Stress Reduction of a V-Based BCC Metal Hydride Bed Using Silicone Oil as a Glidant
Abstract
:1. Introduction
2. Results
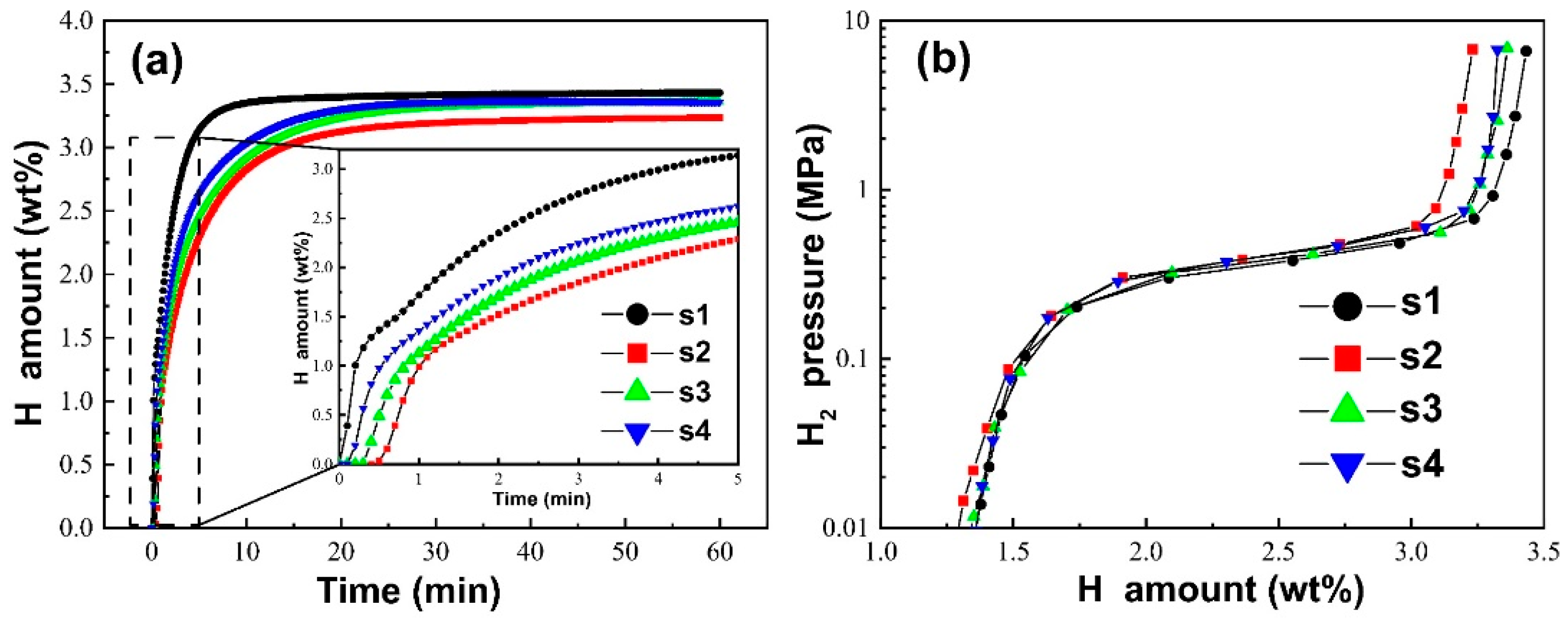
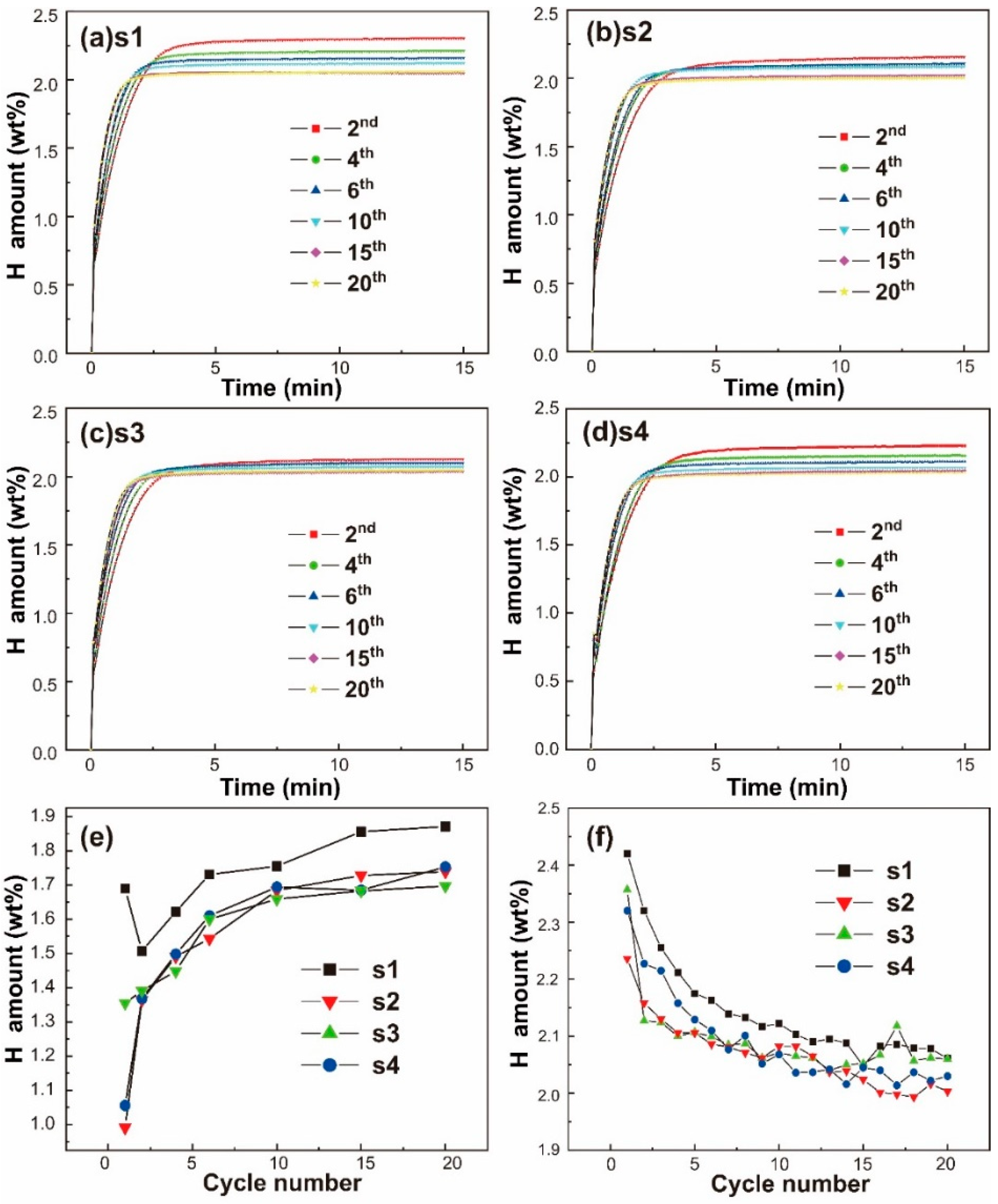
2.1. Hydrogen Storage Properties
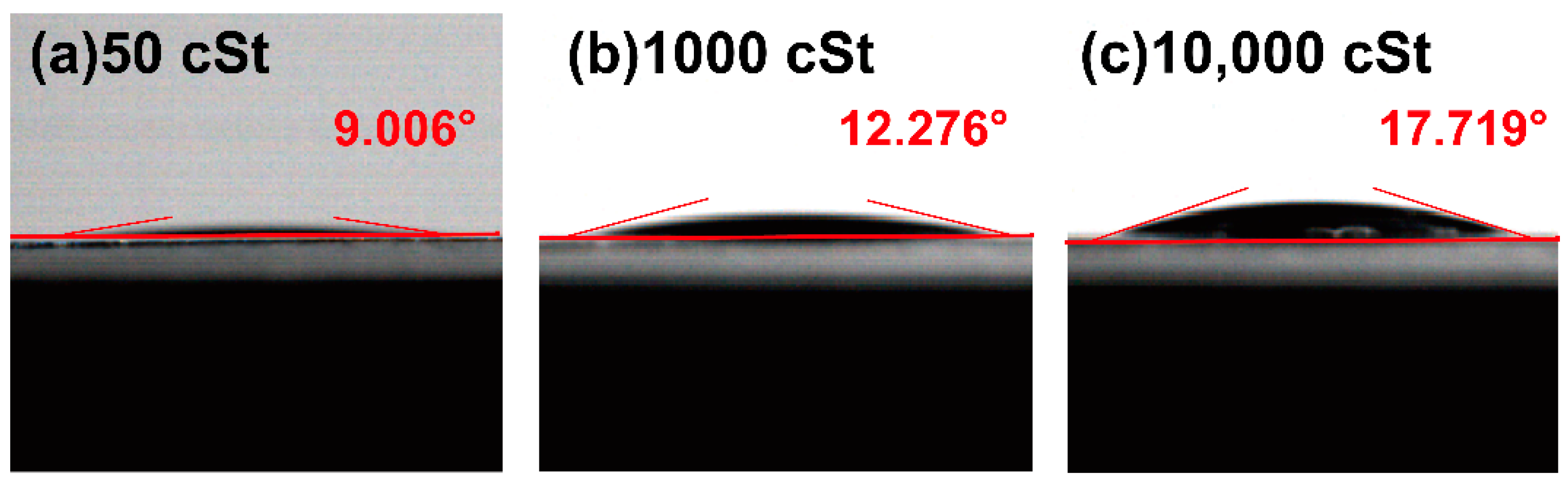
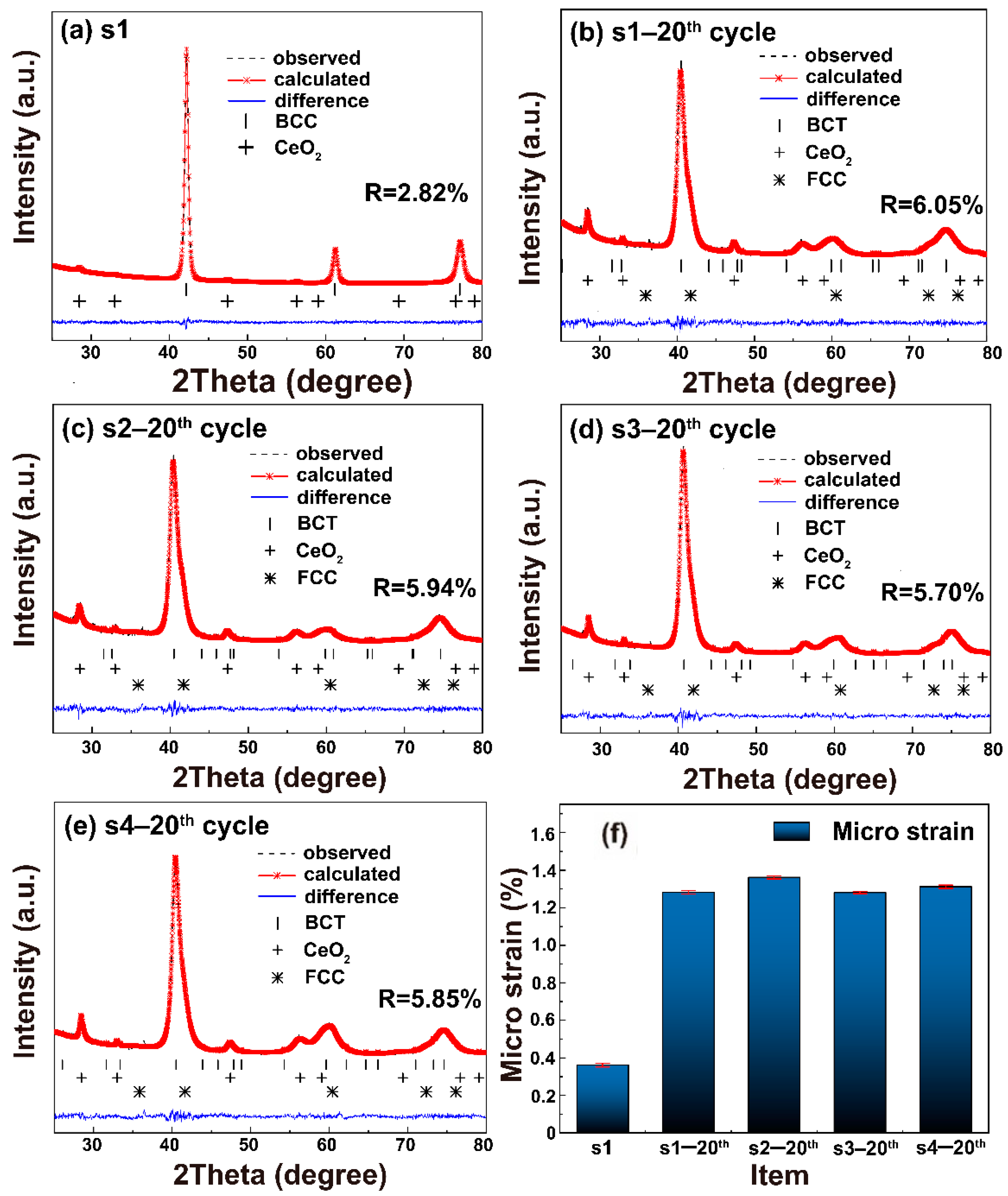
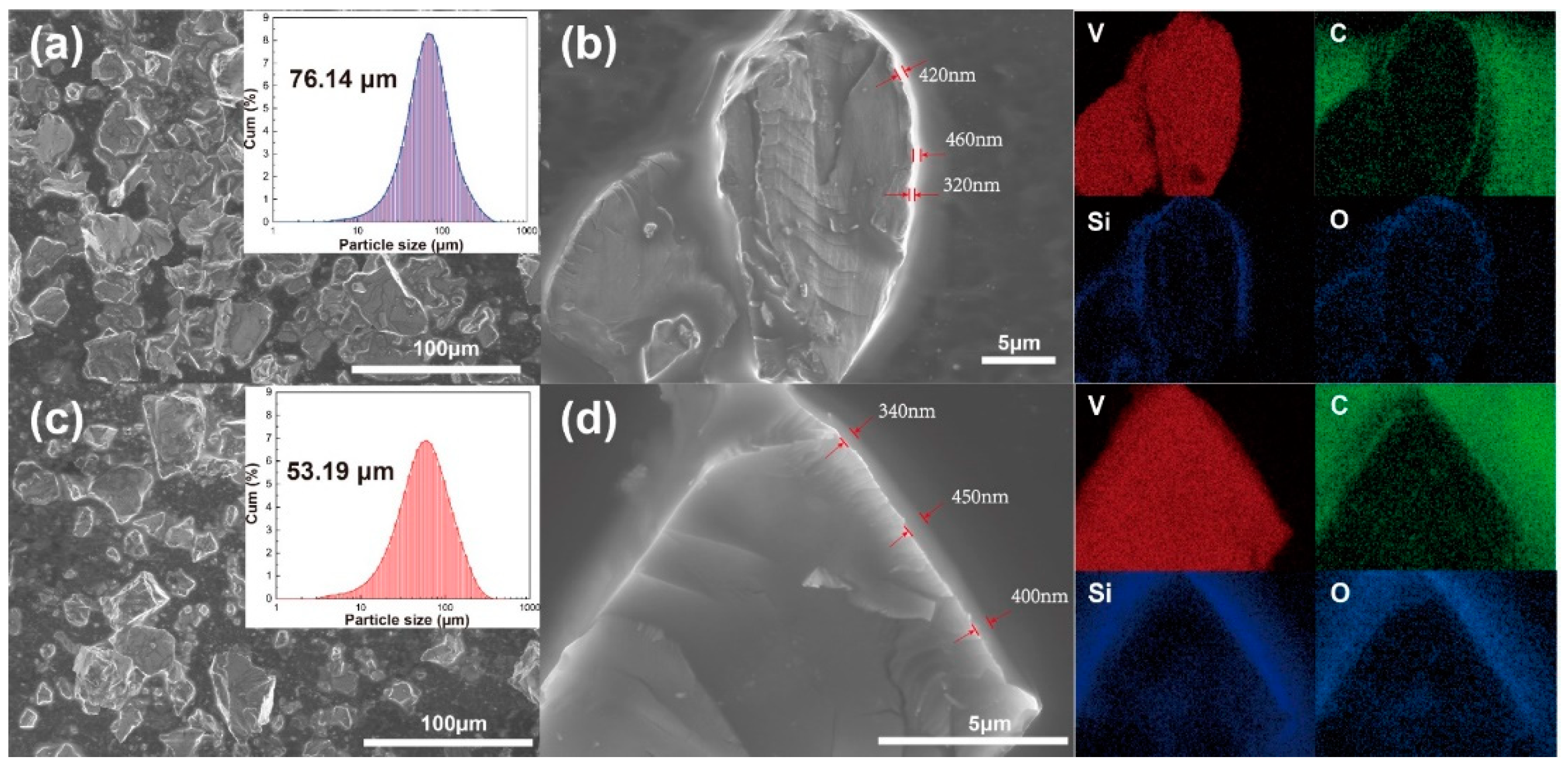
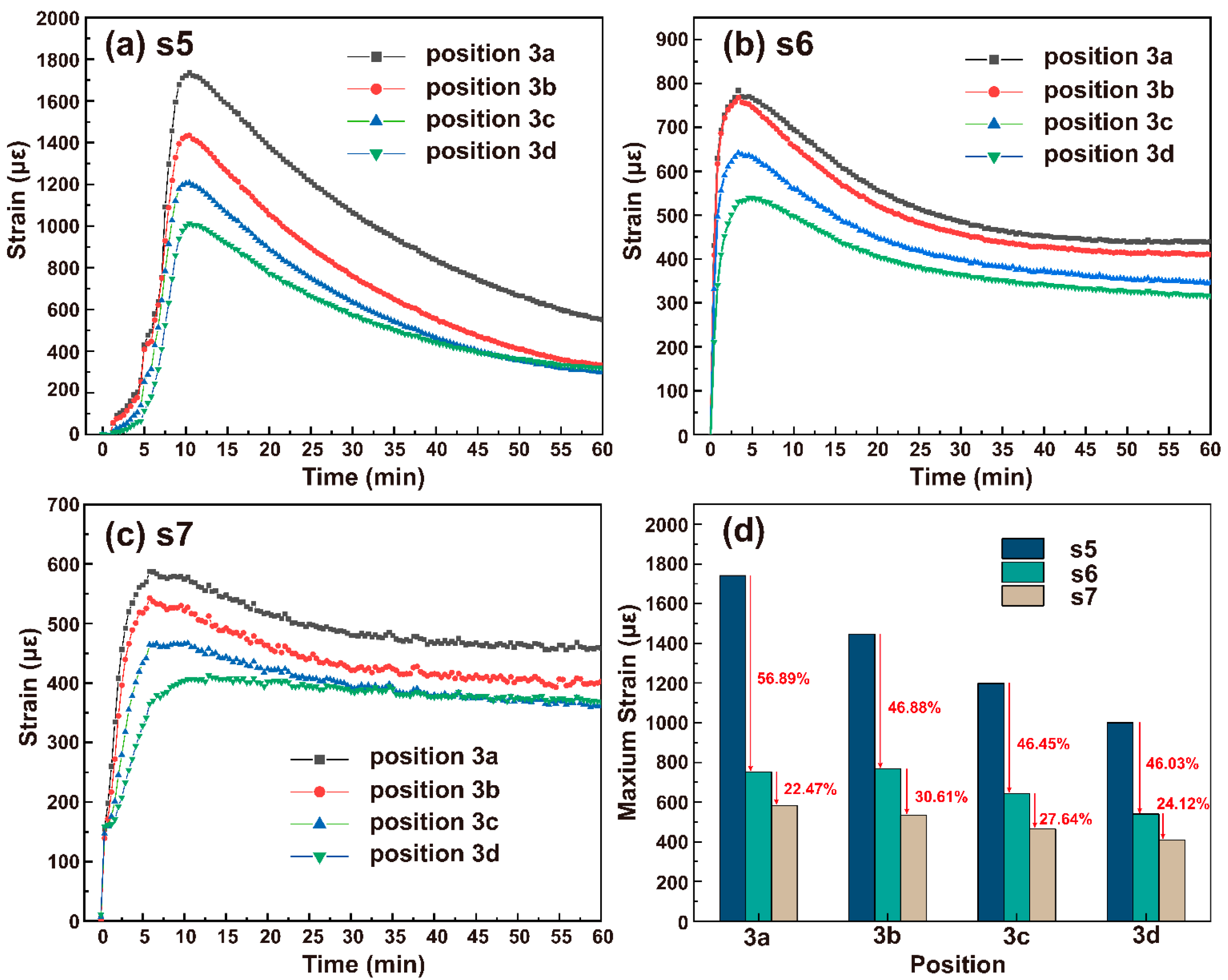
2.2. Influence of the Silicone Oil on Strain Distribution
3. Materials and Methods
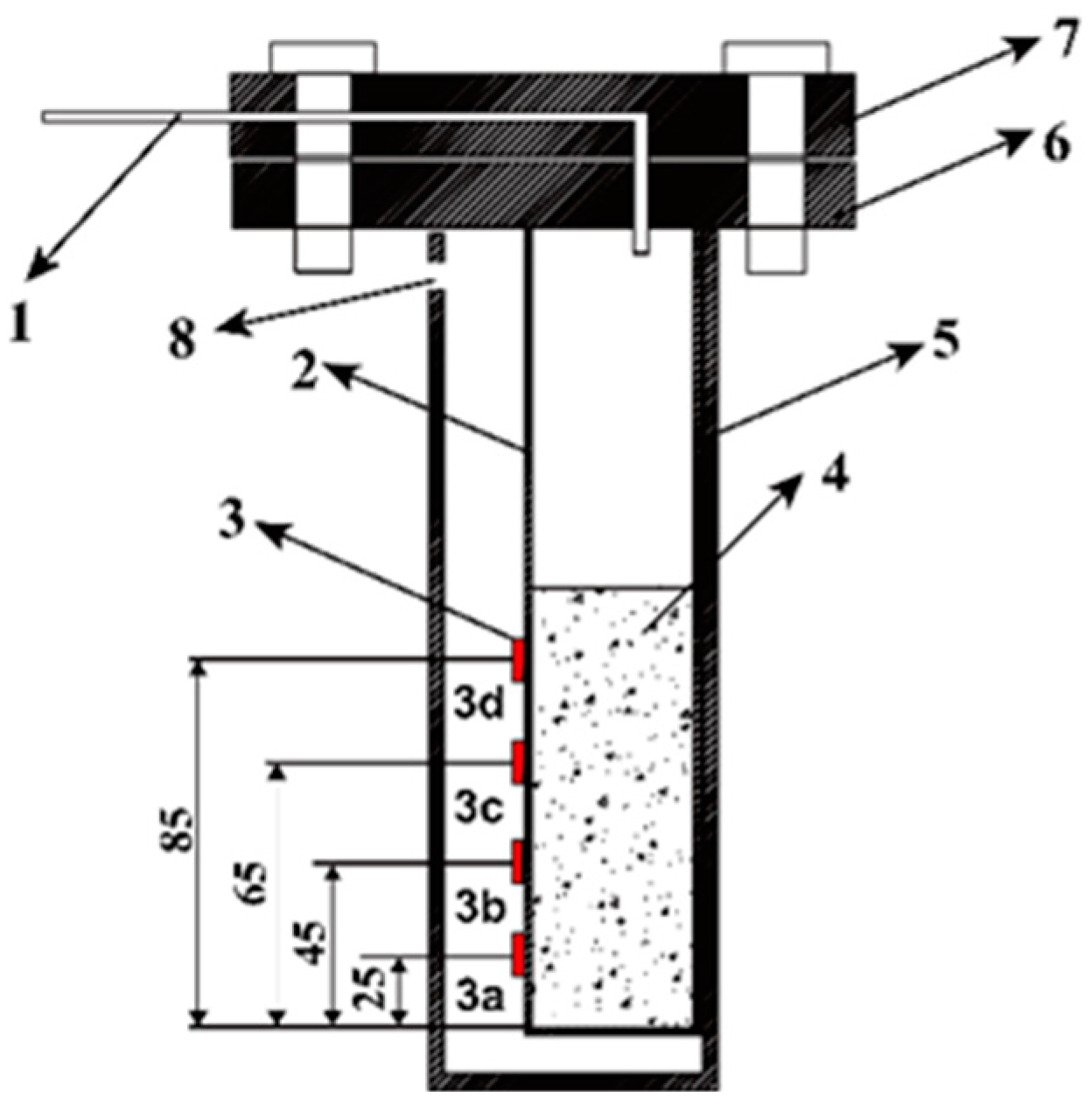
3.1. Sample Preparation
3.2. Strain Test
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef] [PubMed]
- Tarhan, C.; Çil, M.A. A study on hydrogen, the clean energy of the future: Hydrogen storage methods. J. Energy Storage 2021, 40, 102676. [Google Scholar] [CrossRef]
- Manoharan, Y.; Hosseini, S.E.; Butler, B.; Alzhahrani, H.; Senior, B.T.F.; Ashuri, T.; Krohn, J. Hydrogen Fuel Cell Vehicles; Current Status and Future Prospect. Appl. Sci. 2019, 9, 2296. [Google Scholar] [CrossRef] [Green Version]
- Usman, M.R. Hydrogen storage methods: Review and current status. Renew. Sustain. Energy Rev. 2022, 167. [Google Scholar] [CrossRef]
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4574. [Google Scholar] [CrossRef]
- Eberle, U.; Felderhoff, M.; Schüth, F. Chemical and Physical Solutions for Hydrogen Storage. Angew. Chem. Int. Ed. 2009, 48, 6608–6630. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrog. Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Pasini, J.M.; Corgnale, C.; van Hassel, B.; Motyka, T.; Kumar, S.; Simmons, K.L. Metal hydride material requirements for automotive hydrogen storage systems. Int. J. Hydrogen Energy 2013, 38, 9755–9765. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, M.; Monde, M.; Mitsutake, Y. Experimental formula for estimating porosity in a metal hydride packed bed. Int. J. Hydrogen Energy 2013, 38, 7056–7064. [Google Scholar] [CrossRef]
- Matsushita, M.; Tajima, I.; Abe, M.; Tokuyama, H. Experimental study of porosity and effective thermal conductivity in packed bed of nano-structured FeTi for usage in hydrogen storage tanks. Int. J. Hydrogen Energy 2019, 44, 23239–23248. [Google Scholar] [CrossRef]
- Smith, K.C.; Fisher, T.S. Models for metal hydride particle shape, packing, and heat transfer. Int. J. Hydrogen Energy 2012, 37, 13417–13428. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.-K.; Chen, Y.-C. Effects of cyclic hydriding–dehydriding reactions of LaNi5 on the thin-wall deformation of metal hydride storage vessels with various configurations. Renew. Energy 2012, 48, 404–410. [Google Scholar] [CrossRef]
- Ao, B.; Chen, S.; Jiang, G. A study on wall stresses induced by LaNi5 alloy hydrogen absorption–desorption cycles. J. Alloys Compd. 2005, 390, 122–126. [Google Scholar] [CrossRef]
- Tran, X.; McDonald, S.; Gu, Q.; Nogita, K. In-situ synchrotron X-ray diffraction investigation of the hydriding and dehydriding properties of a cast Mg–Ni alloy. J. Alloys Compd. 2015, 636, 249–256. [Google Scholar] [CrossRef]
- Kojima, Y.; Kawai, Y.; Towata, S.-I.; Matsunaga, T.; Shinozawa, T.; Kimbara, M. Development of metal hydride with high dissociation pressure. J. Alloys Compd. 2006, 419, 256–261. [Google Scholar] [CrossRef]
- Hu, H.; Ma, C.; Chen, Q. Mechanism and microstructural evolution of TiCrVFe hydrogen storage alloys upon de-/hydrogenation. J. Alloys Compd. 2021, 877, 160315. [Google Scholar] [CrossRef]
- Saito, T.; Suwa, K.; Kawamura, T. Influence of expansion of metal hydride during hydriding–dehydriding cycles. J. Alloys Compd. 1997, 253–254, 682–685. [Google Scholar] [CrossRef]
- Qin, F. Pulverization, expansion of La0.6Y0.4Ni4.8Mn0.2 during hydrogen absorption–desorption cycles and their influences in thin-wall reactors. Int. J. Hydrogen Energy 2008, 33, 709–717. [Google Scholar] [CrossRef]
- Charlas, B.; Gillia, O.; Doremus, P.; Imbault, D. Experimental investigation of the swelling/shrinkage of a hydride bed in a cell during hydrogen absorption/desorption cycles. Int. J. Hydrogen Energy 2012, 37, 16031–16041. [Google Scholar] [CrossRef]
- Lin, C.-K.; Huang, S.-M.; Jhang, Y.-H. Effects of cyclic hydriding–dehydriding reactions of Mg2Ni alloy on the expansion deformation of a metal hydride storage vessel. J. Alloys Compd. 2011, 509, 7162–7167. [Google Scholar] [CrossRef]
- Wu, K.; Cai, B.; Fan, L.; Qin, L.; Chen, D.; Huang, Y. Stress measurement of MlNi4.5Cr0·45Mn0.05 alloy during hydrogen absorption-desorption process in a cylindrical reactor. Int. J. Hydrogen Energy 2020, 45, 28175–28182. [Google Scholar] [CrossRef]
- Heubner, F.; Hilger, A.; Kardjilov, N.; Manke, I.; Kieback, B.; Gondek, Ł.; Banhart, J.; Röntzsch, L. In-operando stress measurement and neutron imaging of metal hydride composites for solid-state hydrogen storage. J. Power Sources 2018, 397, 262–270. [Google Scholar] [CrossRef]
- Duan, W.; Du, J.; Wang, Z.; Niu, Y.; Huang, T.; Li, Z.; Pu, C.; Wu, Z. Strain variation on the reaction tank of high hydrogen content during hydrogen absorption-desorption cycles. Int. J. Hydrogen Energy 2013, 38, 2347–2351. [Google Scholar] [CrossRef]
- Melnichuk, M.; Cuscueta, D.J.; Silin, N. Effect of glidants on LaNi5 powder flowability. Int. J. Hydrogen Energy 2018, 43, 6219–6228. [Google Scholar] [CrossRef]
- Cuscueta, D.; Silin, N.; Melnichuk, M. Stress reduction in a hydride container by the addition of a glidant agent. Int. J. Hydrogen Energy 2020, 45, 27452–27456. [Google Scholar] [CrossRef]
- Meyer, K.; Zimmermann, I. Effect of glidants in binary powder mixtures. Powder Technol. 2004, 139, 40–54. [Google Scholar] [CrossRef]
- Wang, Q.-D.; Wu, J.; Chen, C.-P.; Li, Z.-P. An investigation of the mechanical behaviour of hydrogen storage metal beds on hydriding and dehydriding and several methods of preventing the damage of hydride containers caused by the expansion of hydrogen storage metals. J. Less Common Met. 1987, 131, 399–407. [Google Scholar] [CrossRef]
- Heubner, F.; Pohlmann, C.; Mauermann, S.; Kieback, B.; Röntzsch, L. Mechanical stresses originating from metal hydride composites during cyclic hydrogenation. Int. J. Hydrogen Energy 2015, 40, 10123–10130. [Google Scholar] [CrossRef]
- Yan, Y.; Chen, Y.; Wu, C.; Tao, M.; Liang, H. A low-cost BCC alloy prepared from a FeV80 alloy with a high hydrogen storage capacity. J. Power Sources 2007, 164, 799–802. [Google Scholar] [CrossRef]
- Yu, H.; Zhai, G.; Zhang, Y.; Li, Y.; Liu, Y.; Liu, Y. Structure and thermal stability study of dimethicone (in Chinese). Shanghai Meas. Test. 2020, 47, 28–32. [Google Scholar]
- Wu, C.; Zheng, X.; Chen, Y.; Tao, M.; Tong, G.; Zhou, J. Hydrogen storage and cyclic properties of V60Ti(21.4+x)Cr(6.6−x)Fe12 (0 ≤ x ≤ 3) alloys. Int. J. Hydrogen Energy 2010, 35, 8130–8135. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, X.; Kong, H.; Chu, D.; Hu, F.; Wang, Y.; Yan, Y.; Wu, C. Stress Reduction of a V-Based BCC Metal Hydride Bed Using Silicone Oil as a Glidant. Inorganics 2022, 10, 167. https://doi.org/10.3390/inorganics10100167
Zheng X, Kong H, Chu D, Hu F, Wang Y, Yan Y, Wu C. Stress Reduction of a V-Based BCC Metal Hydride Bed Using Silicone Oil as a Glidant. Inorganics. 2022; 10(10):167. https://doi.org/10.3390/inorganics10100167
Chicago/Turabian StyleZheng, Xin, Hanyang Kong, Desheng Chu, Faping Hu, Yao Wang, Yigang Yan, and Chaoling Wu. 2022. "Stress Reduction of a V-Based BCC Metal Hydride Bed Using Silicone Oil as a Glidant" Inorganics 10, no. 10: 167. https://doi.org/10.3390/inorganics10100167




