Experimental Application of an Advanced Separation Process for NOM Removal from Surface Drinking Water Supply
Abstract
:1. Introduction
2. Drinking Water NOM Removal: A Current State-Of-The-Art Summary
2.1. Coagulation
2.2. Activated Carbon Adsorption
2.3. Membrane Filtration
2.4. Oxidation and Advanced Oxidation Processes
2.5. Ballasted Flocculation and Separation
3. Materials and Methods
3.1. Experimental Design
3.2. Jar Tests
3.3. Pilot Testing
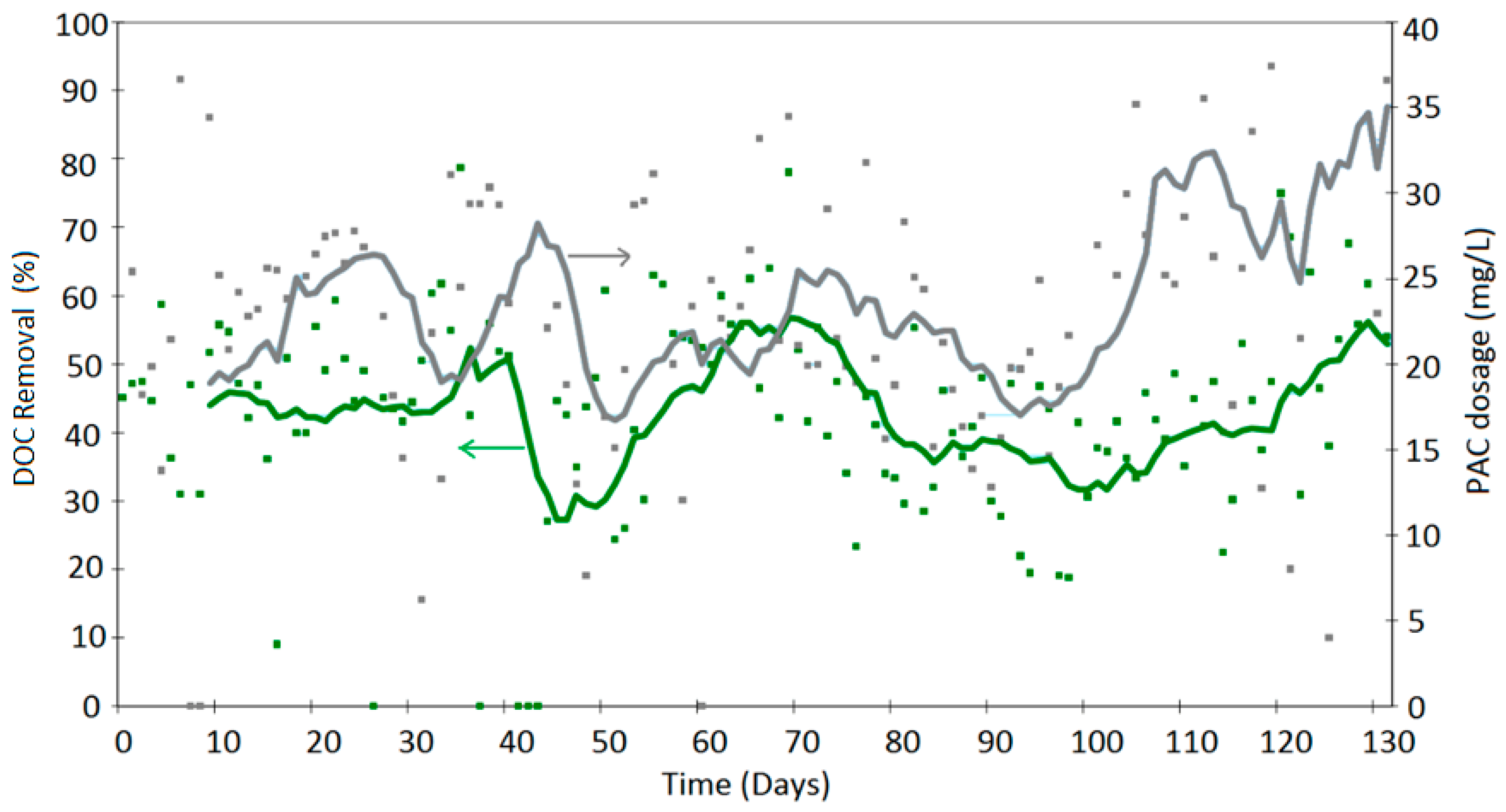
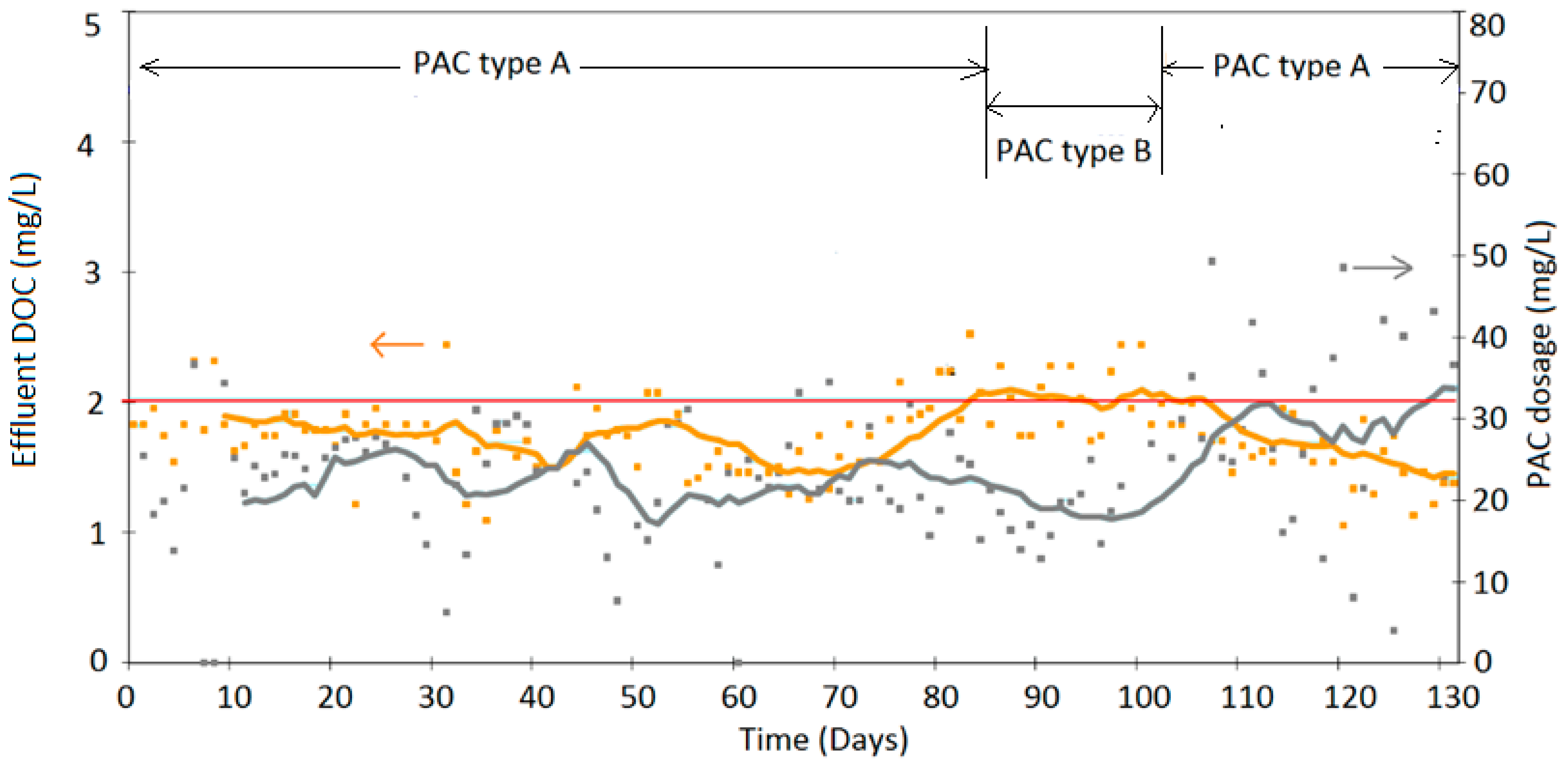
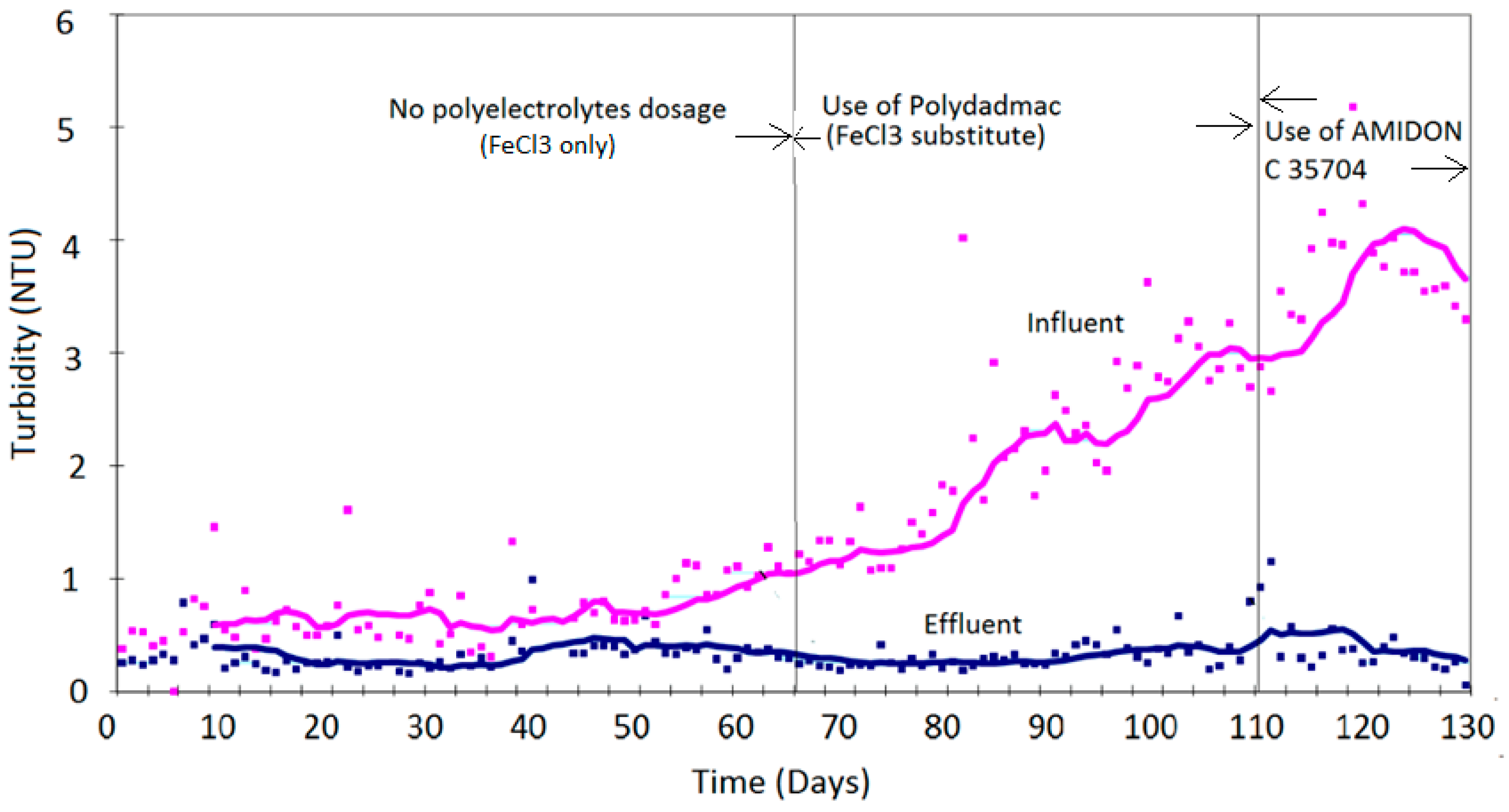
4. Results
Discussion
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Crouè, J.; Korshin, G.V.; Benjamin, M. Characterization of Natural Organic Matter in Drinking Water; American Water Works Association: Denver, CO, USA, 2000; ISBN 1583210156. [Google Scholar]
- Aiken, G.; McKnight, D.; Wershaw, R.; McCarthy, P. Humic Substances in Soil, Sediment and Water; John Wiley & Sons: New York, NY, USA, 1985. [Google Scholar]
- McCreary, J.J.; Snoeyink, V.L. Characterization and activated carbon adsorption of several humic substances. Water Res. 1980, 14, 151–160. [Google Scholar] [CrossRef]
- Leenheer, J.A.; Crouè, J.-P. Characterizing aquatic dissolved organic matter. Environ. Sci. Technol. 2003, 37, 18A–26A. [Google Scholar] [CrossRef] [PubMed]
- Copetti, D.; Valsecchi, L.; Capodaglio, A.G.; Tartari, G. Direct measurement of nutrient concentrations in freshwaters with miniaturized analytical probe: Evaluation and validation. Environ. Monit. Assess. 2017, 189, 144. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. In-stream detection of waterborne priority pollutants, and applications in drinking water Contaminant Warning Systems. Water Sci. Technol. Water Supply 2016, 17, 707–725. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Molognoni, D. Online monitoring of priority and dangerous pollutants in natural and urban waters: A state-of-the-art review. Manag. Environ. Qual. 2016, 27, 507–536. [Google Scholar] [CrossRef]
- CRC. Natural Organic Matter: Understanding and Controlling the Impact on Water Quality and Water Treatment Processes. In Management Implications from the Research Programs of the Cooperative Research Centre for Water Quality and Treatment; CRC: Salisbury, Australia, 2005; ISBN 187661644X. [Google Scholar]
- Luukkonen, T.; Tolonen, E.T.; Runtti, H.; Pellinen, J.; Tao, H.; Rämő, J.; Lassi, U. Removal of total organic carbon (TOC) residue from power plant make-up water by activated carbon. J. Water Process Eng. 2014, 3, 46–52. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Liu, S.; Jiang, F.; Shi, X.; Li, M.; Xu, X. Improvement of the assimilable organic carbon (AOC) analytical method for reclaimed water. Front. Environ. Sci. Eng. 2013, 7, 483–491. [Google Scholar] [CrossRef]
- Worrall, F.; Burt, T.P. Trends in DOC concentration in Great Britain. J. Hydrol. 2007, 346, 81–92. [Google Scholar] [CrossRef]
- Eikebrokk, B.; Vogt, R.D.; Liltved, H. NOM increase in Northern European source waters: Discussion of possible causes and impacts on coagulation/contact filtration processes. Water Sci. Technol. 2004, 4, 47–54. [Google Scholar]
- Matilainen, A.; Gjessing, E.G.; Lahtinen, T.; Hed, L.; Bhatnagar, A.; Sillanpää, A. An overview of the methods used in the characterisation of natural organic matter (NOM) in relation to drinking water treatment. Chemosphere 2011, 83, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Hozalski, R.M.; Bouwer, E.J.; Goel, S. Removal of natural organic matter (NOM) from drinking water supplies by ozone-biofiltration. Water Sci. Technol. 1999, 40, 157–163. [Google Scholar]
- Eikebrokk, B.; Juhna, T.; Melin, E.; Østerhus, S.W. Water Treatment by Enhanced Coagulation and Ozonation-Biofiltration. 2006. Available online: http://www.techneau.org/fileadmin/files/Publications/Publications/Deliverables/D5.3.2.pdf (accessed on 20 September 2017).
- Capodaglio, A.G. Integrated, decentralized wastewater management for resource recovery in rural and peri-urban areas. Resources 2017, 6, 22. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Callegari, A.; Cecconet, D.; Molognoni, D. Sustainability of decentralized wastewater treatment technologies. Water Pract. Technol. 2017, 12, 463–477. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Ghilardi, P.; Boguniewicz-Zablocka, J. New paradigms in urban water management for conservation and sustainability. Water Pract. Technol. 2016, 11, 176–186. [Google Scholar] [CrossRef]
- Capodaglio, A.G.; Muraca, A.; Becchi, G. Accounting for water quality effects of future urbanization: Diffuse pollution loads estimates and control in Mantua’s lakes (Italy). Water Sci. Technol. 2003, 47, 291–298. [Google Scholar] [PubMed]
- Aliverti, N.; Callegari, A.; Capodaglio, A.G.; Sauvignet, P. NOM removal from freshwater supplies by advanced separation technology. In Advanced Water Supply and Wastewater Treatment; Havlinek, P., Winkler, P., Marsalek, J., Mahrikova, I., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 49–61. [Google Scholar]
- Yoon, Y.; Amy, G.; Cho, J.; Her, N. Effects of retained natural organic matter (NOM) on NOM rejection and membrane flux decline with nanofiltration and ultrafiltration. Desalination 2005, 173, 209–221. [Google Scholar] [CrossRef]
- Butler, E.; Hung, Y.T.; Yeh, R.Y.L.; Al Ahmad, M.S. Electrocoagulation in Wastewater Treatment. Water 2011, 3, 495–525. [Google Scholar] [CrossRef]
- Check, J.K. Characterization and Removal of NOM from Raw Waters in Coastal Environments. Ph.D. Thesis, Georgia Institute of Technology, Atlanta, GA, USA, 2005. [Google Scholar]
- Dempsey, B.A. Removal of naturally occurring compounds by coagulation and sedimentation. Crit. Rev. Environ. Control. 1984, 14, 311–331. [Google Scholar] [CrossRef]
- Matilainen, A.; Lindqvist, N.; Korhonen, S.; Tuhkanen, T. Removal of NOM in the different stages of the water treatment process. Environ. Int. 2002, 28, 457–465. [Google Scholar] [CrossRef]
- Matilainen, A.; Vepsäläinen, M.; Sillanpää, M. Natural organic matter removal by coagulation during drinking water treatment: A review. Adv. Colloid Interface Sci. 2010, 159, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, G. Charge vs. porosity—some influences on the adsorption of natural organic matter (NOM) by activated carbon. Water Sci. Technol. 1999, 40, 191–198. [Google Scholar]
- Newcombe, G.; Morrison, J.; Hepplewhite, C. Simultaneous adsorption of MIB and NOM onto activated carbon: I. Characterization of system and NOM adsorption. Carbon 2002, 40, 2135–2146. [Google Scholar] [CrossRef]
- Jacangelo, J.G.; DeMarco, J.; Owen, D.M.; Randtke, S.J. Selected processes for removing NOM: An overview. J. Am. Water Works Assoc. 1995, 87, 64–77. [Google Scholar]
- Szlachta, M.; Adamski, W. Effects of natural organic matter removal by integrated processes: Alum coagulation and PAC-adsorption. Water Sci. Technol. 2009, 59, 1951–1957. [Google Scholar] [CrossRef] [PubMed]
- Boere, J.A. Combined use of ozone and granular activated carbon (GAC) in potable water treatment: Effects on GAC quality after reactivation. Ozone Sci. Eng. 1992, 14. [Google Scholar] [CrossRef]
- Guirguis, W.; Cooper, T.; Harris, J.; Ungar, A. Improved Performance of Activated Carbon by Pre-Ozonation. J. Water Pollut. Control Fed. 1978, 50, 308–320. [Google Scholar]
- Swietlik, J.; Raczyk-Stanislawiak, U.; Bilozor, S.; Ilecki, W.; Nawrocki, J. Adsorption of natural organic matter oxidized with ClO2 on granular activated carbon. Water Res. 2002, 36, 2328–2336. [Google Scholar] [CrossRef]
- Swietlik, J.; Dabrowska, A.; Raczyk-Stanislawiak, U.; Nawrocki, J. Reactivity of natural organic matter fractions with chlorine dioxide and ozone. Water Res. 2004, 38, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Huggins, T.; Wang, H.; Kearns, J.; Jenkins, P.; Ren, Z.J. Biochar as a sustainable electrode material for electricity production in microbial fuel cells. Bioresour. Technol. 2014, 157, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Callegari, A.; Capodaglio, A.G. Properties and beneficial uses of biochar from sewage sludge pyrolysis. In Proceedings of the 5th International Conference on Sustainable Solid Waste Management, Athens, Greece, 21–24 June 2017. [Google Scholar]
- Lidén, A.; Persson, K.M. Comparison between ultrafiltration and nanofiltration hollow-fiber membranes for removal of natural organic matter: A pilot study. J. Water Supply 2016, 65, 43–53. [Google Scholar] [CrossRef]
- Shen, J.; Schäfer, A.I. Factors affecting fluoride and natural organic matter (NOM) removal from natural waters in Tanzania by nanofiltration/reverse osmosis. Sci. Total Environ. 2015, 527–528, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Fan, H.; Guo, H.; Ji, S.; Zhang, G. Natural organic matter fouling behaviors on superwetting nanofiltration membranes. Water Res. 2016, 93, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Ødegaard, H.; Thorsen, T.; Melin, E. Practical experiences from membrane filtration plants for humic substance removal. Water Sci. Technol. 2000, 41, 33–41. [Google Scholar]
- Ødegaard, H.; Østerhus, S.; Melin, E.; Eikebrokk, B. NOM removal technologies –Norwegian experiences, Drink. Water Eng. Sci. 2010, 3, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Acero, J.L.; Javier Benitez, F.; Real, F.J.; Teva, F. Micropollutants removal from retentates generated in ultrafiltration and nanofiltration treatments of municipal secondary effluents by means of coagulation, oxidation, and adsorption processes. Chem. Eng. J. 2016, 289, 48–58. [Google Scholar] [CrossRef]
- Gottschalk, C.; Libra, J.; Saupe, A. Ozonation of Water and Wastewater: A practical Guide to Understanding Ozone and Its Application; Wiley-VCH: New York, NY, USA, 2000. [Google Scholar]
- Murray, C.A.; Parsons, S.A. Removal of NOM from drinking water: Fenton’s and photo-Fenton’s processes. Chemosphere 2004, 54, 1017. [Google Scholar] [CrossRef] [PubMed]
- Matilainen, A.; Sillanpää, M. Removal of natural organic matter from drinking water by advanced oxidation processes. Chemosphere 2010, 80, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Capodaglio, A.G. High-energy oxidation process: An efficient alternative for wastewater organic contaminants removal. Clean Technol. Environ. Policy 2017, 19, 1995–2006. [Google Scholar] [CrossRef]
- Trojanowicz, M.; Bojanowska-Czajka, A.; Capodaglio, A.G. Can radiation chemistry supply a highly efficient AO(R)P process for organics removal from drinking and waste water? A review. Environ. Sci. Pollut. Res. 2017, 24, 20187–20208. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Ghosh, N.C.; Kazmi, A.A. Ballasted sand flocculation for water, wastewater and CSO treatment. Environ. Technol. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Su, Z.Y.; Li, X.; Yang, Y.; Zhou, Z.; Song, W.; Sun, S. Treatment of micro-polluted surface water by ballasted flocculation and its floc structural characteristics. Appl. Mech. Mater. 2014, 448–453, 1147–1150. [Google Scholar] [CrossRef]
- Lee, W.; Westerhoff, P. Dissolved organic nitrogen removal during water treatment by aluminum sulfate and cationic polymer coagulation. Water Res. 2006, 40, 3767–3774. [Google Scholar] [CrossRef] [PubMed]
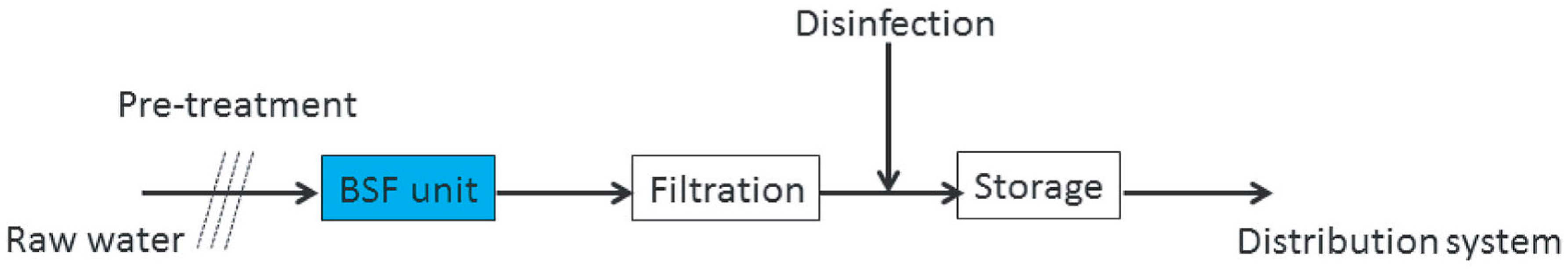
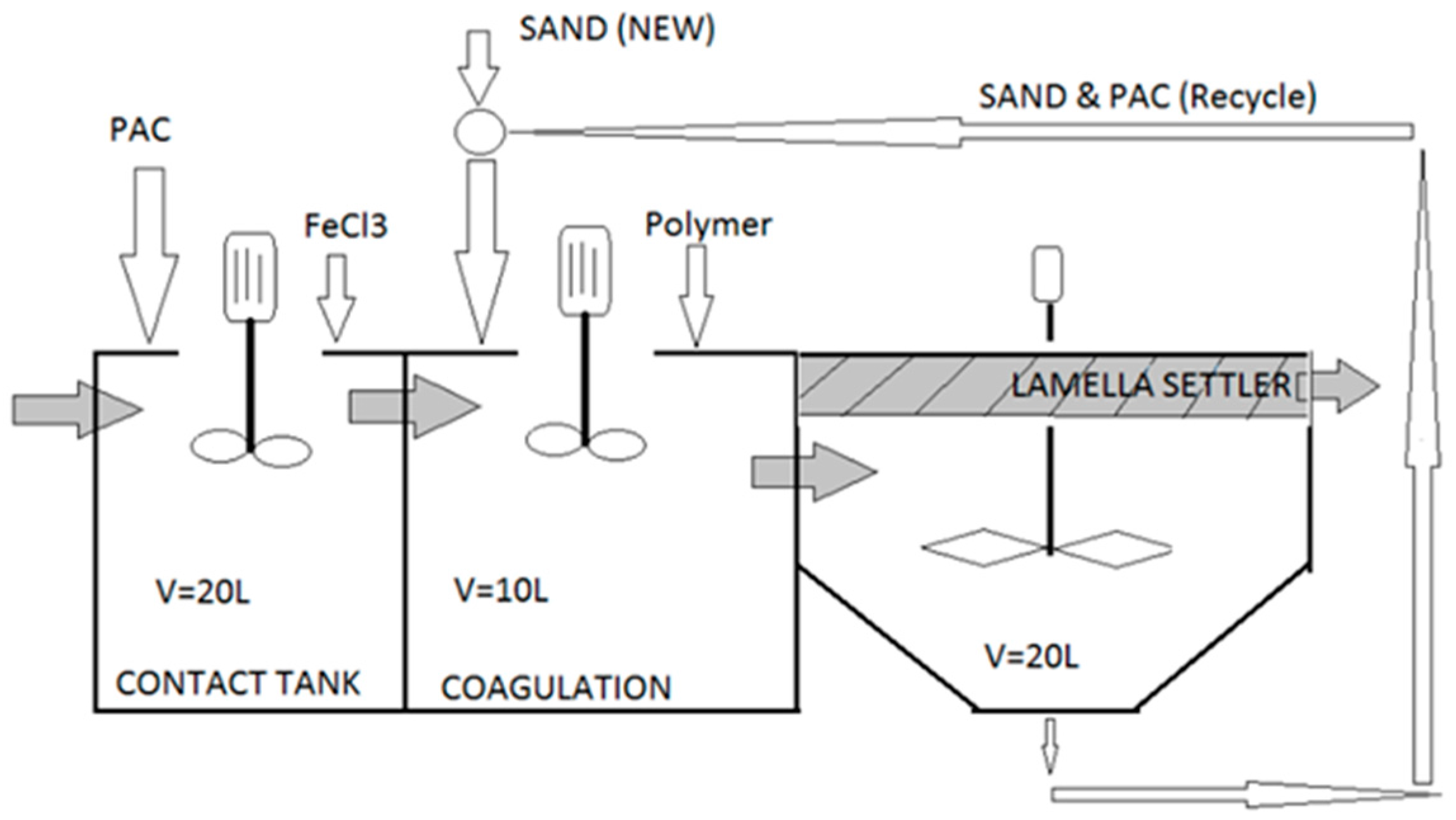

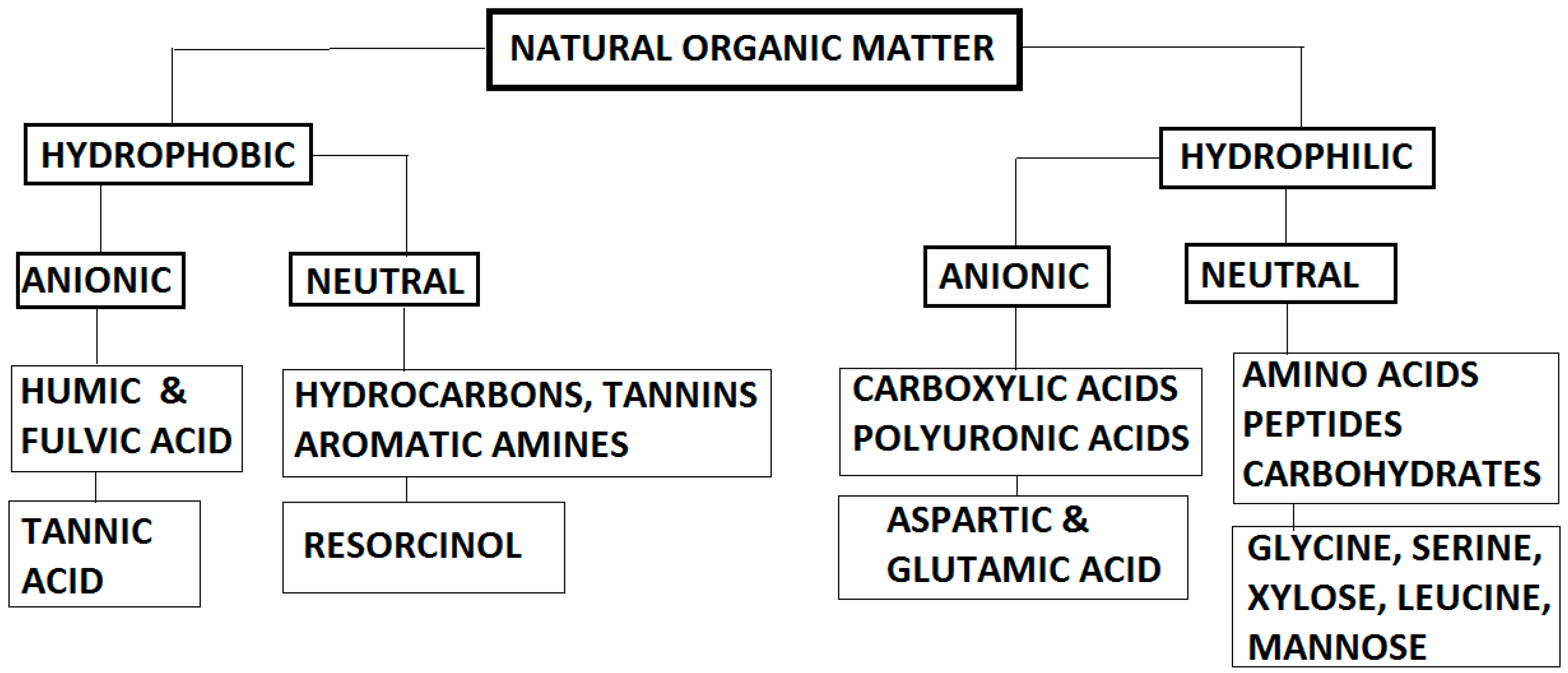
| Parameters | Analytical Tools |
|---|---|
| Colour | Visible spectrophotometry |
| Visual comparators | |
| Aromaticity (UV 1 absorbance) | UV spectrophotometry |
| Total organic carbon | DOC Analyser, LC-OCD 2 liquid chromatography |
| Dissolved organic carbon (DOC) | |
| Biodegradable organic carbon (BDOC) | |
| Assimilable organic carbon (AOC) | Bioassays (Pseudomonas fluorescens P17, Spirillum sp. NOX) or (Stenotrophomonas sp. ZJ2, Pseudomonas saponiphila G3 and Enterobacter sp. G6) |
| Bacterial regrowth | Bacterial regrowth potential (BRP) |
| Molecular weight distribution | High-performance size exclusion Chromatography (HPSEC) |
| Hydrophobicity/hydrophilicity | Rapid fractionation (RF) |
| Trihalomethane formation potential (THMFP) | Gas chromatography (GC) |
| Functional groups (aliphatic, aromatic, nitrogen-containing) | Gas chromatography-mass spectroscopy (GC-MS) |
| Infrared spectroscopy (FTIR) | |
| Nuclear magnetic resonance (NMR) |
| Observed Values | Turbidity (NTU) | DOC (mg/L) | pH | Conductivity (mS/cm) | UV 254 nm Absorption (Abs/m) |
|---|---|---|---|---|---|
| Average | 2.5 | 3.1 | 7.8 | 202 | 23 |
| Max value | 4.8 | 3.8 | 8.4 | 270 | 37.9 |
| Min value | 0.4 | 2.5 | 7.2 | 185 | 18.3 |
| Test No. | Additive(s) Studied | Jar 1 | Jar 2 | Jar 3 | Jar 4 | Jar 5 | Jar 6 |
|---|---|---|---|---|---|---|---|
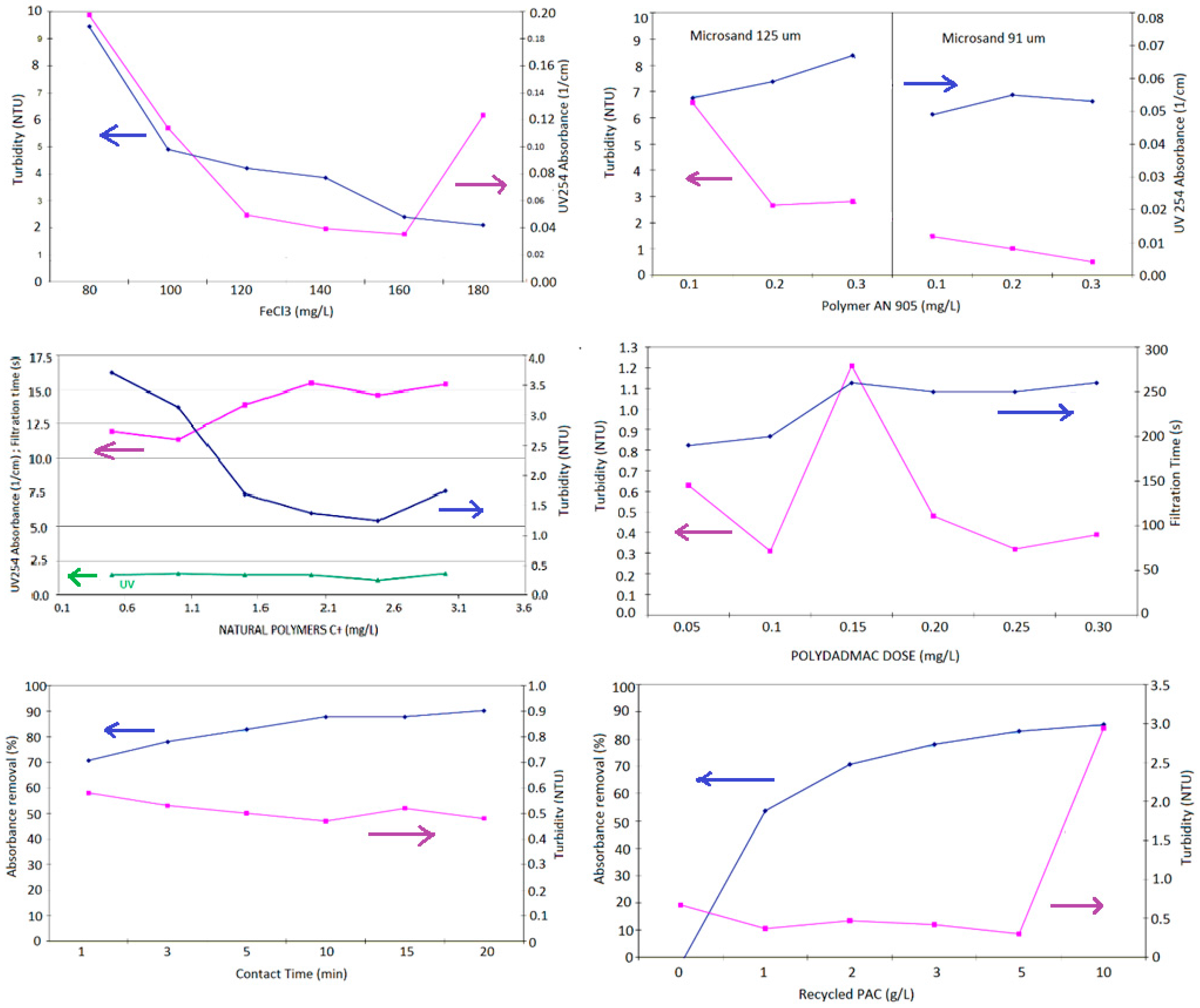
| 0 Non-ballasted | FeCl3 (mg/L) | 80 | 100 | 120 | 140 | 160 | 180 |
| Other test conditions (all jars): polymer AN 905: 0.2 mg/L, no sand HRTs: coagulation 2 min @ 200 rpm, flocculation 4 min @ 100 rpm, clarification 4 min | |||||||
| 1 Coagulant dosage | FeCl3 (mg/L) | 80 | 100 | 120 | 140 | 160 | 180 |
| Other test conditions (all jars): polymer AN 905: 0.2 mg/L, sand D50 91 μm: 5 g/L HRTs: coagulation 2 min @ 200 rpm, flocculation 4 min @ 100 rpm, clarification 4 min | |||||||
| 2 Flocculant dosage, sand type | AN 934 (mg/L) | 0.1 | 0.2 | 0.3 | 0.1 | 0.2 | 0.3 |
| SandD50 (μm) | 91 | 91 | 91 | 125 | 125 | 125 | |
| Other test conditions (all Jars): FeCl3: 160 mg/L, sand D50: 5 g/L (regardless of size) HRTs: coagulation 2 min @ 200 rpm, flocculation 1 min @ 200 rpm, clarification 4 min | |||||||
| 3 Coagulant w/biod. Polymer | FeCl3 (mg/L) | 0.2 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 |
| Other test conditions (all jars): sand D50 91 μm: 5 g/L, AMIDONC* 35704: 1.2 g/L HRTs: coagulation 1 min @ 200 rpm, flocculation 1 min @ 100 rpm, clarification 4 min | |||||||
| 4 Biodegrad. polymer | AMIDONC* 35704 (g/L) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 |
| Other test conditions (all jars): sand D50 91 μm: 5 g/L, FeCl3: 0.5 mg/L HRTs: coagulation 1 min @ 200 rpm, flocculation 1 min @ 100 rpm, clarification 4 min | |||||||
| 5 Organic coagulant | Polydadmac (g/L) | 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 |
| Other test conditions (all jars): sand D50 91 μm: 5 g/L, AN 905: 0.6 mg/L HRTs: coagulation 2 min @ 200 rpm, flocculation 5 min @ 100 rpm, clarification 4 min | |||||||
| 6 PAC contact time | Contact time (min) | 1 | 3 | 5 | 10 | 15 | 20 |
| Other test conditions (all jars): sand D50 91 μm: 5 g/L, AN 905: 0.8 mg/L, FeCl3: 0.3 mg/L, PAC = 3 mg/L HRTs: coagulation 2 min @ 200 rpm, flocculation 5 min @ 100 rpm, clarification 4 min | |||||||
| 7 Recycle PAC dosage | PAC (mg/L) | 0 | 1 | 2 | 3 | 5 | 10 |
| Other test conditions (all jars): Sand D50 91 μm: 5 g/L, AN 905: 0.8 mg/L, FeCl3: 0.3 mg/L HRTs: coagulation 10 min @ 200 rpm, flocculation 2 min @ 100 rpm, clarification 4 min | |||||||
| 8 New PAC dosage | PAC (mg/L) | 0 | 5 | 10 | 15 | 20 | 25 |
| Other test conditions (all jars): Sand D50 91 μm: 5 g/L, AN 905: 0.8 mg/L, FeCl3: 0.3 mg/L HRTs: coagulation 10 min @ 200 rpm, flocculation 2 min @ 100 rpm, clarification 4 min | |||||||
| Parameter/Additive | Range Tested and Units | Value for Which Best Results Were Observed |
|---|---|---|
| Flow | 50 ± 30% (L/h) | Not applicable |
| FeCl3 | Initially 80–180 mg/L, then 0.2–1 mg/L | 0.5 mg/L |
| Polydadmac | 0–5 mg/L | 0.1 mg/L |
| Amidon C 35704 | 0–5 mg/L | 1.2 mg/L |
| Anionic Polymer AN 905 | 0–1 mg/L | 0. |
| Anionic Polymer AN 934 | 0–1 mg/L | Not applicable |
| Sand 91 μm | 0–20 mg/L | 5 mg/L |
| Sand 125 μm | 0–10 mg/L | Not applicable |
| PAC | 0–40 mg/L | 20 mg/L |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callegari, A.; Boguniewicz-Zablocka, J.; Capodaglio, A.G. Experimental Application of an Advanced Separation Process for NOM Removal from Surface Drinking Water Supply. Separations 2017, 4, 32. https://doi.org/10.3390/separations4040032
Callegari A, Boguniewicz-Zablocka J, Capodaglio AG. Experimental Application of an Advanced Separation Process for NOM Removal from Surface Drinking Water Supply. Separations. 2017; 4(4):32. https://doi.org/10.3390/separations4040032
Chicago/Turabian StyleCallegari, Arianna, Joanna Boguniewicz-Zablocka, and Andrea G. Capodaglio. 2017. "Experimental Application of an Advanced Separation Process for NOM Removal from Surface Drinking Water Supply" Separations 4, no. 4: 32. https://doi.org/10.3390/separations4040032