Thiosemicarbazones and Derived Antimony Complexes: Synthesis, Structural Analysis, and In Vitro Evaluation against Bacterial, Fungal, and Cancer Cells
Abstract
:1. Introduction
2. Results and Discussion
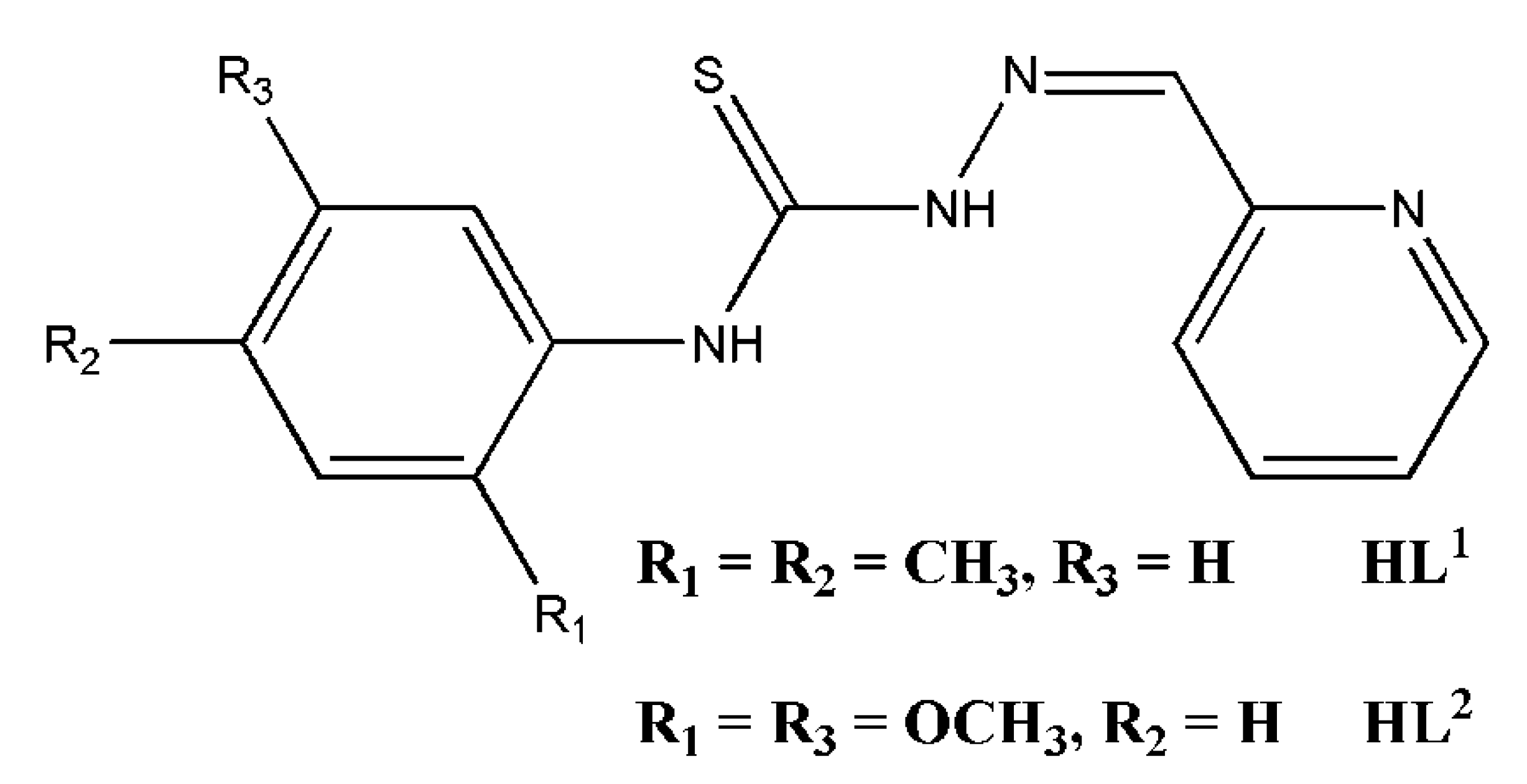
2.1. Synthesis and Spectroscopic Characterization
2.2. X-ray Crystallography
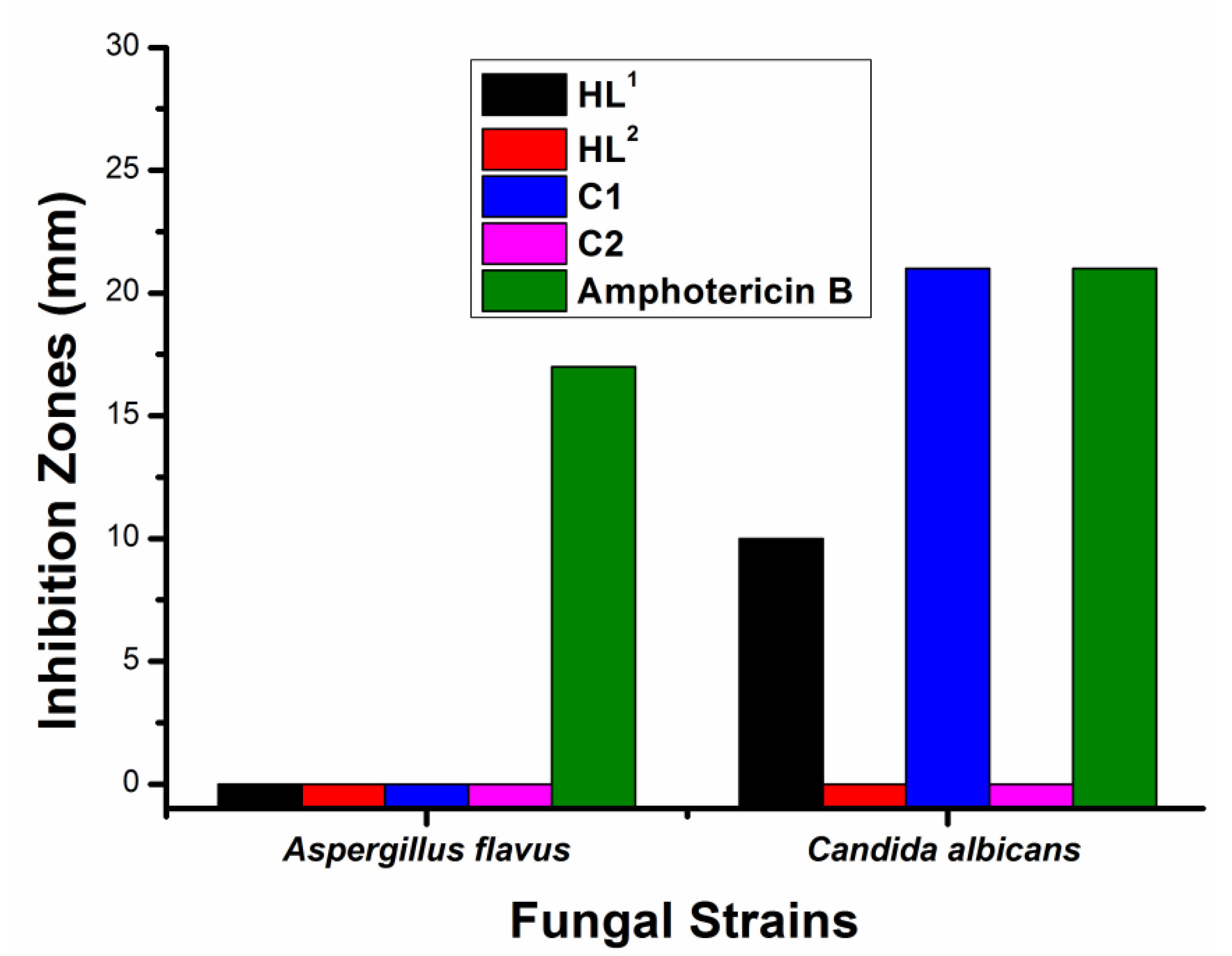
2.3. Antibacterial and Antifungal Activity Screening
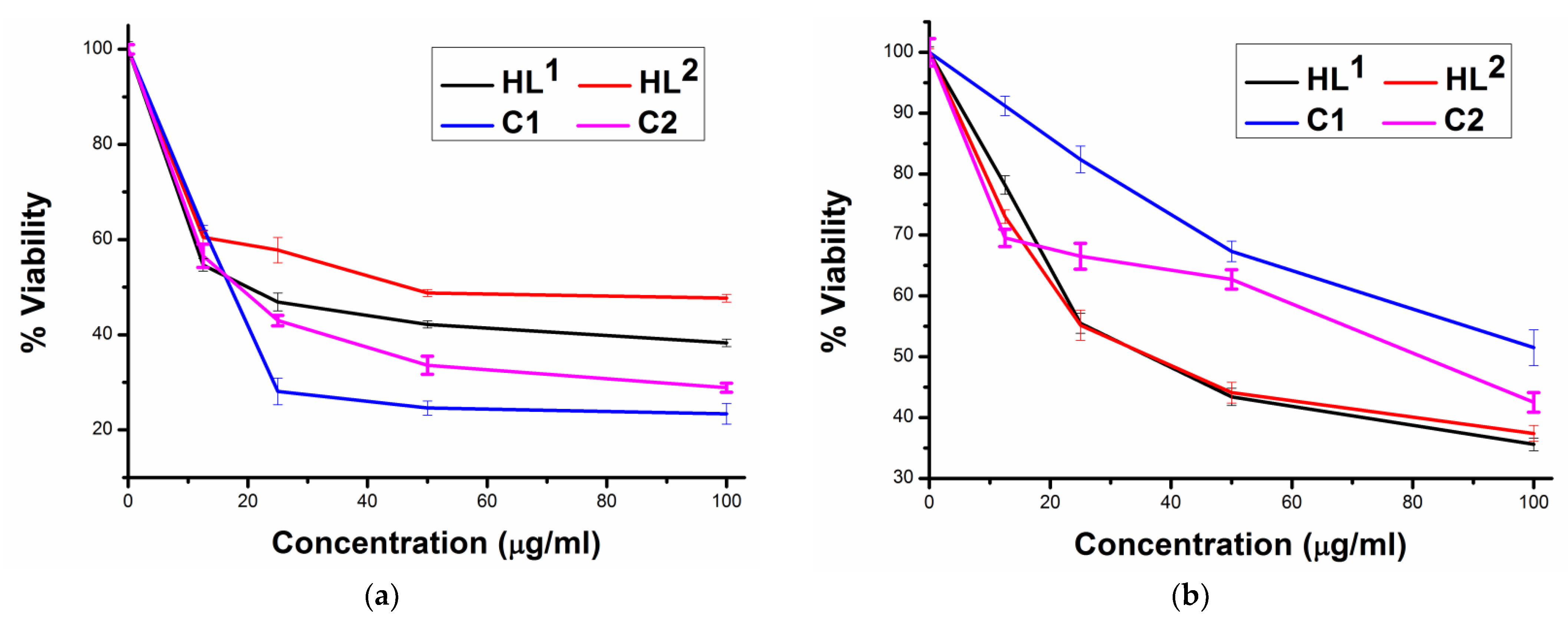
2.4. Cytotoxicity in Cancer and Normal Cells
3. Materials and Methods
3.1. Chemicals and Instruments
3.2. Single Crystal X-ray Diffraction Analysis
3.3. Preparation of the Complexes
3.4. Evaluation of Antibacterial and Antifungal Activities
3.5. Cytotoxicity against MCF-7 Cancer and BHK Normal Cells
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Da Silva, J.G.; Azzolini, L.S.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Increasing the antibacterial activity of gallium(III) against Pseudomonas aeruginosa upon coordination to pyridine-derived thiosemicarbazones. Polyhedron 2009, 28, 2301–2305. [Google Scholar] [CrossRef]
- Mendes, I.C.; Moreira, J.P.; Ardisson, J.D.; Dos Santos, R.G.; Da Silva, P.R.O.; Garcia, I.; Castiñeiras, A.; Beraldo, H. Organotin(IV) complexes of 2-pyridineformamide-derived thiosemicarbazones: Antimicrobial and cytotoxic effects. Eur. J. Med. Chem. 2008, 43, 1454–1461. [Google Scholar] [CrossRef] [PubMed]
- Parrilha, G.L.; Da Silva, J.G.; Gouveia, L.F.; Gasparoto, A.K.; Dias, R.P.; Rocha, W.R.; Santos, D.A.; Speziali, N.L.; Beraldo, H. Pyridine-derived thiosemicarbazones and their tin(IV) complexes with antifungal activity against Candida spp. Eur. J. Med. Chem. 2011, 46, 1473–1482. [Google Scholar] [CrossRef]
- Reis, D.C.; Pinto, M.C.X.; Souza-Fagundes, E.M.; Wardell, S.M.S.V.; Wardell, J.L.; Beraldo, H. Antimony(III) complexes with 2-benzoylpyridine-derived thiosemicarbazones: Cytotoxicity against human leukemia cell lines. Eur. J. Med. Chem. 2010, 45, 3904–3910. [Google Scholar] [CrossRef] [PubMed]
- Pathan, A.H.; Ramesh, A.K.; Bakale, R.P.; Naik, G.N.; Kumar, H.G.R.; Frampton, C.S.; Rao, G.M.A.; Gudasi, K.B. Association of late transition metal complexes with ethyl 2-(2-(4-chlorophenylcarbamothioyl)hydrazono)propanoate: Design, synthesis and in vitro anticancer studies. Inorg. Chim. Acta 2015, 430, 216–224. [Google Scholar] [CrossRef]
- Mendes, I.C.; Soares, M.A.; Dos Santos, R.G.; Pinheiro, C.; Beraldo, H. Gallium(III) complexes of 2-pyridineformamide thiosemicarbazones: Cytotoxic activity against malignant glioblastoma. Eur. J. Med. Chem. 2009, 44, 1870–1877. [Google Scholar] [CrossRef] [PubMed]
- Klayman, D.L.; Bartosevich, J.F.; Griffin, T.S.; Mason, C.J.; Scovill, J.P. 2-Acetylpyridine thiosemicarbazones. 1. A new class of potential antimalarial agents. J. Med. Chem. 1979, 22, 855–862. [Google Scholar] [CrossRef]
- Merlino, A.; Benitez, D.; Chavez, S.; Da Cunha, J.; Hernandez, P.; Tinoco, L.W.; Campillo, N.E.; Paez, J.A.; Cerecetto, H.; Gonzalez, M. Development of second generation amidinohydrazones, thio- and semicarbazones as Trypanosoma cruzi-inhibitors bearing benzofuroxan and benzimidazole 1,3-dioxide core scaffolds. Med. Chem. Commun. 2010, 1, 216–228. [Google Scholar] [CrossRef]
- Parrilha, G.L.; Dias, R.P.; Rocha, W.R.; Mendes, I.C.; Benítez, D.; Varela, J.; Cerecetto, H.; Gonzalez, M.; Melo, C.M.L.; Neves, J.K.A.L.; et al. 2-Acetylpyridine- and 2-benzoylpyridine-derived thiosemicarbazones and their antimony(III) complexes exhibit high anti-trypanosomal activity. Polyhedron 2012, 31, 614–621. [Google Scholar] [CrossRef]
- Finch, R.A.; Liu, M.C.; Grill, S.P.; Rose, W.C.; Loomis, R.; Vasquez, K.M.; Cheng, Y.C.; Sartorelli, A.C. Triapine (3-aminopyridine-2-carboxaldehyde-thiosemicarbazone): A potent inhibitor of ribonucleotide reductase activity with broad spectrum antitumor activity. Biochem. Pharmacol. 2000, 59, 983–991. [Google Scholar] [CrossRef]
- Gojo, I.; Tidwell, M.L.; Greer, J.; Takebe, N.; Seiter, K.; Pochron, M.F.; Johnson, B.; Sznol, M.; Karp, J.E. Phase I and pharmacokinetic study of Triapine®, a potent ribonucleotide reductase inhibitor, in adults with advanced hematologic malignancies. Leuk. Res. 2007, 31, 1165–1173. [Google Scholar] [CrossRef] [PubMed]
- Wadler, S.; Makower, D.; Clairmont, C.; Lambert, P.; Fehn, K.; Sznol, M. Phase I and pharmacokinetic study of the ribonucleotide reductase inhibitor, 3-aminopyridine-2-carboxaldehyde thiosemicarbazone, administered by 96-h intravenous continuous infusion. J. Clin. Oncol. 2004, 22, 1553–1563. [Google Scholar] [CrossRef] [PubMed]
- Karp, J.E.; Giles, F.J.; Gojo, I.; Morris, L.; Greer, J.; Johnson, B.; Thein, M.; Sznol, M.; Low, J. A Phase I study of the novel ribonucleotide reductase inhibitor 3-aminopyridine-2-carboxaldehyde thiosemicarbazone (3-AP, Triapine®) in combination with the nucleoside analog fludarabine for patients with refractory acute leukemias and aggressive myeloproliferative disorders. Leuk. Res. 2008, 32, 71–77. [Google Scholar] [PubMed] [Green Version]
- Soares, M.A.; Lessa, J.A.; Mendes, I.C.; Da Silva, J.G.; Dos Santos, R.G.; Salum, L.B.; Daghestani, H.; Andricopulo, A.D.; Day, B.W.; Vogt, A.; et al. N4-Phenyl-substituted 2-acetylpyridine thiosemicarbazones: Cytotoxicity against human tumor cells, structure–activity relationship studies and investigation on the mechanism of action. Bioorg. Med. Chem. 2012, 20, 3396–3409. [Google Scholar] [CrossRef] [PubMed]
- Rebolledo, A.P.; Ayala, J.D.; Lima, G.M.; Marchini, N.; Bombieri, G.; Zani, C.L.; Souza-Fagundes, E.M.; Beraldo, H. Structural studies and cytotoxic activity of N(4)-phenyl-2-benzoylpyridine thiosemicarbazone Sn(IV) complexes. Eur. J. Med. Chem. 2005, 40, 467–472. [Google Scholar] [CrossRef]
- Rebolledo, A.P.; Vieites, M.; Gambino, D.; Piro, O.E.; Castellano, E.E.; Zani, C.L.; Fagundes, E.M.S.; Teixeira, L.R.; Batista, A.A.; Beraldo, H. Palladium(II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: Spectral characterization, structural studies and cytotoxic activity. J. Inorg. Biochem. 2005, 99, 698–706. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; Plaisier, J.R.; Shalaby, E.M. Synthesis, structural and antimicrobial studies of binary and ternary complexes of a new tridentate thiosemicarbazone. Future Med. Chem. 2018, 10, 2507–2519. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; Mayer, P. Copper complexes of new thiosemicarbazone ligands: Synthesis, structural studies and antimicrobial activity. Inorg. Chem. Commun. 2018, 94, 127–132. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; Mayer, P. Synthesis, structural studies and antimicrobial evaluation of nickel (II) complexes of NNS tridentate thiosemicarbazone based ligands. Appl. Organomet. Chem. 2019, 33, e4883. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; Santos, I.C.; Paulo, A. Nickel Complexes Bearing SNN and SS Donor Atom Ligands: Synthesis, Structural Characterization and Biological activity. Appl. Organomet. Chem. 2019, 33, e5088. [Google Scholar] [CrossRef]
- Ibrahim, A.B.M.; Farh, M.K.; El-Gyar, S.A.; EL-Gahami, M.A.; Fouad, D.M.; Silva, F.; Santos, I.C.; Paulo, A. Synthesis, structural studies and antimicrobial activities of manganese, nickel and copper complexes of two new tridentate 2-formylpyridine thiosemicarbazone ligands. Inorg. Chem. Commun. 2018, 96, 194–201. [Google Scholar] [CrossRef]
- Mahmoud, G.A.-E.; Ibrahim, A.B.M.; Mayer, P. Zn(II) and Cd(II) thiosemicarbazones for stimulation/inhibition of kojic acid biosynthesis from Aspergillus flavus and the fungal defense behavior against the metal complexes’ excesses. J. Biol. Inorg. Chem. 2020, 25, 797–809. [Google Scholar] [CrossRef]
- Demicheli, C.; Ochoa, R.; Da Silva, J.B.B.; Falcao, C.A.B.; Rossi-Bergmann, B.; de Melo, A.L.; Sinisterra, R.D.; Frezard, F. Oral delivery of meglumine antimoniate-β-cyclodextrin complex for treatment of leishmaniasis. Antimicrob. Agents Chemother. 2004, 48, 100–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gencarelli, R.A.; Hughes, K.A.; Sierakowski, J.F. Extreme Pressure Additive for Lubricants. U.S. Patent 3988249A, 26 October 1976. [Google Scholar]
- Willingham, G.L. Use of Antimony Salt Stabilizers for 3-Isothiazolones. U.S. Patent 5145981A, 8 September 1992. [Google Scholar]
- Ozturk, I.I.; Yarar, S.; Gürgan, M.; Ceyhan, D.; Banti, C.N.; Hadjikakou, S.K.; Manoli, M.; Moushi, E.; Tasiopoulos, A.J. Synthesis, characterization and biological evaluation of novel antimony(III) iodide complexes with tetramethylthiourea and N-ethylthiourea. Inorg. Chim. Acta 2019, 491, 14–24. [Google Scholar] [CrossRef]
- Duffin, J.; Campling, B.G. Therapy and disease concepts: The history (and future?) of antimony in cancer. J. Hist. Med. Allied Sci. 2002, 57, 61–78. [Google Scholar] [CrossRef] [PubMed]
- Hadjikakou, S.K.; Ozturk, I.I.; Banti, C.N.; Kourkoumelis, N.; Hadjiliadis, N. Recent advances on antimony(III/V) compounds with potential activity against tumor cells. J. Inorg. Biochem. 2015, 153, 293–305. [Google Scholar] [CrossRef]
- Tripathi, U.N.; Solanki, J.S.; Ahmad, M.S.; Bhardwaj, A. Synthesis, spectral and antimicrobial studies of chloroantimony(III)di [3(2′-hydroxyphenyl)-5-(4-substitutedphenyl)pyrazolinates]. J. Coord. Chem. 2009, 62, 636–644. [Google Scholar] [CrossRef]
- Ozturk, I.I.; Banti, C.N.; Manos, M.J.; Tasiopoulos, A.J.; Kourkoumelis, N.; Charalabopoulos, K.; Hadjikakou, S.K. Synthesis, characterization and biological studies of new antimony(III) halide complexes with ω-thiocaprolactam. J. Inorg. Biochem. 2012, 109, 57–65. [Google Scholar] [CrossRef]
- Sharma, P.; Perez, D.; Cabrera, A.; Rosas, N.; Arias, J.L. Perspectives of antimony compounds in oncology. Acta Pharmacol. Sin. 2008, 29, 881–890. [Google Scholar] [CrossRef]
- Geary, W.J. The use of conductivity measurements in organic solvents for the characterization of coordination compounds. Coord. Chem. Rev. 1971, 7, 81–122. [Google Scholar] [CrossRef]
- Fekete, T.; Tumah, H.; Woodwell, J.; Truant, A.; Satischandran, V.; Axelrod, P.; Kreter, B. A comparison of serial plate agar dilution, bauer-kirby disk diffusion, and the vitek automicrobic system for the determination of susceptibilities of Klebsiella spp., Enterobacter spp., and Pseudomonas aeruginosa to ten antimicrobial agents. Diagn. Microbiol. Infect. Dis. 1994, 18, 251–258. [Google Scholar] [CrossRef]
- Khan, N.U.H.; Nadeem, K.S.H. Synthesis, characterization and antibacterial activity of new antimony (III) complexes of some tosyl-sulfonamide derivatives. Middle East J. Sci. Res. 2013, 16, 1109–1115. [Google Scholar]
- Abdolmaleki, S.; Yarmohammadi, N.; Adibi, H.; Ghadermazi, M.; Ashengroph, M.; Rudbari, H.A.; Bruno, G. Synthesis, X-ray studies, electrochemical properties, evaluation as in vitro cytotoxic and antibacterial agents of two antimony (III) complexes with dipicolinic acid. Polyhedron 2019, 159, 239–250. [Google Scholar] [CrossRef]
- Ferraz, K.S.O.; Silva, N.F.; da Silva, J.G.; de Miranda, L.F.; Romeiro, C.F.D.; Souza-Fagundes, E.M.; Mendes, I.C.; Beraldo, H. Investigation on the pharmacological profile of 2,6-diacetylpyridine bis(benzoylhydrazone) derivatives and their antimony(III) and bismuth(III) complexes. Eur. J. Med. Chem. 2012, 53, 98–106. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
- Rigaku. CrysAlisPro Software System; Rigaku Oxford Diffraction: Tokyo, Japan, 2019. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. The anatomy of a comprehensive constrained, restrained refinement program for the modern computing environment—Olex2 dissected. Acta Crystallogr. 2015, A71, 59–75. [Google Scholar]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Crystallogr. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Pennington, W.T. DIAMOND—Visual crystal structure information system. J. Appl. Cryst. 1999, 32, 1028–1029. [Google Scholar] [CrossRef]
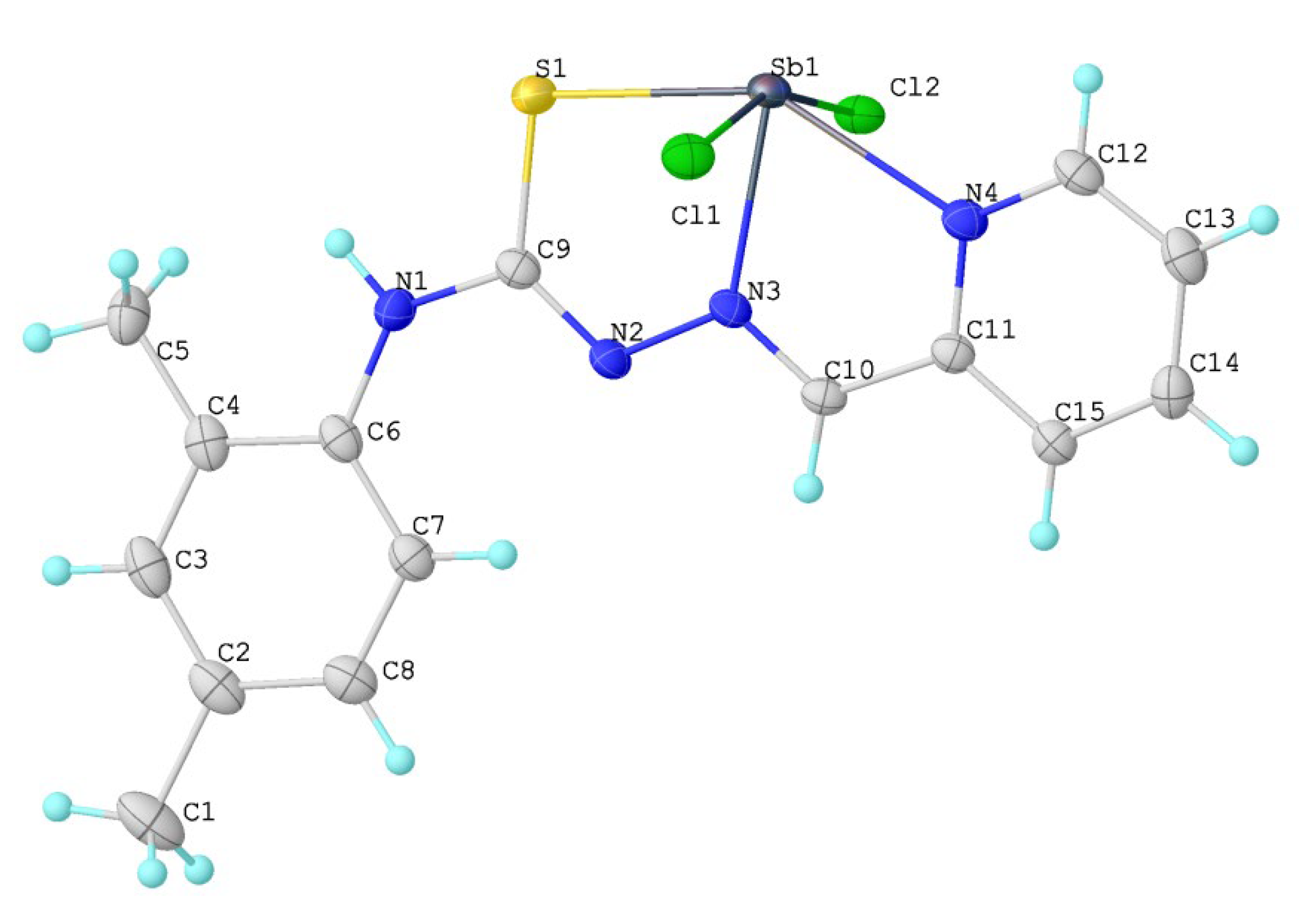


| Empirical formula | C15H15N4SCl2Sb | µ (mm−1) | 16.395 |
| Formula weight | 476.04 | F(000) | 470.9 |
| Crystal system | Triclinic | θ range for data collection (ᵒ) | 3.81 to 73.95 |
| Space group | P-1 | Reflections collected | 141,369 |
| a (Å) | 8.1520(1) | Unique refl. collected (Rint) | 3546 (0.0690) |
| b (Å) | 9.3163(1) | Completeness to theta | 100% |
| c (Å) | 11.7748(1) | Parameters (Restraints) | 210(0) |
| α (ᵒ) | 82.279(1) | Max. and min. transmission | 0.481 and 0.717 |
| β (ᵒ) | 83.243(1) | GOF on F2 | 1.116 |
| γ (ᵒ) | 85.761(1) | R1 [I > 2σ(I)] | 0.0213 (3536) |
| Volume (Å3) | 878.457(16) | wR2 (all data) | 0.0561 |
| Z | 2 | Largest diff. peak, hole/e Å−3 | 0.860 and −0.502 |
| Density (g/cm3) | 1.800 | CCDC number | 2207805 |
| Atoms | Distance (Ǻ) | Atoms | Angle (ᵒ) | Atoms | Angle (ᵒ) |
|---|---|---|---|---|---|
| Sb1—Cl2 | 2.6048(6) | Cl2—Sb1—N4 | 83.69(5) | C11—N4—C12 | 118.8(2) |
| Sb1—Cl1 | 2.5786(6) | Cl1—Sb1—S1 | 92.12(2) | N3—C10—C11 | 119.8(2) |
| Sb1—S1 | 2.5215(6) | Cl1—Sb1—N3 | 80.90(5) | N3—C10—H10 | 120.1(3) |
| Sb1—N3 | 2.239(2) | Cl1—Sb1—N4 | 84.34(5) | S1—C9—N2 | 127.4(2) |
| Sb1—N4 | 2.410(2) | S1—Sb1—N3 | 76.43(5) | S1—C9—N1 | 113.6(2) |
| S1—C9 | 1.746(3) | S1—Sb1—N4 | 146.25(5) | N3—N2—C9 | 114.9(2) |
| N3—N2 | 1.372(3) | N3—Sb1—N4 | 69.86(6) | C6—N1—C9 | 132.0(2) |
| N3—C10 | 1.294(3) | Sb1—S1—C9 | 97.19(9) | C6—N1—H8 | 114.0(3) |
| N4—C11 | 1.350(3) | Sb1—N3—N2 | 123.9(1) | C9—N1—H8 | 114.0(3) |
| N4—C12 | 1.337(3) | Sb1—N3—C10 | 120.5(2) | N1—C6—C7 | 123.8(2) |
| N2—C9 | 1.311(4) | N2—N3—C10 | 115.7(2) | N1—C6—C4 | 116.0(2) |
| N1—C6 | 1.418(3) | Sb1—N4—C11 | 114.9(1) | N2—C9—N1 | 118.9(2) |
| N1—C9 | 1.361(3) | Sb1—N4—C12 | 126.3(2) | C8—C2—C1 | 120.7(2) |
| Hydrogen bonding interaction parameters | |||||
| D—H….A | d(D—H) | d(H….A) | d(D….A) | <(D—H….A) | |
| C7—H7….Cl2 (i) | 0.930(4) | 2.8608(3) | 3.6137(3) | 138.927(2) | |
| C12—H12….Cl1 (ii) | 0.930(4) | 2.7470(3) | 3.6692(3) | 171.218(3) | |
| (i) 1 − x, 1 − y, 1 − z | (ii) 2 − x, −y, 1 − z | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fathy, A.; Ibrahim, A.B.M.; Abd Elkhalik, S.; Meurer, F.; Bodensteiner, M.; Abbas, S.M. Thiosemicarbazones and Derived Antimony Complexes: Synthesis, Structural Analysis, and In Vitro Evaluation against Bacterial, Fungal, and Cancer Cells. Inorganics 2022, 10, 172. https://doi.org/10.3390/inorganics10100172
Fathy A, Ibrahim ABM, Abd Elkhalik S, Meurer F, Bodensteiner M, Abbas SM. Thiosemicarbazones and Derived Antimony Complexes: Synthesis, Structural Analysis, and In Vitro Evaluation against Bacterial, Fungal, and Cancer Cells. Inorganics. 2022; 10(10):172. https://doi.org/10.3390/inorganics10100172
Chicago/Turabian StyleFathy, Amany, Ahmed B. M. Ibrahim, S. Abd Elkhalik, Florian Meurer, Michael Bodensteiner, and S. M. Abbas. 2022. "Thiosemicarbazones and Derived Antimony Complexes: Synthesis, Structural Analysis, and In Vitro Evaluation against Bacterial, Fungal, and Cancer Cells" Inorganics 10, no. 10: 172. https://doi.org/10.3390/inorganics10100172





