In order to enhance the properties of silk fibroin nanoparticles, SFm-NPs and silk fibroin–bovine serum albumin nanoparticles (SF-BSA-NPs) were prepared via the introduction of magnetic nanoparticles (Fe3O4) and BSA during the silk fibroin nanoparticle formulation, and the drug encapsulation and release efficiency of these silk fibroin nanoparticles were examined in detail. Since propranolol presented the highest encapsulation and release performance, it was used as the model drug for the performance of the two modified types of silk fibroin nanoparticles.
3.5.1 Characterization of Modified Nanoparticles
DLS measurements for silk fibroin-bovine serum albumin nanoparticles and magnetic silk fibroin nanoparticles are presented in
Table 5. Both samples indicated a small polydispersity index of 0.30 and 0.36, respectively, indicating a uniform diameter distribution of nanoparticles.
The encapsulation efficiency of magnetic silk fibroin and silk fibroin–bovine serum albumin nanoparticles is presented in
Table 6. From the Table, it can be seen that the modification of silk fibroin with magnetic nanoparticles and bovine serum albumin increased the drug encapsulation compared to the pure silk fibroin nanoparticles. Propranolol is a hydrophilic drug and the presence of bovine serum albumin makes the silk fibroin–bovine serum albumin nanoparticles more hydrophilic; for this reason the encapsulation efficiency was increased. Also, the increased encapsulation efficiency of magnetic silk fibroin loaded with propranolol may be attributed to the adsorption of propranolol on Fe
3O
4.
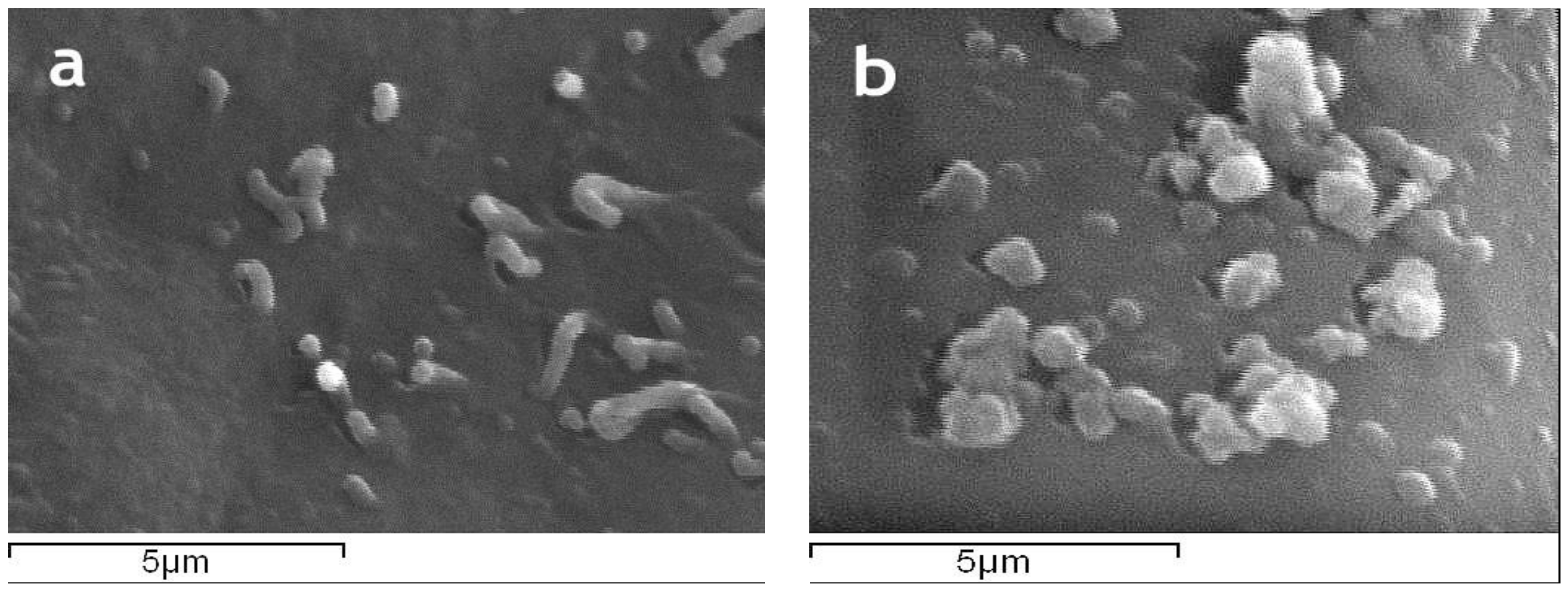
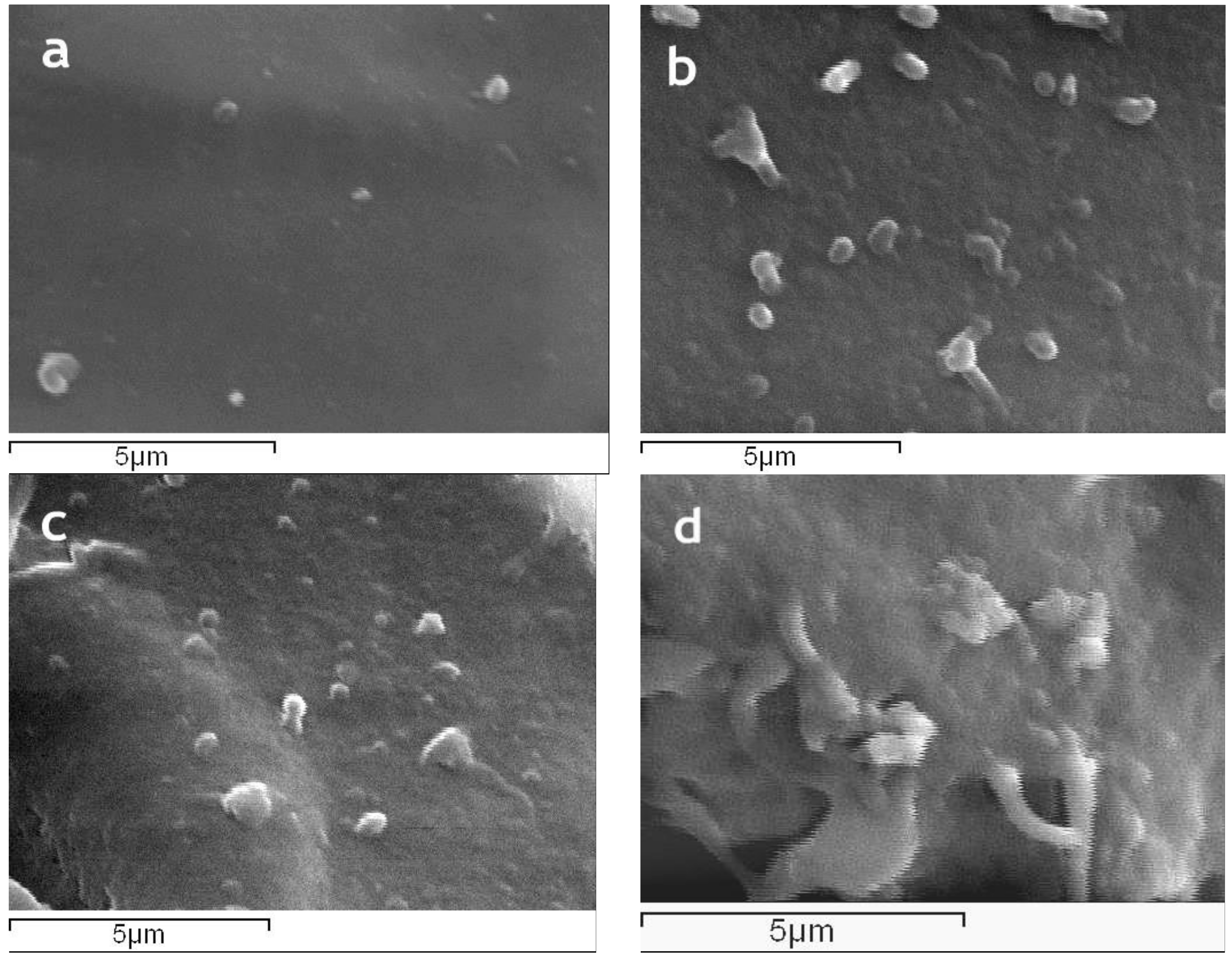
The surface morphology of magnetic silk fibroin particles and the silk fibroin-bovine serum albumin nanoparticles as well as their encapsulated-with-propranolol counterparts, illustrated by SEM images, is presented in
Figure 5. The magnetic silk fibroin (SFm) and silk fibroin-bovine serum albumin nanoparticles (SF-BSA) exhibited a spherical shape with a diameter of about 288 nm, while the propranolol-encapsulated silk fibroin-bovine serum albumin nanoparticles (SF-BSA + propranolol) and propranolol-encapsulated magnetic silk fibroin nanoparticles (SFm + propranolol) exhibited a particle size of about 384 nm and 490 nm, respectively (
Figure 5).
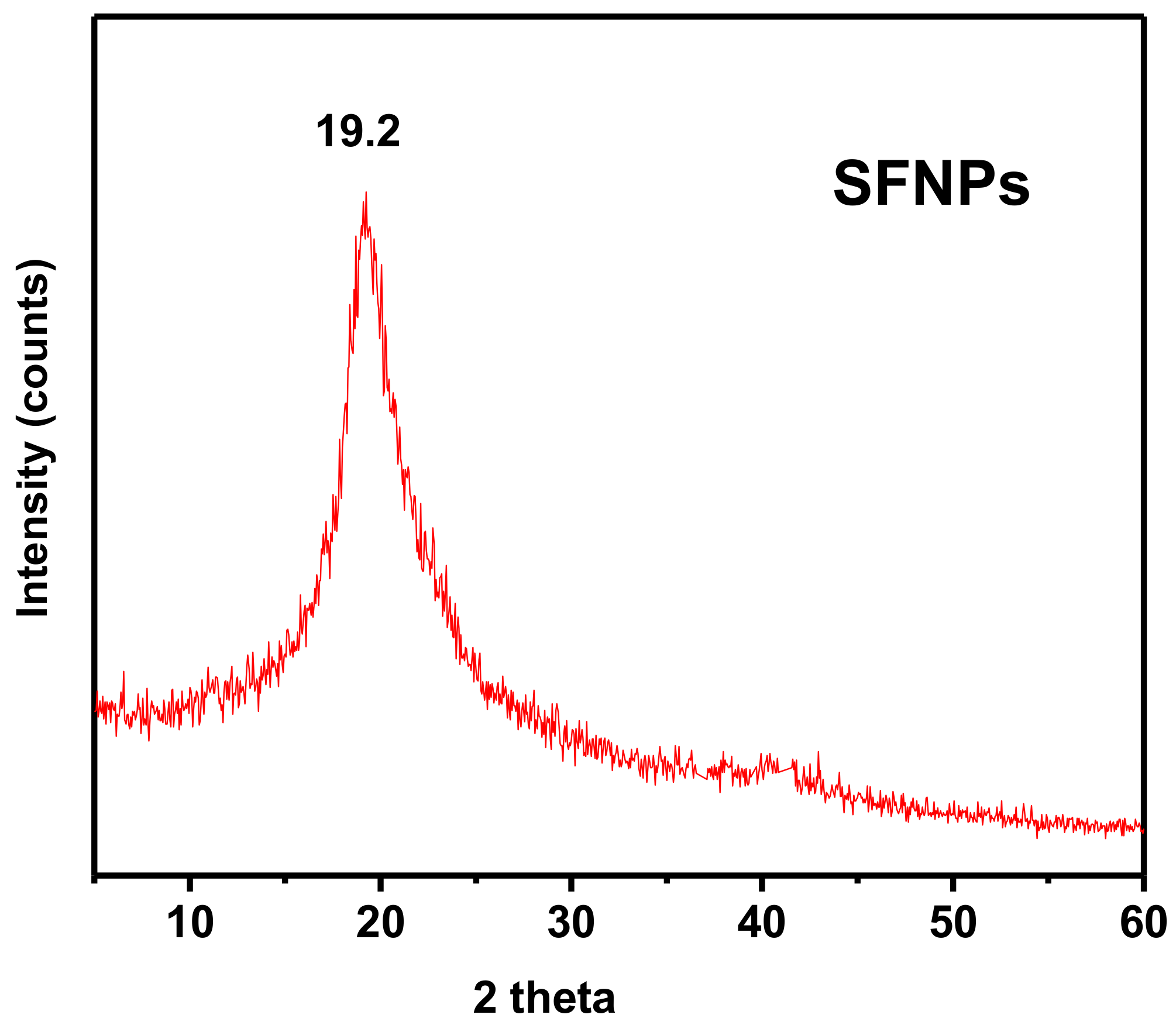
The 2θ diffraction peak presented in the XRD pattern of the magnetic silk fibroin nanoparticles (
Figure 6a), that appeared at 2θ = 19.2°, could be attributed to silk I, a random coil of silk fibroin, while for the encapsulated-with-propranolol magnetic silk fibroin nanoparticles, the characteristic peak at 2θ = 20.7° could be attributed to silk II, a β-sheet of fibroin. In addition, diffraction peaks corresponding to the (3 1 1), (4 0 0), and (4 4 0) planes of the cubic crystal structure (fcc) of Fe
3O
4, show the formation of magnetic silk fibroin nanoparticles [
19]. The aforementioned XRD results are in agreement with the FTIR results.
Prior to the measurements, the instrument was calibrated against a NIST-certified Ni standard. Each sample had approximately the same dimensions and an additional calibration procedure was performed in order to minimize shape effects. All measurements were performed in a controlled room temperature of 22 °C and consisted of a gradual increase of the applied external magnetic field up to about +1.9 T (μ
0H) then down to −1.9 T and again up to +1.9 T while measuring the magnetic moment of the sample. The magnetic properties are attributed to the Fe
3O
4 particles and the overall appearance of the loop is typical for nanoparticles of that composition and size. All measurements have exactly the same appearance, i.e., initial slope, curvature, and saturation plateau, indicating that there are no significant differences in the Fe
3O
4 particles between the samples and that the procedure for the preparation of the magnetic silk fibroin nanoparticles (SFm-NPs) does not affect significantly the magnetic properties of the Fe
3O
4 particles. Remanence, Mr, the Mass Magnetization which corresponds to a zero field after the first application of the maximum external field equals to 5–7% of the saturation magnetization and coercivity, μ
0H
c, the reverse external field which zeroes the magnetic moment of the sample is about 0.01 T. Both the remanence and the coercivity are very low in all cases as expected for a soft ferromagnetic material. Saturation magnetization, the maximum Mass Magnetization which could be observed, was obtained for the sample via extrapolation from data of
Figure 6b,c and was found to be σ
s = 27.0 (8b) and 27.9 (8c) emu/g. These results correspond to about 50% wt. Fe
3O
4 content, since the saturation magnetization depends on the Fe
3O
4 content which is diluted in the silk fibroin, confirmed that Fe
3O
4 particles are successfully incorporated into the silk fibroin and that their magnetic properties are preserved after drug encapsulation.
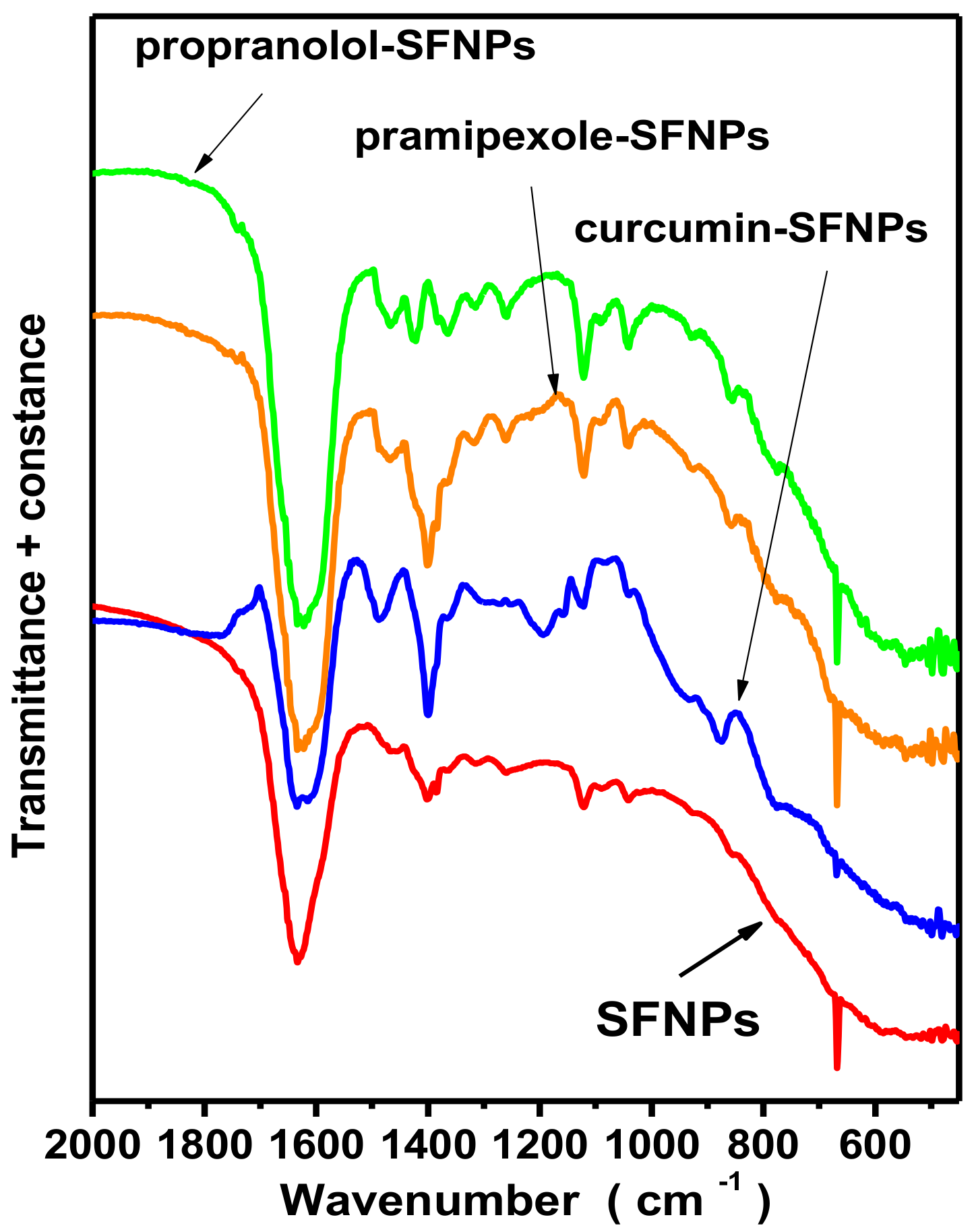
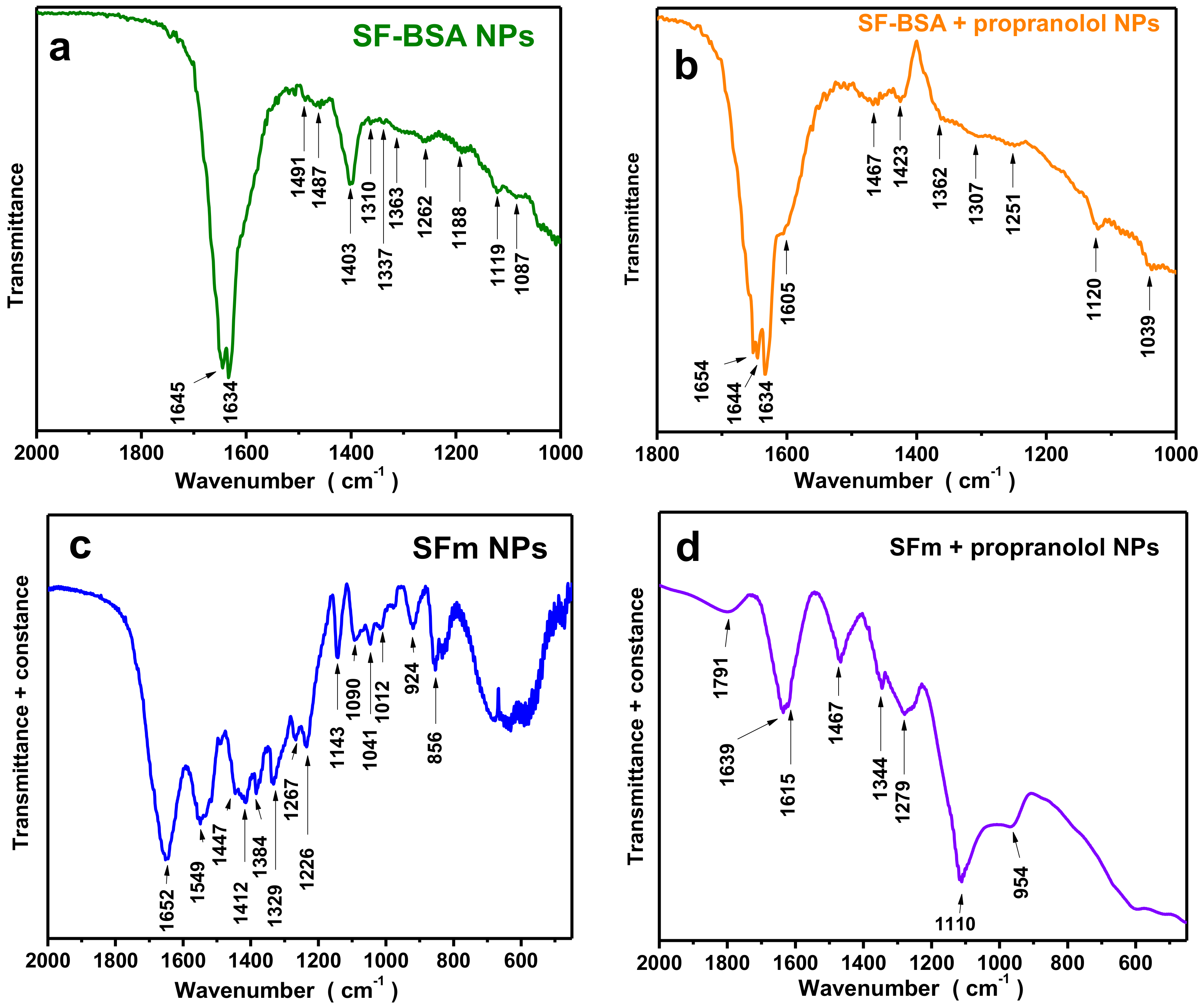
FTIR results of silk fibroin-bovine serum albumin nanoparticles and propranolol-encapsulated silk fibroin-bovine serum albumin nanoparticles are presented in
Figure 7a,b, respectively, and the characteristic peaks are presented in
Table 7. The bands at 1645 cm
−1 and 1491 cm
−1 are due to the BSA modification and can be attributed to amide I (C=O stretching) and amide II (C–N stretching and N–H bending) vibrations of BSA, respectively (
Table 7). The bands of BSA at 1392 cm
−1 (–CH
2 bending) and ~1260 cm
−1 (amide III, C–N stretching, and N–H bending) may be overlapped by the bands attributed to silk fibroin [
7]. A slight shift in the spectra for the propranolol-loaded nanoparticles could be due to drug binding.
In
Figure 7c,d, the FTIR results of fibroin magnetic nanoparticles (SFm) and propranolol-encapsulated fibroin magnetic nanoparticles (SFm + propranolol) are presented, while the characteristic peaks are also presented in detail in
Table 7. The spectra presented characteristic bands at 471 and 588 cm
−1 and 506 and 595 cm
−1, respectively, attributed to Fe–O bonds [
4], which confirmed the successful preparation of magnetic silk fibroin nanoparticles (
Figure 7c). From the spectra, the existence of both β-sheets and a-helices for magnetic fibroin nanoparticles could be observed, while for the propranolol-encapsulated silk fibroin magnetic nanoparticles β-sheets could be also observed.