Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)silver(I) Perchlorate, [Agpy2ClO4]·0.5py
Abstract
:1. Introduction
2. Results and Discussion
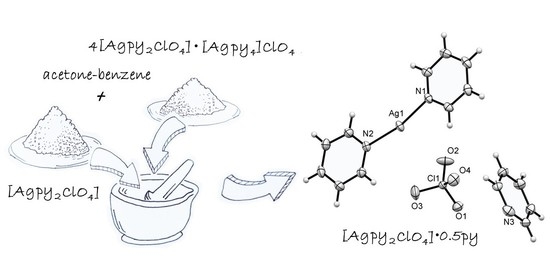
2.1. Preparation of [Agpy2ClO4]·0.5py (Compound 1)
2.2. Structure of [Agpy2ClO4]·0.5py (Compound 1)
2.3. Vibrational Spectroscopic Analysis of [Agpy2ClO4]·0.5py (Compound 1)
2.4. Assignment of the Vibrational Modes in the Spectra of Compound 1
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Béres, K.A.; Homonnay, Z.; Kvitek, L.; Dürvanger, Z.; Kubikova, M.; Harmat, V.; Szilágyi, F.; Czégény, Z.; Németh, P.; Bereczki, L.; et al. Thermally-induced solid-phase quasi-intramolecular redox reactions of [hexakis(urea-O)iron(III)] permanganate: An easy way to prepare (Fe,Mn)Ox catalysts for CO2 hydrogenation. Inorg. Chem. 2022, in press. [Google Scholar]
- Béres, K.A.; Sajó, I.E.; Lendvay, G.; Trif, L.; Petruševski, V.M.; Barta-Holló, B.; Korecz, L.; Franguelli, F.P.; László, K.; Szilágyi, I.M.; et al. Solid-Phase “Self-Hydrolysis” of [Zn(NH3)4MoO4@2H2O] Involving Enclathrated Water—An Easy Route to a Layered Basic Ammonium Zinc Molybdate Coordination Polymer. Molecules 2021, 26, 4022. [Google Scholar] [CrossRef] [PubMed]
- Franguelli, F.P.; Kováts, É.; Czégény, Z.; Bereczki, L.; Petruševski, V.M.; Barta Holló, B.; Béres, K.A.; Farkas, A.; Szilágyi, I.M.; Kótai, L. Multi-Centered Solid-Phase Quasi-Intramolecular Redox Reactions of [(Chlorido)Pentaamminecobalt(III)] Permanganate—An Easy Route to Prepare Phase Pure CoMn2O4 Spinel. Inorganics 2022, 10, 18. [Google Scholar] [CrossRef]
- Franguelli, F.P.; Barta Holló, B.; Petruševski, V.M.; Sajó, I.E.; Klébert, S.; Farkas, A.; Bódis, E.; Szilágyi, I.M.; Pawar, R.P.; Kótai, L. Thermal decomposition and spectral characterization of di[carbonatotetraamminecobalt(III)] sulfate trihydrate and the nature of its thermal decomposition products. J. Therm. Anal. Calorim. 2021, 145, 2907–2923. [Google Scholar] [CrossRef]
- Sajó, I.E.; Bakos, L.P.; Szilágyi, I.M.; Lendvay, G.; Magyari, J.; Mohai, M.; Szegedi, A.; Farkas, Á.; Jánosity, A.; Klébert, S.; et al. Unexpected Sequential NH3/H2O Solid/Gas Phase Ligand Exchange and Quasi-Intramolecular Self-Protonation Yield [NH4Cu(OH)MoO4], a Photocatalyst Misidentified before as (NH4)2Cu(MoO4)2. Inorg. Chem. 2018, 57, 13679–13692. [Google Scholar] [CrossRef]
- Bereczki, L.A.; Fogaça, L.A.; Dürvanger, Z.; Harmat, V.; Kamarás, K.; Németh, G.; Barta Holló, B.; Petruševski, V.M.; Bódis, E.; Farkas, A.; et al. Dynamic disorder in the high-temperature polymorph of bis[diamminesilver(I)] sulfate—reasons and consequences of simultaneous ammonia release from two different polymorphs. Coord. Chem. 2021, 74, 2144–2162. [Google Scholar] [CrossRef]
- Fogaca, L.A.; Kováts, É.; Németh, G.; Kamarás, K.; Béres, K.A.; Németh, P.; Petruševski, V.; Bereczki, L.; Berta, B.H.; Sajó, I.E.; et al. Solid-Phase Quasi-Intramolecular Redox Reaction of [Ag(NH3)2]MnO4: An Easy Way to Prepare Pure AgMnO2. Inorg. Chem. 2021, 60, 3749–3760. [Google Scholar] [CrossRef]
- Fogaça, L.A.; Bereczki, L.; Petruševski, V.M.; Barta-Holló, B.; Franguelli, F.P.; Mohai, M.; Béres, K.A.; Sajó, I.E.; Szilágyi, I.M.; Kotai, L. A Quasi-Intramolecular Solid-Phase Redox Reaction of Ammonia Ligands and Perchlorate Anion in Diamminesilver(I) Perchlorate. Inorganics 2021, 9, 38. [Google Scholar] [CrossRef]
- Petruševski, V.M.; Béres, K.A.; Bombicz, P.; Farkas, A.; Kótai, L.; Bereczki, L. Structural and Raman Spectroscopic Characterization of Tetrapyridinesilver(I) Perrhenate, [Agpy4]ReO4. Maced. J. Chem. Chem. Eng. 2022, 41, 37–46. [Google Scholar] [CrossRef]
- Kovács, G.B.; May, N.V.; Bombicz, P.A.; Klébert, S.; Németh, P.; Menyhárd, A.; Novodárszki, G.; Petruševski, V.M.; Franguelli, F.P.; Magyari, J.; et al. An unknown component of a selective and mild oxidant: Structure and oxidative ability of a double salt-type complex having κ1O-coordinated permanganate anions and three- and four-fold coordinated silver cations. RSC Adv. 2019, 9, 28387–28398. [Google Scholar] [CrossRef]
- Holló, B.B.; Petruševski, V.M.; Kovács, G.B.; Franguelli, F.P.; Farkas, A.; Menyhárd, A.; Lendvay, G.; Sajó, I.E.; Bereczki, L.; Pawar, R.P.; et al. Thermal and spectroscopic studies on a double-salt-type pyridine–silver perchlorate complex having κ1-O coordinated perchlorate ions. J. Therm. Anal. Calorim. 2019, 138, 1193–1205. [Google Scholar] [CrossRef] [Green Version]
- Franguelli, F.P.; Béres, K.A.; Kótai, L. Pyridinesilver Tetraoxometallate Complexes: Overview of the Synthesis, Structure, and Properties of Pyridine Complexed AgXO4 (X = Cl, Mn, Re) Compounds. Inorganics 2021, 9, 79. [Google Scholar] [CrossRef]
- Sajó, I.E.; Kovács, G.B.; Pasinszki, T.; Bombicz, P.A.; May, Z.; Szilágyi, I.M.; Jánosity, A.; Banerji, K.K.; Kant, R.; Kótai, L. The chemical identity of “[Ag(py)2]MnO4" organic solvent-soluble oxidizing agent and new synthetic routes for the preparation of [Ag(py)n]XO4 (X = Mn, Cl, and Re, n = 2–4) complexes. J. Coord. Chem. 2018, 71, 2884–2904. [Google Scholar] [CrossRef]
- Constable, E.C. Silver Coordination Polymers -From Simple Complexes to Functional Networks. Aust. J. Chem. 2006, 59, 1–2. [Google Scholar] [CrossRef]
- Meyer, G.; Sehabi, M.; Pantenburg, I. Coordinative Flexibility of Monovalent Silver in [AgI←L1]L2 Complexes. In Design and Construction of Coordination Polymers; Hong, M.-C., Chen, L., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2009; Chapter 1; pp. 1–23. [Google Scholar]
- Chen, C.-L.; Kang, B.-S.; Su, C.-Y. Recent Advances in Supramolecualr Design and Asembly of Silver(I) Cooridnation Polymers. Aust. J. Chem. 2006, 59, 3–18. [Google Scholar] [CrossRef]
- Ali, A.; Khalid, M.; Abid, S.; Tahir, M.N.; Iqbal, J.; Ashfaq, M.; Kanwal, F.; Lu, C.; Rehman, M.F. Green Synthesis, SC-XRD, Non-Covalent Interactive Potential and Electronic Communication via DFT Exploration of Pyridine-Based Hydrazone. Crystals 2020, 10, 778. [Google Scholar] [CrossRef]
- Fitchett, C.M.; Steel, P.J. Chiral Heterocyclic Ligands. XIII. Synthesis and X-Ray Crystal Structure of a Chiral, Unidirectional, Silver Coordination Polymer. Aust. J. Chem. 2006, 59, 19–21. [Google Scholar] [CrossRef]
- Duriska, M.B.; Batten, S.R.; Price, D.J. An Interpenetrating Coordination Polymer Containing Weak Hydrogen Bonds and Argentophilic Interactions. Aust. J. Chem. 2006, 59, 26–29. [Google Scholar] [CrossRef]
- Constable, E.C.; Housecroft, C.E.; Kariuki, B.M.; Smith, C.B. A Planar Silver(I) Complex with a ‘Simple’ 2,2′-Bipyridine Ligand. Aust. J. Chem. 2006, 59, 30–33. [Google Scholar] [CrossRef]
- Chen, C.Y.; Zeng, J.Y.; Lee, H.M. Argentophilic interaction and anionic control of supramolecular structures in simple silver pyridine complexes. Inorg. Chim. Acta 2007, 360, 21. [Google Scholar] [CrossRef]
- Dyason, J.C.; Healy, P.C.; Engelhardt, L.M.; White, A.H. Lewis-Base Adducts of Group 1B Metal(I) Compounds. XXII. Crystal Structure of ’Bis(pyridine)silver(I) Perchlorate. Aust. J. Chem. 1985, 38, 1325–1328. [Google Scholar] [CrossRef]
- Nilsson, K.; Oskarsson, A. The crystal structure of tetrapyridinecopper(I) perchlorate and tetrapyridinesilver(I) perchlorate at 260 K. Acta Chem. Scand. 1982, A36, 605–610. [Google Scholar] [CrossRef]
- Majzik, E.; Franguelli, F.P.; Lendvay, G.; Trif, L.; Németh, C.; Farkas, A.; Klébert, S.; Bereczki, L.; Szilágyi, I.M.; Kótai, L. Deuteration and Vibrational Spectra of Dimethylammonium Paratungstate-B Hydrates. Z. Anorg. Allg. Chem 2021, 637, 593–598. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. Appl. J. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Kline, C.H., Jr.; Turkevich, J. The Vibrational Spectrum of Pyridine and the Thermodynamic Properties of Pyridine Vapors. J. Chem. Phys. 1944, 12, 300–309. [Google Scholar] [CrossRef]
- Corrsin, L.; Fax, B.J.; Lord, R.C. The Vibrational Spectra of Pyridine and Pyridine-d5. J. Chem. Phys. 1953, 21, 1170–1176. [Google Scholar] [CrossRef]
- Partal Ureña, F.; Fernández Gómez, M.; López González, J.J.; Martínez Torres, E. A new insight into the vibrational analysis of pyridine. Spectrochim. Acta Part A Mol. Biomol. Spectr. 2003, 59, 2815–2839. [Google Scholar] [CrossRef]
- Bowmaker, G.A.; Lim, K.C.; Skelton, B.W.; Sukarianingsih, D.; White, A.H. Syntheses, structures and vibrational spectroscopy of some 1:2 and 1:3 adducts of silver(I) oxyanion salts with pyridine and piperidine bases containing non-coordinating 2(,6)-substituents. Inorg. Chim. Acta 2005, 358, 4342–4370. [Google Scholar] [CrossRef]
- Alvisi, U. Researches on the Perchlorates, Luteocobaltiammine perchlorate and observations on amminemetal complexes. Gazz. Chim. Ital. 1901, 31, 289–301. [Google Scholar]
- Sheldrick, G.M. Short History of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar] [CrossRef]
- CrystalClear SM, Version 1 4.0; Rigaku/MSC Inc.: The Woodlands, TX, USA, 2008.
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision, E.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.; Handy, N. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Schultz, N.E.; Truhlar, D.G. Exchange-correlation functional with broad accuracy for metallic and nonmetallic compounds, kinetics, and noncovalent interactions. J. Chem. Phys. 2005, 123, 161103. [Google Scholar] [CrossRef]











| Compound 1 | Compound 2 | |
|---|---|---|
| Temperature | 103(2) K | 293(2) K |
| Crystal system | monoclinic | monoclinic |
| Space group | C2/c | C2/c |
| Unit cell dimensions | ||
| a | 19.1093(16) Å | 19.410(1) Å |
| b | 7.7016(8) Å | 7.788(1) Å |
| c | 20.6915(19) Å | 21.177(1) Å |
| β | 105.515(7)° | 104.20(1)° |
| Cell volume | 2934.2(5) Å3 | 3103.4(1) Å3 |
| Anion | ClO4− not disordered | MnO4− disordered 66:34 |
| KPI | 69.8% | 67.4% |
| Pyridine | 527.2 Å3, 18.0% | 604.5 Å3, 19.5% |
| Ag∙∙∙Ag | 3.3873(5) Å | 3.4213(9) Å |
| Ag-O2 | 2.840(2) Å | 2.548(14) Å |
| Ag-O2′ | 2.8749(16) Å | 2.745(9) Å |
| Cα21-H21α∙∙∙O2XO4 | 2.355 Å | 2.602 Å |
| 2.701 Å | 2.770 Å | |
| π−π N1∙∙∙N1 | 3.745 Å | 3.782 Å |
| π−π N1∙∙∙N2 | 3.666 Å | 3.740 Å |
| D-H∙∙∙A | D-H [Å] | H∙∙∙A [Å] | D∙∙∙A [Å] | D-H∙∙∙A [o] | Symmetry Operation |
|---|---|---|---|---|---|
| C21-H21∙∙∙O3 | 0.93 | 2.360 | 3.228(3) | 156 | intra |
| C15-H15∙∙∙O4 | 0.93 | 2.701 | 3.621(3) | 170 | intra |
| C24-H24∙∙∙O4 | 0.93 | 2.598 | 3.261(3) | 129 | x, 1−y, −1/2 + z |
| C13-H13∙∙∙O1 | 0.93 | 2.600 | 3.376(3) | 141 | −1/2 + x, 1/2 + y, z |
| C23-H23∙∙∙O1 | 0.93 | 2.595 | 3.504(3) | 166 | x, 1−y, −1/2 + z |
| C32pyr-H32∙∙∙O4 | 0.93 | 2.64 | 3.555(3) | 169 | x, 1 + y, z |
| C2v | ν | Vibrational Mode | Compounds | Pyridine [26,27,28] | DFT Calculations | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4 [8] | Pyridine | Agpy2ClO4 Dimer | Dimer and 2 py | |||||||
| IR | Raman | IR | Raman | IR | Raman | ||||||
| A1 (in- plane) | 1 | C-H stretch | 3120 | 3150 | 3094 | 3082 | 3076 | 3076 | 3261 | 3268–3272 | 3258–3269 |
| 2 | C-H stretch | 3074 | 3085 | 3062 | 3066 | 3060 | 3060 | 3236 | 3236–3242 | (3218–3243) b | |
| 3 | C-H stretch | 3046 | 3068 | 3043 | 3030 | 3030 | 3030 | 3191 | 3223, 3229 | (3218–3238) b | |
| 4 | Ring stretch | 1574 | 1586 | 1592 1571 | 1602 1571 | 1578 | 1578 | 1652 | 1668–1670 | 1660–1673 | |
| 5 | Ring stretch | 1488 | 1488 1485 | 1482 | 1483 | 1483 | 1483 | 1479 | 1515–1520 | 1519–1524 | |
| 6 | C-H wag | 1222 | 1224 | 1215 | 1215 | 1217 | 1217 | 1246 | 1247–1257 | 1244–1267 | |
| 7 | C-H wag | 1069 a | 1062 a | 1079 | 1072 | 1071 | 1071 | 1094 | 1088, 1096, 1097 b | 1095, 1097 1103 | |
| 8 | Ring bend | 1040 a | 1042 a | 1034 | 1034 | 1031 | 1031 | 1002 | 1024–1027 | 1006, 1024, 1026, 1029, 1030 | |
| 9 | Ring breathing | 880 | 863 | - | 865 | 858 | 858 | 1046 | 1024–1027 | 1043–1049 | |
| 10 | Ring bend | 619 a | 621 a | - | - | 601 | 601 | 606 | 630–636 | 610, 611, 632–640 | |
| A2 (out of plane) | 11 | C-H wag | 983 | 992 | 983 | 985 | 982 | 982 | 1001 | 1020–1023 | (1004–1040) c |
| 12 | C-H wag | 881 | - | 885 | 878 | 887 | 887 | 897 | 897–903 | 898–929 | |
| 13 | Ring bend | - | - | - | - | - | - | 375 | 389–395 | 389–405 | |
| B1 (out of plane) | 14 | C-H wag | 1014 | 1013 | 1003 | 1010 | 997 | 997 | 1005 | 1011–1015 | (1005–1016) c |
| 15 | C-H wag | 949 | 929 * | 944 | - | - | - | 966 | 988–991 | 973–996 | |
| 16 | C-H wag | 751 | - | 751 | - | - | - | 713 | 714–717 | 715–729 | |
| 17 | Ring bend | 704, 692, 674 | - | 700 | - | - | - | 763 | 765–770 | 767–783 | |
| 18 | Ring bend | - | - | - | - | - | - | 412, | 416–420 | 415–425 | |
| B2 (in- plane) | 19 | C-H stretch | 3074 | 3068 | 3063 | 3066 | 3066 | 3066 | 3253 | 3256–3263 | (3248–3253) b |
| 20 | C-H stretch | 3006 | 3009, 3028 | 3043 | 3032 | 3030 | 3030 | 3188 | 3223–3225 | (3218–3236) b | |
| 21 | Ring stretch | 1574 | 1571 | 1571 | 1574 | 1577 | 1577 | 1651 | 1648–1650 | 1644–1654 | |
| 22 | Ring stretch | 1447 | 1446 | 1440 | 1444 | 1443 | 1443 | 1473 | 1482–1483 | 1477–1487 | |
| 23 | C-H wag | 1373 | 1380 | 1384 | 1355 | - | - | 1370 | 1375–1380 | 1377–1389 | |
| 24 | Ring stretch | 1233 | 1230 | 1232 | 1227 | 1227 | 1227 | 1333 | 1334–1336 | 1335–1341 | |
| 25 | C-H wag | 1155 | 1165 | 1155 | 1155 | - | 1163 | 1169–1170 | 1167–1177 | ||
| 26 | C-H wag | 1068 a | 1065 *, 1072 * | 1064 | 1072 | 1068 | 1082 | 1079, 1094, 1095, 1097 b, 1098, 1104 | (1167–1177) | ||
| 27 | Ring bend | 651 | 649 | - | 649 | 654 | 668 | 660–663 | 660–667 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
May, N.V.; Bayat, N.; Béres, K.A.; Bombicz, P.; Petruševski, V.M.; Lendvay, G.; Farkas, A.; Kótai, L. Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)silver(I) Perchlorate, [Agpy2ClO4]·0.5py. Inorganics 2022, 10, 123. https://doi.org/10.3390/inorganics10090123
May NV, Bayat N, Béres KA, Bombicz P, Petruševski VM, Lendvay G, Farkas A, Kótai L. Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)silver(I) Perchlorate, [Agpy2ClO4]·0.5py. Inorganics. 2022; 10(9):123. https://doi.org/10.3390/inorganics10090123
Chicago/Turabian StyleMay, Nóra V., Niloofar Bayat, Kende Attila Béres, Petra Bombicz, Vladimir M. Petruševski, György Lendvay, Attila Farkas, and László Kótai. 2022. "Structure and Vibrational Spectra of Pyridine Solvated Solid Bis(Pyridine)silver(I) Perchlorate, [Agpy2ClO4]·0.5py" Inorganics 10, no. 9: 123. https://doi.org/10.3390/inorganics10090123