Synthesis, Structure and Mg2+ Ionic Conductivity of Isopropylamine Magnesium Borohydride
Abstract
:1. Introduction
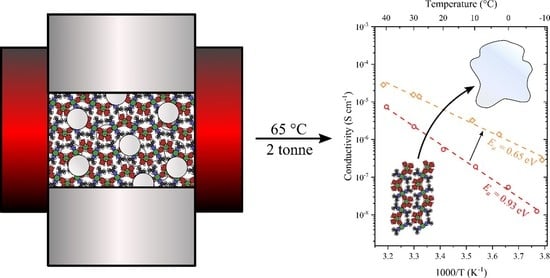
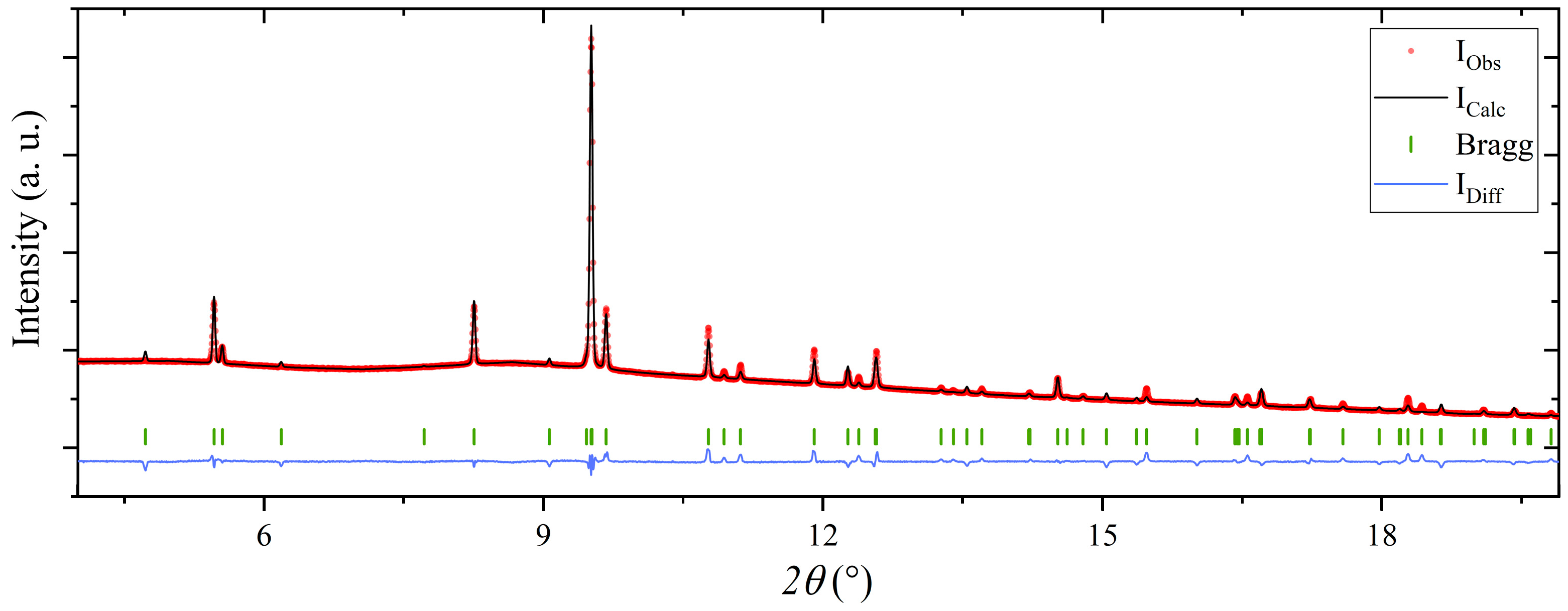
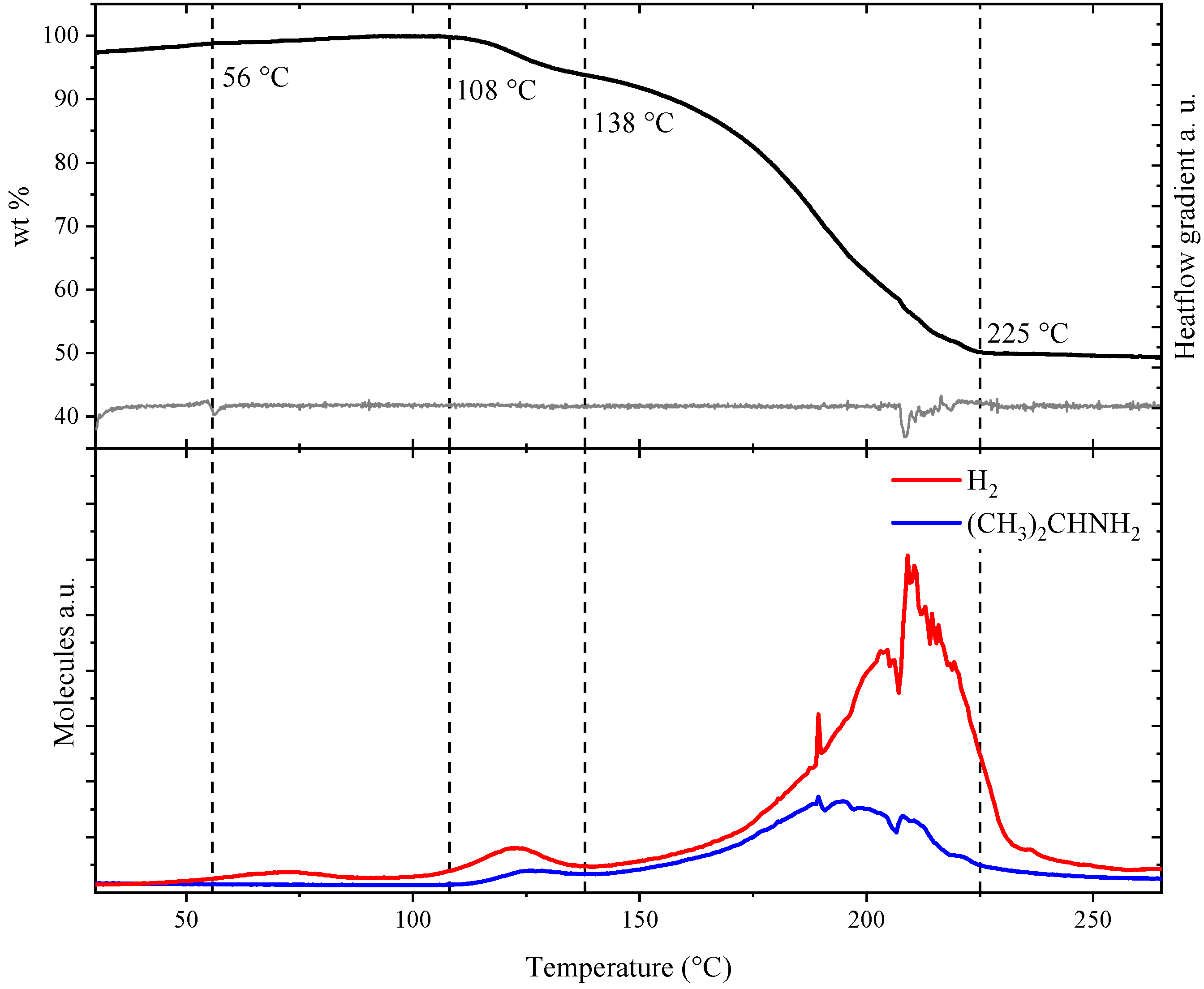
2. Results
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Maier, J.; Yu, Y. Guidelines and Trends for Next-Generation Rechargeable Lithium and Lithium-Ion Batteries. Chem. Soc. Rev. 2020, 49, 1569–1614. [Google Scholar] [CrossRef] [PubMed]
- Kulova, T.L.; Fateev, V.N.; Seregina, E.A.; Grigoriev, A.S. A Brief Review of Post-Lithium-Ion Batteries. Int. J. Electrochem. Sci. 2020, 15, 7242–7259. [Google Scholar] [CrossRef]
- U.S. Geological Survey. Mineral Commodity Summaries 2022; Mineral Commodity Summaries: Reston, VA, USA, 2022; Volume 2022, p. 202. [Google Scholar] [CrossRef]
- Famprikis, T.; Canepa, P.; Dawson, J.A.; Islam, M.S.; Masquelier, C. Fundamentals of Inorganic Solid-State Electrolytes for Batteries. Nat. Mater. 2019, 18, 1278–1291. [Google Scholar] [CrossRef] [PubMed]
- Skov, L.N.; Grinderslev, J.B.; Rosenkranz, A.; Lee, Y.-S.; Jensen, T.R. Towards Solid-State Magnesium Batteries: Ligand-Assisted Superionic Conductivity. Batter. Supercaps 2022, 5, e202200163. [Google Scholar] [CrossRef]
- Kristensen, L.G.; Amdisen, M.B.; Skov, L.N.; Jensen, T.R. Fast Magnesium Ion Conducting Isopropylamine Magnesium Borohydride Enhanced by Hydrophobic Interactions. Phys. Chem. Chem. Phys. 2022, 24, 18185–18197. [Google Scholar] [CrossRef]
- Yan, Y.; Dononelli, W.; Jørgensen, M.; Grinderslev, J.B.; Lee, Y.-S.; Cho, Y.W.; Černý, R.; Hammer, B.; Jensen, T.R. The Mechanism of Mg2+ Conduction in Ammine Magnesium Borohydride Promoted by a Neutral Molecule. Phys. Chem. Chem. Phys. 2020, 22, 9204–9209. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Jørgensen, M.; Skov, L.N.; Skibsted, J.; Jensen, T.R. Ammine Magnesium Borohydride Nanocomposites for All-Solid-State Magnesium Batteries. ACS Appl. Energy Mater. 2020, 3, 9264–9270. [Google Scholar] [CrossRef]
- Kisu, K.; Kim, S.; Inukai, M.; Oguchi, H.; Takagi, S.; Orimo, S. Magnesium Borohydride Ammonia Borane as a Magnesium Ionic Conductor. ACS Appl. Energy Mater. 2020, 3, 3174–3179. [Google Scholar] [CrossRef]
- Xiao, N.; McCulloch, W.D.; Wu, Y. Reversible Dendrite-Free Potassium Plating and Stripping Electrochemistry for Potassium Secondary Batteries. J. Am. Chem. Soc. 2017, 139, 9475–9478. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, J.; Han, L.; Wang, Z.; Wang, H.; Zhao, Q.; Liu, J.; Pan, F. A MOF-Based Single-Ion Zn2+ Solid Electrolyte Leading to Dendrite-Free Rechargeable Zn Batteries. Nano Energy 2019, 56, 92–99. [Google Scholar] [CrossRef]
- Cao, C.; Li, Y.; Feng, Y.; Peng, C.; Li, Z.; Feng, W. A Solid-State Single-Ion Polymer Electrolyte with Ultrahigh Ionic Conductivity for Dendrite-Free Lithium Metal Batteries. Energy Storage Mater. 2019, 19, 401–407. [Google Scholar] [CrossRef]
- Xia, S.; Wu, X.; Zhang, Z.; Cui, Y.; Liu, W. Practical Challenges and Future Perspectives of All-Solid-State Lithium-Metal Batteries. Chem 2019, 5, 753–785. [Google Scholar] [CrossRef]
- Yan, Y.; Grinderslev, J.B.; Lee, Y.-S.; Jørgensen, M.; Cho, Y.W.; Černý, R.; Jensen, T.R. Ammonia-Assisted Fast Li-Ion Conductivity in a New Hemiammine Lithium Borohydride, LiBH4·1/2NH3. Chem. Commun. 2020, 56, 3971–3974. [Google Scholar] [CrossRef]
- Blanchard, D.; Nale, A.; Sveinbjörnsson, D.; Eggenhuisen, T.M.; Verkuijlen, M.H.W.; Suwarno; Vegge, T.; Kentgens, A.P.M.; Jongh, P.E. de Nanoconfined LiBH4 as a Fast Lithium Ion Conductor. Adv. Funct. Mater. 2015, 25, 184–192. [Google Scholar] [CrossRef]
- Huot, J.; Cuevas, F.; Deledda, S.; Edalati, K.; Filinchuk, Y.; Grosdidier, T.; Hauback, B.C.; Heere, M.; Jensen, T.R.; Latroche, M.; et al. Mechanochemistry of Metal Hydrides: Recent Advances. Materials 2019, 12, 2778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richter, B.; Grinderslev, J.B.; Møller, K.T.; Paskevicius, M.; Jensen, T.R. From Metal Hydrides to Metal Borohydrides. Inorg. Chem. 2018, 57, 10768–10780. [Google Scholar] [CrossRef] [PubMed]
- Černý, R.; Filinchuk, Y.; Hagemann, H.; Yvon, K. Magnesium Borohydride: Synthesis and Crystal Structure. Angew. Chem. Int. Ed. 2007, 46, 5765–5767. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, L.H.; Ban, V.; Møller, K.T.; Lee, Y.-S.; Cho, Y.W.; Besenbacher, F.; Filinchuk, Y.; Skibsted, J.; Jensen, T.R. Synthesis, Crystal Structure, Thermal Decomposition, and 11B MAS NMR Characterization of Mg(BH4)2(NH3BH3)2. J. Phys. Chem. C 2014, 118, 12141–12153. [Google Scholar] [CrossRef]
- Jepsen, L.H.; Ley, M.B.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Tailoring the Properties of Ammine Metal Borohydrides for Solid-State Hydrogen Storage. ChemSusChem 2015, 8, 1452–1463. [Google Scholar] [CrossRef]
- Goursot, A.; Mineva, T.; Kevorkyants, R.; Talbi, D. Interaction between N-Alkane Chains: Applicability of the Empirically Corrected Density Functional Theory for Van Der Waals Complexes. J. Chem. Theory Comput. 2007, 3, 755–763. [Google Scholar] [CrossRef]
- Roedern, E.; Kühnel, R.-S.; Remhof, A.; Battaglia, C. Magnesium Ethylenediamine Borohydride as Solid-State Electrolyte for Magnesium Batteries. Sci. Rep. 2017, 7, 46189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grinderslev, J.B.; Skov, L.N.; Andreasen, J.G.; Ghorwal, S.; Skibsted, J.; Jensen, T.R. Methylamine Lithium Borohydride as Electrolyte for All-Solid-State Batteries. Angew. Chem. Int. Ed. 2022, 61, e202203484. [Google Scholar] [CrossRef] [PubMed]
- Grinderslev, J.B.; Ley, M.B.; Lee, Y.-S.; Jepsen, L.H.; Jørgensen, M.; Cho, Y.W.; Skibsted, J.; Jensen, T.R. Ammine Lanthanum and Cerium Borohydrides, M(BH4)3·nNH3; Trends in Synthesis, Structures, and Thermal Properties. Inorg. Chem. 2020, 59, 7768–7778. [Google Scholar] [CrossRef]
- Grinderslev, J.B.; Jensen, T.R. Trends in the Series of Ammine Rare-Earth-Metal Borohydrides: Relating Structural and Thermal Properties. Inorg. Chem. 2021, 60, 2573–2589. [Google Scholar] [CrossRef] [PubMed]
- Zanella, P.; Crociani, L.; Masciocchi, N.; Giunchi, G. Facile High-Yield Synthesis of Pure, Crystalline Mg(BH4)2. Inorg. Chem. 2007, 46, 9039–9041. [Google Scholar] [CrossRef]
- Filinchuk, Y.; Richter, B.; Jensen, T.R.; Dmitriev, V.; Chernyshov, D.; Hagemann, H. Porous and Dense Magnesium Borohydride Frameworks: Synthesis, Stability, and Reversible Absorption of Guest Species. Angew. Chem. Int. Ed. Engl. 2011, 50, 11162–11166. [Google Scholar] [CrossRef] [PubMed]
- Thompson, S.P.; Parker, J.E.; Potter, J.; Hill, T.P.; Birt, A.; Cobb, T.M.; Yuan, F.; Tang, C.C. Beamline I11 at Diamond: A New Instrument for High Resolution Powder Diffraction. Rev. Sci. Instrum. 2009, 80, 075107. [Google Scholar] [CrossRef]
- Ravnsbæk, D.B.; Sørensen, L.H.; Filinchuk, Y.; Besenbacher, F.; Jensen, T.R. Screening of Metal Borohydrides by Mechanochemistry and Diffraction. Angew. Chem., Int. Ed. 2012, 51, 3582–3586. [Google Scholar] [CrossRef]
- Favre-Nicolin, V.; Černý, R. FOX, ‘free Objects for Crystallography’: A Modular Approach Toab Initiostructure Determination from Powder Diffraction. J. Appl. Crystallogr. 2002, 35, 734–743. [Google Scholar] [CrossRef]
- Favre-Nicolin, V. Free Objects for Crystallography. Available online: https://sourceforge.net/projects/objcryst/ (accessed on 18 November 2022).
- Mortensen, J.J.; Hansen, L.B.; Jacobsen, K.W. Real-Space Grid Implementation of the Projector Augmented Wave Method. Phys. Rev. B 2005, 71, 035109. [Google Scholar] [CrossRef] [Green Version]
- Enkovaara, J.; Rostgaard, C.; Mortensen, J.J.; Chen, J.; Dułak, M.; Ferrighi, L.; Gavnholt, J.; Glinsvad, C.; Haikola, V.; Hansen, H.A.; et al. Electronic Structure Calculations with GPAW: A Real-Space Implementation of the Projector Augmented-Wave Method. J. Phys. Condens. Matter 2010, 22, 253202. [Google Scholar] [CrossRef]
- Larsen, A.H.; Mortensen, J.J.; Blomqvist, J.; Castelli, I.E.; Christensen, R.; Dułak, M.; Friis, J.; Groves, M.N.; Hammer, B.; Hargus, C.; et al. The Atomic Simulation Environment—a Python Library for Working with Atoms. J. Phys. Condens. Matter 2017, 29, 273002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellendorff, J.; Lundgaard, K.T.; Møgelhøj, A.; Petzold, V.; Landis, D.D.; Nørskov, J.K.; Bligaard, T.; Jacobsen, K.W. Density Functionals for Surface Science: Exchange-Correlation Model Development with Bayesian Error Estimation. Phys. Rev. B 2012, 85, 235149. [Google Scholar] [CrossRef]
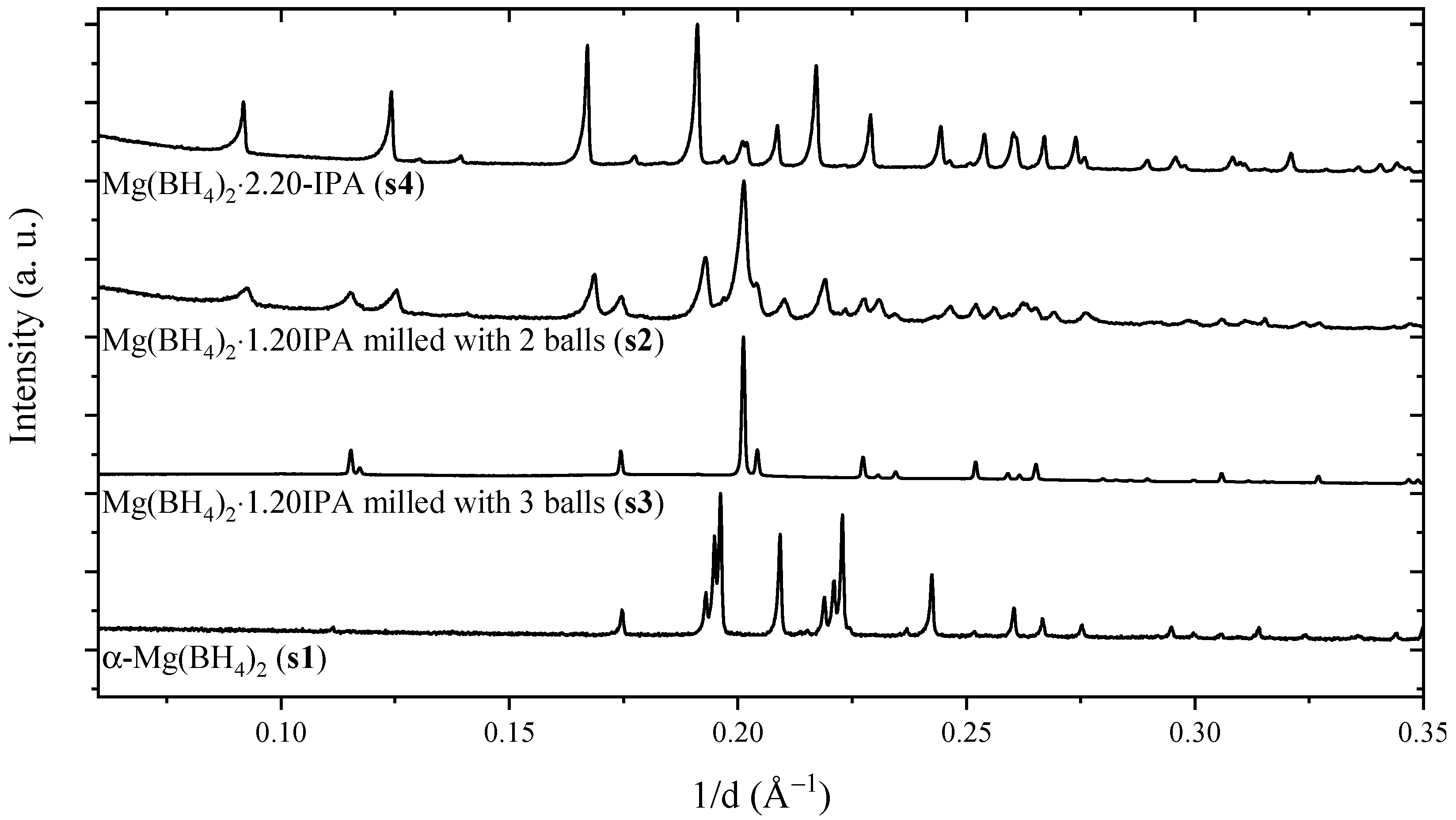



| Sample | Synthesis Method | Reactants | Reactant Ratio (s3/(s1 + s3)) | Crystalline Compounds | IPA Content (1H NMR) |
|---|---|---|---|---|---|
| s1 | Solvent-based | - | - | α-Mg(BH4)2 | - |
| s2 | Mechanochem. | s1 & s4 | 0.44 | Mg(BH4)2∙(CH3)2CHNH2 | 1.20 |
| s3 | Mechanochem. | s1 & s4 | 0.44 | Mg(BH4)2∙(CH3)2CHNH2 | 1.20 |
| s4 | Solvent-based | - | - | Mg(BH4)2∙2(CH3)2CHNH2 | 2.20 |
| s5 | Mechanochem. | s3 & Al2O3 | - | Mg(BH4)2∙(CH3)2CHNH2 + Al2O3 (50 wt%) | 1.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kristensen, L.G.; Amdisen, M.B.; Andersen, M.; Jensen, T.R. Synthesis, Structure and Mg2+ Ionic Conductivity of Isopropylamine Magnesium Borohydride. Inorganics 2023, 11, 17. https://doi.org/10.3390/inorganics11010017
Kristensen LG, Amdisen MB, Andersen M, Jensen TR. Synthesis, Structure and Mg2+ Ionic Conductivity of Isopropylamine Magnesium Borohydride. Inorganics. 2023; 11(1):17. https://doi.org/10.3390/inorganics11010017
Chicago/Turabian StyleKristensen, Lasse G., Mads B. Amdisen, Mie Andersen, and Torben R. Jensen. 2023. "Synthesis, Structure and Mg2+ Ionic Conductivity of Isopropylamine Magnesium Borohydride" Inorganics 11, no. 1: 17. https://doi.org/10.3390/inorganics11010017