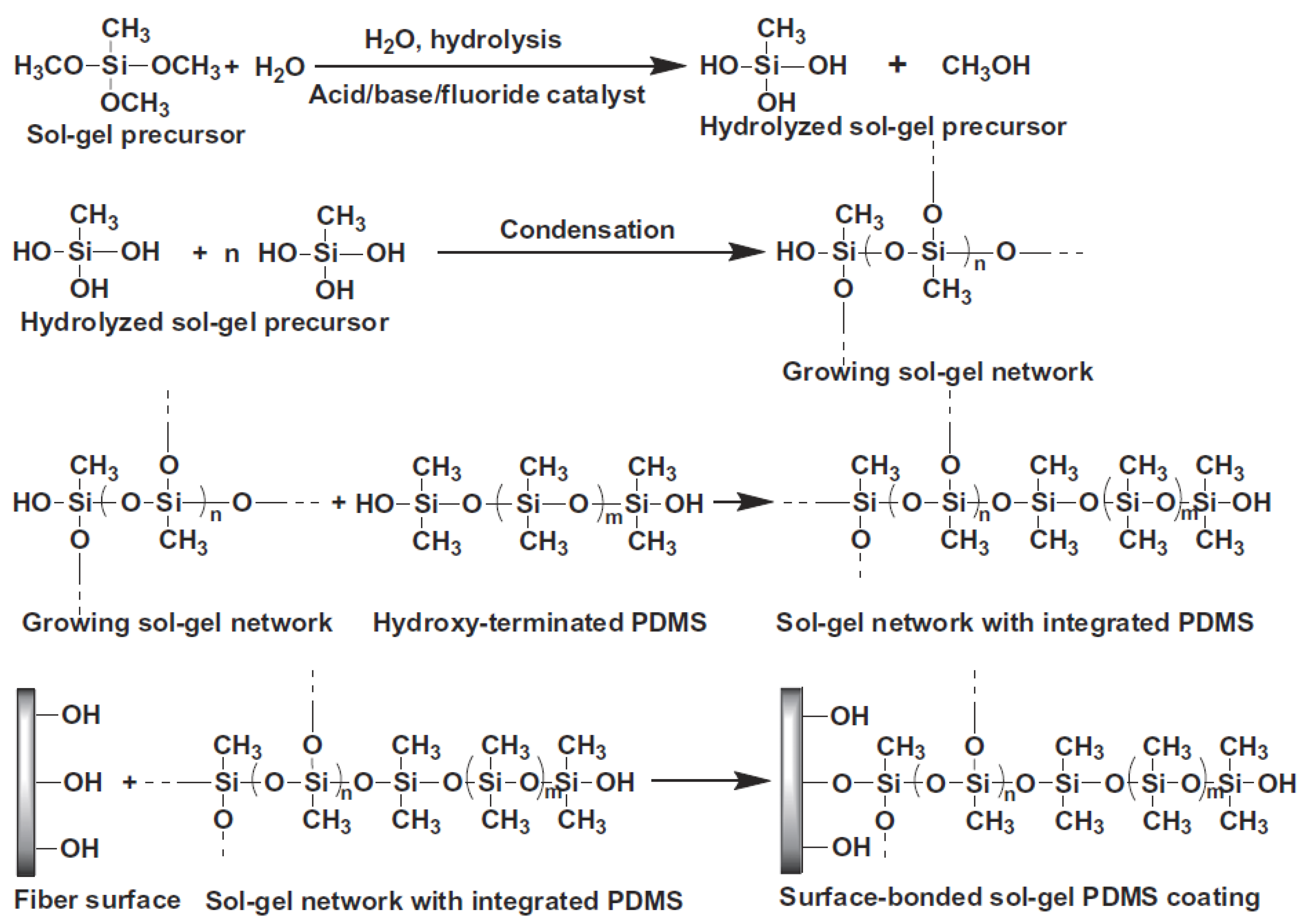
3.2. Preparation of the Sol Solution for the Sol–gel Coating Process
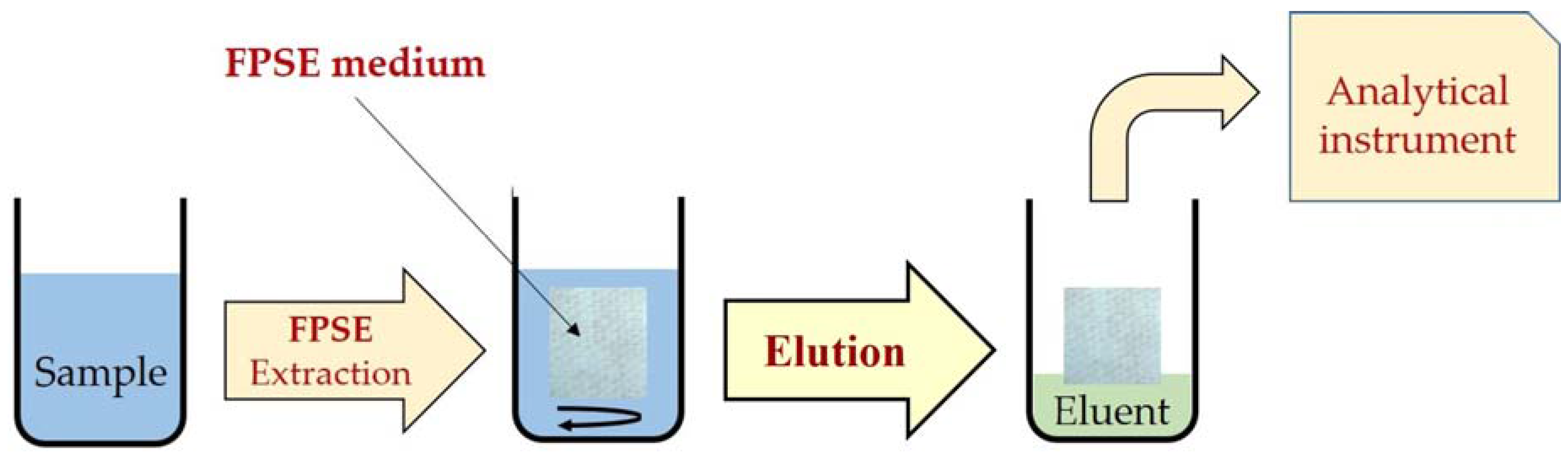
4. Applications of Fabric Phase Sorptive Extraction
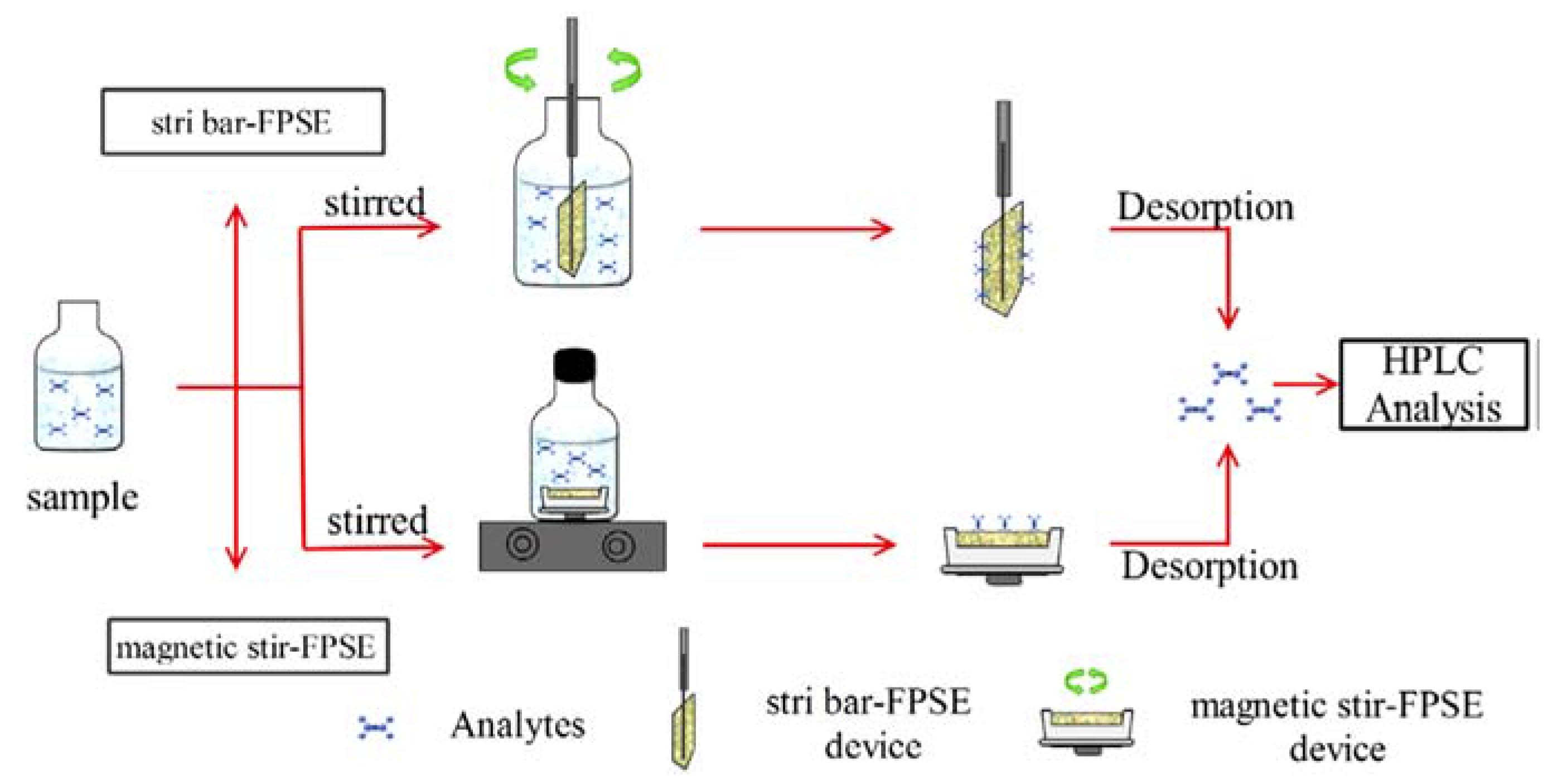
5. Stir Fabric Phase Sorptive Extraction
6. Dynamic Fabric Phase Sorptive Extraction
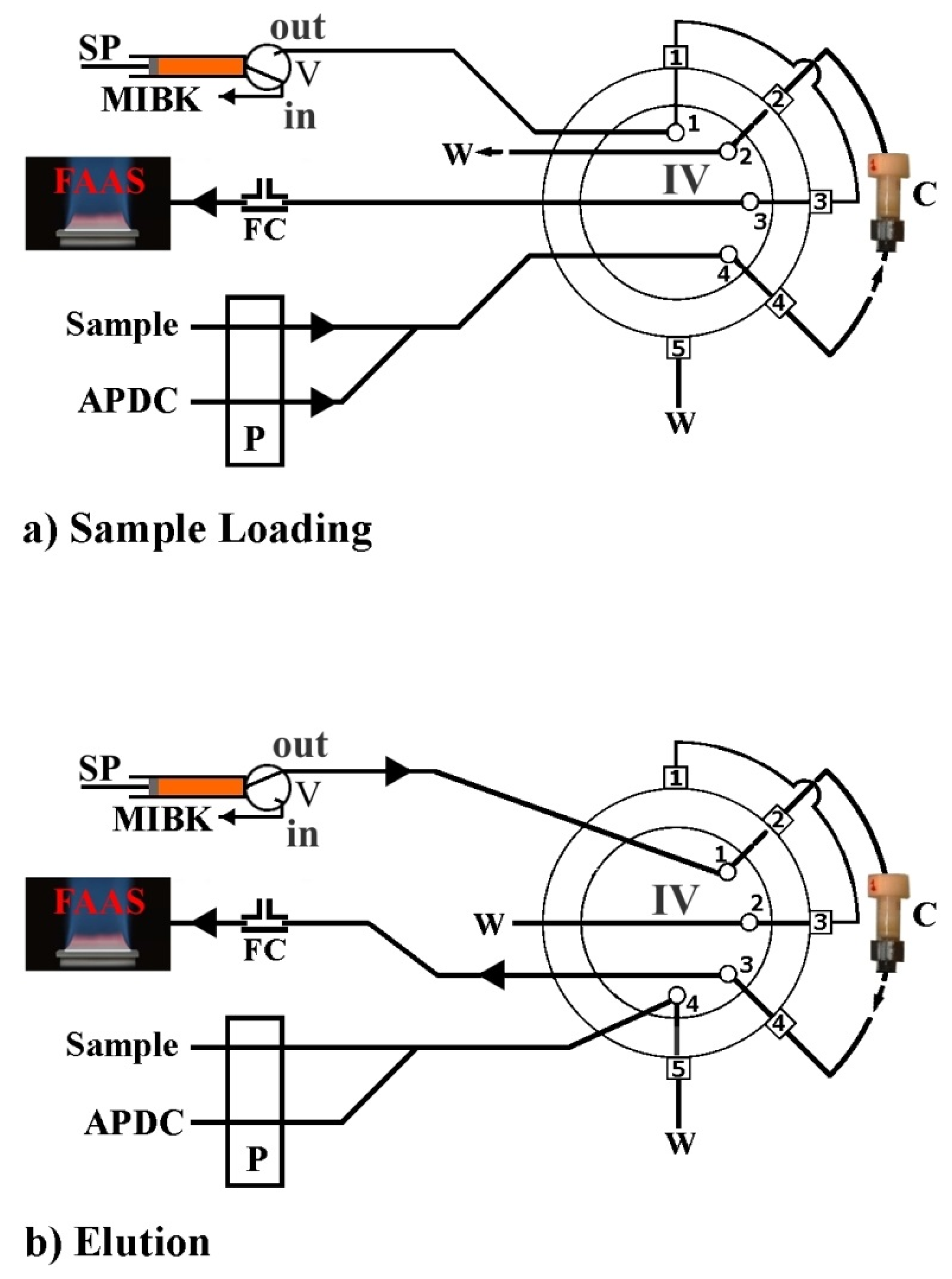
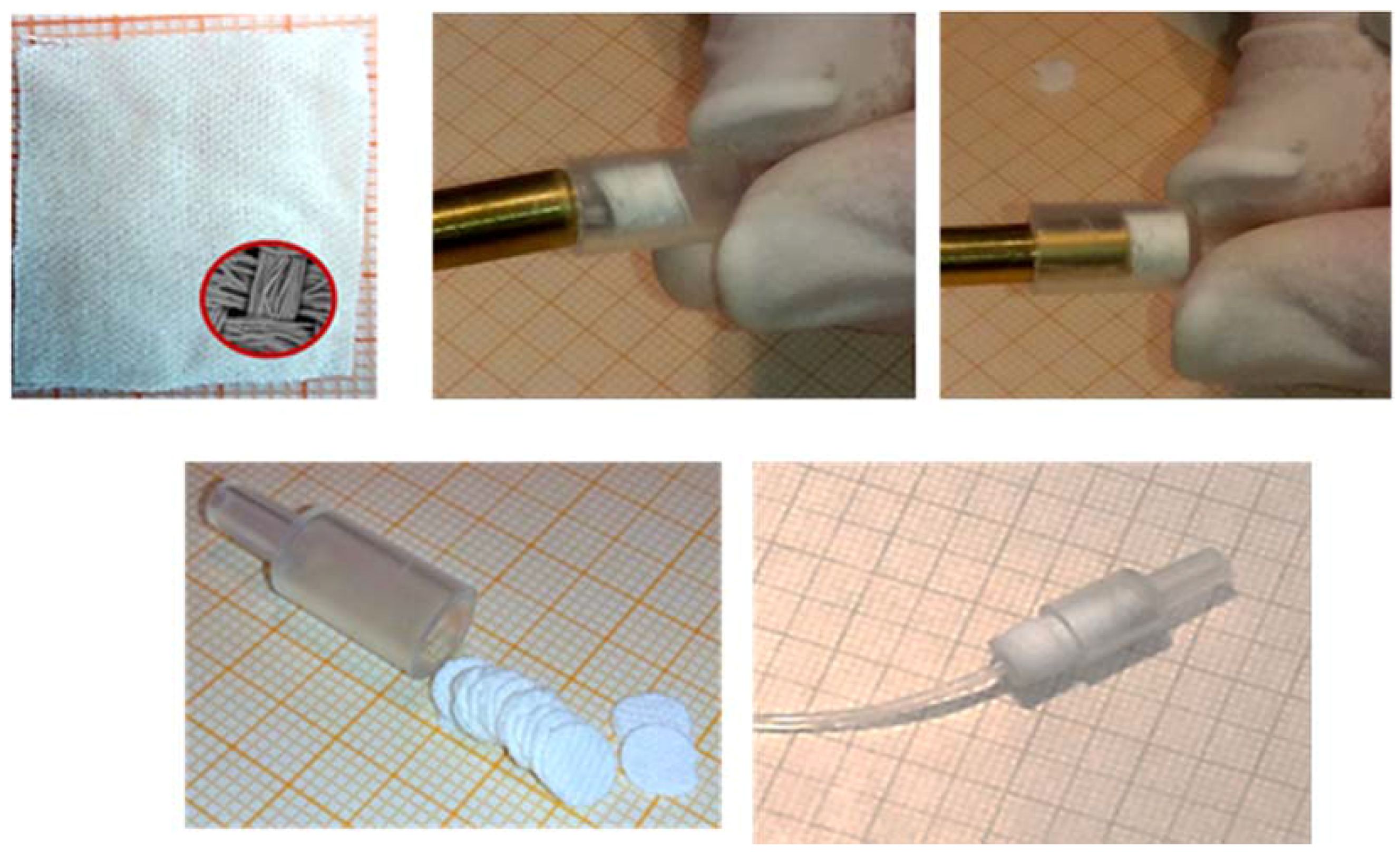
7. Automated Fabric Phase Sorptive Extraction
8. Conclusions and Future Outlook
Author Contributions
Conflicts of Interest
Abbreviations
References
- Płotka-Wasylka, J.; Szczepańska, N.; Guardia de la, M.; Namieśnik, J. Modern trends in solid phase extraction: New sorbent media. TrAC–Trends Anal. Chem. 2016, 77, 23–43. [Google Scholar] [CrossRef]
- Płotka-Wasylka, J.; Szczepańska, N.; Guardia de la, M.; Namieśnik, J. Miniaturized solid-phase extraction techniques. TrAC–Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Armenta, S.; Garrigues, S.; de la Guardia, M. Green analytical chemistry. Trends Anal. Chem. 2008, 27, 497–511. [Google Scholar] [CrossRef]
- Gałuszka, A.; Migaszewski, Z.; Namiesnik, J. The 12 principles of green analytical chemistry and the significance mnemonic of green analytical practices. Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Anthemidis, A.N.; Miró, M. Recent developments in flow injection/sequential injection liquid-liquid extraction for atomic spectrometric determination of metals and metalloids. Appl. Spectrosc. Rev. 2009, 44, 140–167. [Google Scholar] [CrossRef]
- Miró, M.; Hansen, E.H. On-line sample processing involving microextraction techniques as a front-end to atomic spectrometric detection for trace metal assays: a review. Anal. Chim. Acta 2013, 782, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.K.; Kaur, V.; Verma, N. A review on solid phase microextraction—High performance liquid chromatography as a novel tool for the analysis of toxic metal ions. Talanta 2006, 68, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Shen, Z.; Wu, D.; Guan, Y. Recent developments in solid-phase microextraction for on-site sampling and sample preparation. Trends Anal. Chem. 2011, 30, 1568–1574. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Baltussen, E.; Sandra, P.; David, F.; Cramers, C. Stir bar sorptive extraction (SBSE), a novel extraction technique for aquatus samples: Theory and principles. J. Microcolumn Sep. 1999, 10, 737–747. [Google Scholar] [CrossRef]
- Bruheim, I.; Liu, X.; Pawliszyn, J. Thin-film microextraction. Anal. Chem. 2003, 75, 1002–1010. [Google Scholar] [CrossRef] [PubMed]
- Giakisikli, G.; Anthemidis, A.N. Magnetic materials as sorbents for metal/metalloid preconcentration and/or separation. A review. Anal. Chim. Acta 2013, 789, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Heena; Malik, A.K.; Kabir, A.; Furton, K.G. Efficient analysis of selected estrogens using fabric phase sorptive extraction and high performance liquid chromatography-fluorescence detection. J. Chromatogr. A 2014, 1359, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Kermani, F.R.; Pawliszyn, J. Sorbent Coated Glass Wool Fabric as a Thin Film Microextraction Device. Anal. Chem. 2012, 84, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Grandy, J.J.; Pawliszyn, J. Development of a Carbon Mesh Supported Thin Film Microextraction Membrane As a Means to Lower the Detection Limits of Benchtop and Portable GC/MS Instrumentation. Anal. Chem. 2016, 88, 1760–1767. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Furton, K.G. Fabric Phase Sorptive Extractor (FPSE). U.S. Patent and Trademark Office 14,216,121, 17 March 2014. [Google Scholar]
- Kabir, A.; Furton, K.G.; Malik, A. Innovations in sol–gel microextraction phases for solvent-free sample preparation in analytical chemistry. Trends Anal. Chem. 2013, 45, 197–218. [Google Scholar] [CrossRef]
- Samanidou, V.; Galanopoulos, L.D.; Kabir, A.; Furton, K.G. Fast extraction of amphenicols residues from raw milk using novel fabric phase sorptive extraction followed by high-performance liquid chromatography-diode array detection. Anal. Chim. Acta 2015, 855, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Augusto, F.; Carasek, E.; Silva, R.G.C.; Rivellino, S.R.; Batista, A.D.; Martendal, E. New sorbents for extraction and microextraction techniques. J. Chromatogr. A 2010, 1217, 2533–2542. [Google Scholar] [CrossRef] [PubMed]
- Fumes, B.H.; Silva, M.R.; Andrade, F.N.; Nazario, C.E.D.; Lanças, F.M. Recent advances and future trends in new materials for sample preparation. TrAC–Trends Anal. Chem. 2015, 71, 9–25. [Google Scholar] [CrossRef]
- Chong, S.L.; Wang, D.; Hayes, J.D.; Wilhite, B.W.; Malik, A. Sol–gel coating technology for the preparation of solid-phase microextraction fibers of enhanced thermal stability. Anal. Chem. 1997, 69, 3889–3898. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gaurav; Malik, A.K.; Tewary, D.K.; Singh, B. A review on development of solid phase microextraction fibers by sol–gel methods and their applications. Anal. Chim. Acta 2008, 610, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Valcárcel, M.; Cárdenas, S.; Lucena, R. Novel Sol–gel Sorbents in Sorptive Microextraction Analytical Microextraction Techniques. In Analytical Microextraction Techniques; Bentham Science: Sharjah, United Arab Emirates, 2016; pp. 28–69. [Google Scholar]
- Karageorgou, E.; Manousi, N.; Samanidou, V.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction for the fast isolation of sulfonamides residues from raw milk followed by high performance liquid chromatography with ultraviolet detection. Food Chem. 2016, 196, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Racamonde, I.; Rodil, R.; Quintana, J.B.; Sieira, B.J.; Kabir, A.; Furton, K.G.; Cela, R. Fabric phase sorptive extraction: A new sorptive microextraction technique for the determination of non-steroidal anti-inflammatory drugs from environmental water samples. Anal. Chim. Acta 2015, 865, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Kaltzi, I.; Kabir, A.; Furton, K.G. Simplifying sample preparation using fabric phase sorptive extraction technique for the determination of benzodiazepines in blood serum by high-performance liquid chromatography. Biomed. Chromatogr. 2015, 30, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana-Rodríguez, J.J. Fabric phase sorptive extraction followed by UHPLC-MS/MS for the analysis of benzotriazole UV stabilizers in sewage samples. Anal. Bioanal. Chem. 2015, 407, 8137–8150. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guerra, R.B.; Montesdeoca-Esponda, S.; Sosa-Ferrera, Z.; Kabir, A.; Furton, K.G.; Santana- Rodríguez, J.J. Rapid monitoring of residual UV-stabilizers in seawater samples from beaches using fabric phase sorptive extraction and UHPLC-MS/MS. Chemosphere 2016, 164, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Marcé, R.M.; Fontanals, N. Dynamic fabric phase sorptive extraction for a group of pharmaceuticals and personal care products from environmental waters. J. Chromatogr. A 2016, 1456, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Roldán-Pijuán, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M.; Kabir, A.; Furton, K.G. Stir fabric phase sorptive extraction for the determination of triazine herbicides in environmental waters by liquid chromatography. J. Chromatogr. A 2015, 1376, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Lakade, S.S.; Borrull, F.; Furton, K.G.; Kabir, A.; Fontanals, N.; Marce, R.M. Comparative study of different fabric phase sorptive extraction sorbents to determine emerging contaminants from environmental water using liquid chromatography-tandem mass spectrometry. Talanta 2015, 144, 1342–1351. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Dong, S.; Zhang, M.; Zhang, H.; Huang, T. Fabric phase sorptive extraction: Two practical sample pretreatment techniques for brominated flame retardants in water. Water Res. 2016, 101, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gaurav; Kabir, A.; Furton, K.G.; Malik, A.K. Development of a fabric phase sorptive extraction with high-performance liquid chromatography and ultraviolet detection method for the analysis of alkyl phenols in environmental samples. J. Sep. Sci. 2015, 38, 3228–3238. [Google Scholar] [CrossRef] [PubMed]
- Aznar, M.; Alfaro, P.; Nerin, C.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction: An innovative sample preparation approach applied to the analysis of specific migration from food packaging. Anal. Chim. Acta 2016, 936, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Guedes-Alonso, R.; Ciofi, L.; Sosa-Ferrera, Z.; Santana-Rodríguez, J.J.; Del Bubba, M.; Kabir, A.; Furton, K.G. Determination of androgens and progestogens in environmental and biological samples using fabric phase sorptive extraction coupled to ultra-high performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1437, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Anthemidis, A.; Kazantzi, V.; Samanidou, V.; Kabir, A.; Furton, K.G. An automated flow injection system for metal determination by flame atomic absorption spectrometry involving on-line fabric disk sorptive extraction technique. Talanta 2016, 156–157, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.; Michaelidou, K.; Kabir, A.; Furton, K.G. Fabric phase sorptive extraction of selected penicillin antibiotic residues from intact milk followed by high performance liquid chromatography with diode array detection. Food Chem. 2017, 224, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Alcudia-León, M.C.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Stir membrane extraction: A useful approach for liquid simple preparation. Anal. Chem. 2009, 81, 8957–8961. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.L.; Wang, J.H. Recent advances in flow-based sample pretreatment for the determination of metal species by atomic spectrometry. Chi. Sci. Bull. 2013, 58, 1992–2002. [Google Scholar] [CrossRef]

| Sol–gel Coating | Sorbent Loading (mg·cm−2) | Fabric Substrate | Polarity |
|---|---|---|---|
| PDMDPS | 1.93 | Cellulose/Polyester | Non-polar |
| C18 | 2.4 | Cellulose | Non-polar |
| PDMS | 2.3 | Cellulose | Non-polar |
| PTHF | 3.96 | Cellulose | Medium polar |
| PEO-PPO-PEO | 5.68 | Cellulose | Polar |
| Graphene | 7.57 | Cellulose | Polar |
| PEG-PPG-PEG | 5.68 | Cellulose | Polar |
| PEG | 8.64 | Cellulose | Highly polar |
| Sol–gel Precursor | Operation | |
| Unchanged: | TEOS, Titanium isopropoxide, Zirconium butoxide, Tetramethoxygermane, Alumina | Inorganic component to the hybrid polymeric network. Offers active hydroxyl group to facilitate chemical bonding to the fiber/capillary surface. |
| Organically modified: | MTMS, C18-MTMS, C8-TMS, TMSPA, VTEOS, PTMOS | Forms inorganic backbone of the hybrid material. Organic moieties provide intermolecular interactions between analytes and the sorbent. |
| Catalyst | Operation | |
| Acid/Fluoride/Base: | GAA, HCl, HNO3, Citric acid, TFA, HF, NH4OH, NaOH, Basic amino acids | Catalyzes the hydrolysis and condensation reactions and controls the network structure and porosity. Acid catalyzed sol–gel materials possess weakly branched microporous structures. Base catalyzed sol–gel materials have highly branched particulate structures with large pore sizes. |
| FPSE Media | Substrate | Polymer | Precursor | Organic Solvent System | Catalyst | Reference |
|---|---|---|---|---|---|---|
| Sol–gel PDMDPS | Polyester | Poly(dimethyldiphenylsiloxane) | MTMS | Methylene chloride:acetone | TFA | [25,26,27,28,29,30] |
| Sol–gel PDMDPS | Polyester | Poly(dimethyldiphenylsiloxane) | 3-CPTEOS | Methylene chloride | TFA | [31] |
| Sol–gel PDMDPS | Cellulose | Poly(dimethyldiphenylsiloxane) | MTMS | Methylene chloride:acetone | TFA | [32] |
| Sol–gel PTHF | Cellulose | Poly(tetrahydrofuran) | MTMS | Methylene chloride:acetone | TFA | [13,24,25,26,28,29,30,32,33,34,35] |
| Sol–gel PTHF | Cellulose | Poly(tetrahydrofuran) | 3-CPTEOS | Methylene chloride | TFA | [31] |
| Sol–gel PEO-PPO-PEO | Cellulose | Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) triblock copolymer | MTMS | Methylene chloride: acetone | TFA | [36] |
| Sol–gel Graphene | Cellulose | Graphene | MTMS | Methylene chloride: acetone | TFA | [36] |
| Sol–gel PEG-PPG-PEG | Cellulose | Poly(ethyleneglycol)–block-poly(propyleneglycol)–block-Poly(ethyleneglycol) triblock copolymer | MTMS | Methylene chloride: acetone | TFA | [24], [29], [31], [37], |
| Sol–gel PEG | Cellulose | Poly(ethyleneglycol) | MTMS | Methylene chloride: acetone | TFA | [18,24,25,26,28,29,30,31,32,34,37] |
| Sol–gel C18 | Cellulose | Octadecyl carbon chain | MTMS | Methylene chloride:acetone | TFA | [24], [37] |
| Sol–gel PDMS | Cellulose | Poly(dimethylsiloxane) | MTMS | Methylene chloride:acetone | TFA | [34] |
| Analytical Technique | Sol–gel Coating | Fabric Substrate | Elution Solvent | E.T. min | Sample | Analyte | Type of Analyte | EF | LOD *, ng·L−1 a, ng·g−1 | LOQ ** ng·L−1 b, ng·g−1 | CCα μg ·kg−1 | CCβ μg ·kg−1 | R(%); c, Rapp (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FPSE-HPLC-FLD | PTHF | Cellulose | Methanol | 20 | Urine, Ground-River-Drinking water | BPA | Estrogens | 13.9 | 42 | 139 | − | − | 88.7–96.4 | [13] |
| E2 | 14.4 | 20 | 66 | 89.4–97.4 | ||||||||||
| EE2 | 14.7 | 36 | 119 | 89.0–98.0 | ||||||||||
| FPSE-HPLC-DAD | PEG | Cellulose | Methanol- acetonitrile | 20 | Blood serum | BRZ | Drugs Benzodiazepines | − | 10 | 30 | − | − | 91.6–97.6 | [26] |
| DZP | 90.0–102.2 | |||||||||||||
| LRZ | 87.6–95.5 | |||||||||||||
| APZ | 93.2–106 | |||||||||||||
| FPSE-HPLC-DAD | PEG | Cellulose | Acetonitrile | 40 | Intact milk | PENG | Antibiotics Penicillin | − | 3.0 a | 10.0 b | 11.2 | 12.3 | 89.2–104.3 | [37] |
| CLO | 6.0 a | 20.0 b | 32.8 | 35.4 | 82.8–88.1 | |||||||||
| DICLO | 7.5 a | 25.0 b | 33.2 | 36.1 | 80.8–92.4 | |||||||||
| OXA | 9.0 a | 30.0 b | 33.0 | 36.7 | 82.6–90.5 | |||||||||
| FPSE-HPLC-DAD | PEG | Cellulose | Methanol- acetonitrile | 30 | Raw milk | TAP | Antibiotics Amphenicols | − | − | − | 52.49 | 56.8 | 90.5–103.3 | [18] |
| FF | 55.23 | 58.99 | 92.3–103.3 | |||||||||||
| CAP | 53.8 | 55.9 | 97.0–106.6 | |||||||||||
| FPSE-HPLC-UV | PEG | Cellulose | Methanol- acetonitrile | 13 | Raw milk | SMTH | Antibiotics Sulfonamides | − | − | − | 116.5 | 120.4 | 94.7–107 | [24] |
| SIX | 114.4 | 118.5 | 93.0–104.6 | |||||||||||
| SDMX | 94.7 | 104.1 | 96.1–102.7 | |||||||||||
| FPSE-HPLC-UV | PTHF | Cellulose | Methanol | 25 | Ground-River water, Treated water, Soil, Sludge | 4-TBP | Chemicals Alkyl phenols | − | 182 | 601 | − | − | 90.1–95.0 | [33] |
| 4-SBP | 179 | 599 | 90.6–95.7 | |||||||||||
| 4-TAP | 192 | 640 | 89.0–96.1 | |||||||||||
| 4-CP | 161 | 531 | 91.1–96.0 | |||||||||||
| FPSE-UPLC-MS | PTHF | Cellulose | Acetonitrile | 20 | Food simulants | DEP | Chemicals non-volatile Migrants | 3.1 | 5.0 a | 15 b | − | − | 67.6 | [34] |
| TBC | 6.4 | 1.0 a | 3 b | 104.8 | ||||||||||
| DBM | 6.6 | 3.0 a | 10 b | 112.0 | ||||||||||
| TBoAC | 7.3 | 1.0 a | 3 b | 83.3 | ||||||||||
| TXIB | 5.1 | 1.0 a | 3 b | 87.4 | ||||||||||
| HAA C12 | − | 7.0 a | 20 b | 53.1 | ||||||||||
| DBP | 5.8 | 10 a | 30 b | 91.5 | ||||||||||
| TINU326 | 11.0 | 10 a | 25 b | 72.1 | ||||||||||
| CHIMA81 | 1.8 | 2.0 a | 10 b | 100.8 | ||||||||||
| TINU327 | 3.2 | 10 a | 30 b | 80.6 | ||||||||||
| 2EHAdip | - | 1.0 a | 3 b | 9.1 | ||||||||||
| 2EHSeb | 2.9 | 1.0 a | 3 b | 64.7 | ||||||||||
| CYA1084 | − | 12 a | 30 b | 86.5 | ||||||||||
| IRGA38 | − | 1.0 a | 3 b | 78.1 | ||||||||||
| TOPAC | − | 5.0 a | 15 b | 33.3 | ||||||||||
| IRGA1076 | 12.0 | 3.0 a | 10 b | 80.4 | ||||||||||
| IRGA168 | − | 3.0 a | 10 b | 45.7 | ||||||||||
| IRGA1010 | − | 3.0 a | 10 b | 67.6 | ||||||||||
| FPSE-UHPLC-MS/MS | PTHF | Cellulose | Methanol- acetonitrile | 20 | Tap water, Osmosis effluent wastewater, Untreated effluent/ biological wastewater | NORET | Drugs Androgens & Progestogens | - | 33.5 | − | − | − | 80.6–94.4 | [35] |
| NOR | 1.7 | 94.1–103.5 | ||||||||||||
| MGA | 21.4 | 102.2–121.2 | ||||||||||||
| PRO | 6.9 | 79.8–84.2 | ||||||||||||
| BOL | 46.9 | 66.6–76.2 | ||||||||||||
| NAN | 50.7 | 82.8–102.4 | ||||||||||||
| ADTD | 19.4 | 65.9–77.9 | ||||||||||||
| DHEA | 264 | 77.6–87.4 | ||||||||||||
| TES | 2.2 | 76.6–81.4 | ||||||||||||
| AND | 63.6 | 92.2–98.9 | ||||||||||||
| FPSE-UHPLC-MS/MS | PTHF | Cellulose | Methanol- acetonitrile | 20 | Urine | NORET | Drugs Androgens & Progestogens | - | 33.5 | − | − | − | − | [35] |
| NOR | 1.7 | |||||||||||||
| MGA | 11.1 | |||||||||||||
| PRO | 12.8 | |||||||||||||
| BOL | 37.9 | |||||||||||||
| NAN | 50.1 | |||||||||||||
| ADTD | 25.6 | |||||||||||||
| DHEA | 110.6 | |||||||||||||
| TES | 8.9 | |||||||||||||
| AND | 80.0 | |||||||||||||
| FPSE-UHPLC-MS/MS | PDMDPS | Polyester | Methanol | 60 | Seawater | UV P | UV Stabilizers Personal care | - | 5.63 | 18.8 | − | − | − | [28] |
| UV 329 | 4.33 | 14.5 | ||||||||||||
| UV 326 | 8.96 | 29.9 | ||||||||||||
| UV 328 | 1.63 | 5.44 | ||||||||||||
| UV 327 | 1.06 | 3.54 | ||||||||||||
| UV 360 | 2.72 | 9.08 | ||||||||||||
| FPSE-UHPLC-MS/MS | PDMDPS | Polyester | Methanol | 60 | Sewage | UV P | UV Stabilizers Personal care | - | 12.8–25.3 | 42.7–84.3 | 82–96 | [27] | ||
| UV 329 | 12.2–19.8 | 40.7–66.0 | 48–61 | |||||||||||
| UV 326 | 51.6–60.7 | 172–202 | 49–58 | |||||||||||
| UV 328 | 9.44–18.1 | 31.5–60.3 | 43–59 | |||||||||||
| UV 327 | 36.2–38.6 | 121–129 | 65–73 | |||||||||||
| UV 571 | 40.0–44.3 | 133–148 | 49–53 | |||||||||||
| UV 360 | 6.01–7.34 | 20.0–24.5 | 35–46 | |||||||||||
| FPSE-LC-MS/MS | PEG | Cellulose | Methanol | 240 | River water, Effluent-Influent wastewater | MPB | Pharmaceuticals Personal care | - | 10 | 50 | − | − | 9–27 c | [31] |
| CBZ | 10 | 50 | 20–92 c | |||||||||||
| PrPB | 2 | 20 | 41–65 c | |||||||||||
| DHB | 5 | 50 | 44–74 c | |||||||||||
| BzPB | 1 | 20 | 45–67 c | |||||||||||
| DHMB | 2 | 20 | 50–74 c | |||||||||||
| DICLO | 1 | 20 | 44–73 c | |||||||||||
| BP-3 | 2 | 20 | 59–93 c | |||||||||||
| TCC | 3 | 10 | 57–59 c | |||||||||||
| TCS | 50 | 200 | 43–54 c | |||||||||||
| FPSE-GC-MS | PEG | Cellulose | Ethyl acetate | 120 | River water, Wastewater | IBU | Drugs anti-inflammatory | 418 | 0.8 | 3 | − | − | 82–109 | [25] |
| NAP | 263 | 2 | 3 | 93–111 | ||||||||||
| KET | 223 | 5 | 15 | 92–108 | ||||||||||
| DIC | 162 | 2 | 7 | 94–116 | ||||||||||
| Stir-FPSE-UPLC-DAD | PEG | Cellulose | Methanol | 60 | River water | Simazine | Herbicides | 444 | 140 | 460 | − | − | 84–124 | [30] |
| Atrazine | 729 | 240 | 790 | 75–126 | ||||||||||
| Secbumeton | 988 | 80 | 260 | 76–103 | ||||||||||
| Terbumeton | 1165 | 80 | 260 | 75–104 | ||||||||||
| Propazine | 996 | 110 | 360 | 75–97 | ||||||||||
| Prometryn | 1286 | 470 | 1500 | 78–111 | ||||||||||
| Terbutryn | 1411 | 80 | 260 | 78–99 | ||||||||||
| Stir-FPSE-HPLC-DAD | PTHF | Cellulose | Acetonitrile | 15 | Wastewater, Reservoir water | TBBPA | Flame Retardants | − | 30 | − | − | − | 93 | [32] |
| TBBPA-BAE | 20 | 95 | ||||||||||||
| TBBPA-BDBPE | 40 | 92–99 | ||||||||||||
| Stir-bar-FPSE-HPLC-DAD | PTHF | Cellulose | Acetonitrile | 10 | Wastewater, Reservoir water | TBBPA | Flame Retardants | − | 10 | − | − | − | 92–95 | [32] |
| TBBPA-BAE | 50 | 90–97 | ||||||||||||
| TBBPA-BDBPE | 10 | 91–98 | ||||||||||||
| DPSE-LC-MS/MS | PEG | Cellulose | Ethyl acetate | 10 | River water, Influent-Effluent wastewater | MPB | Pharmaceuticals Personal care | − | 4 | 50 | − | − | 12–30 c | [29] |
| CBZ | 4 | 50 | 18–53 c | |||||||||||
| PrPB | 2 | 50 | 20–64 c | |||||||||||
| DHB | 2 | 50 | 21–68 c | |||||||||||
| BzPB | 2 | 50 | 33–70 c | |||||||||||
| DHMB | 2 | 20 | 39–76 c | |||||||||||
| DICLO | 2 | 50 | 23–50 c | |||||||||||
| BP-3 | 2 | 100 | 45–52 c | |||||||||||
| TCC | 8 | 50 | 15–49 c | |||||||||||
| TCS | 20 | 100 | 22–43 c | |||||||||||
| FDSE-FI-FAAS | PDMDPS | Polyester | Methyl isobutyl ketone | 1.5 | River-Coastal-Ditch water | Lead | Toxic Metals | 140 | 1.8 μg ·L-1 | 6.0 μg ·L-1 | − | − | 95.0–101.0 | [36] |
| Cadmium | 38 | 0.4 μg ·L-1 | 1.2 μg ·L-1 | 94.0–98.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantzi, V.; Anthemidis, A. Fabric Sol–gel Phase Sorptive Extraction Technique: A Review. Separations 2017, 4, 20. https://doi.org/10.3390/separations4020020
Kazantzi V, Anthemidis A. Fabric Sol–gel Phase Sorptive Extraction Technique: A Review. Separations. 2017; 4(2):20. https://doi.org/10.3390/separations4020020
Chicago/Turabian StyleKazantzi, Viktoria, and Aristidis Anthemidis. 2017. "Fabric Sol–gel Phase Sorptive Extraction Technique: A Review" Separations 4, no. 2: 20. https://doi.org/10.3390/separations4020020
APA StyleKazantzi, V., & Anthemidis, A. (2017). Fabric Sol–gel Phase Sorptive Extraction Technique: A Review. Separations, 4(2), 20. https://doi.org/10.3390/separations4020020






