Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices
Abstract
:1. Introduction
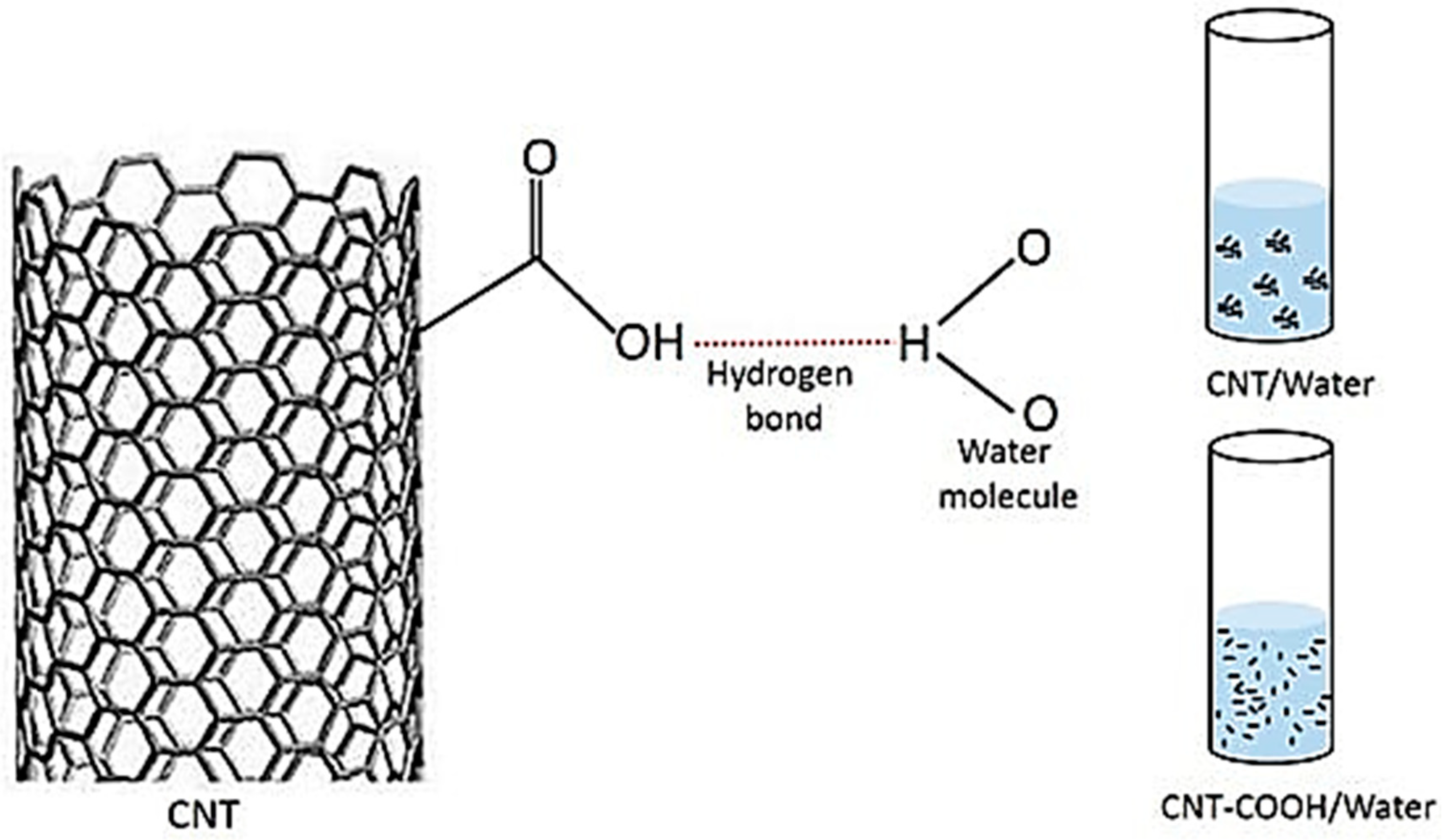
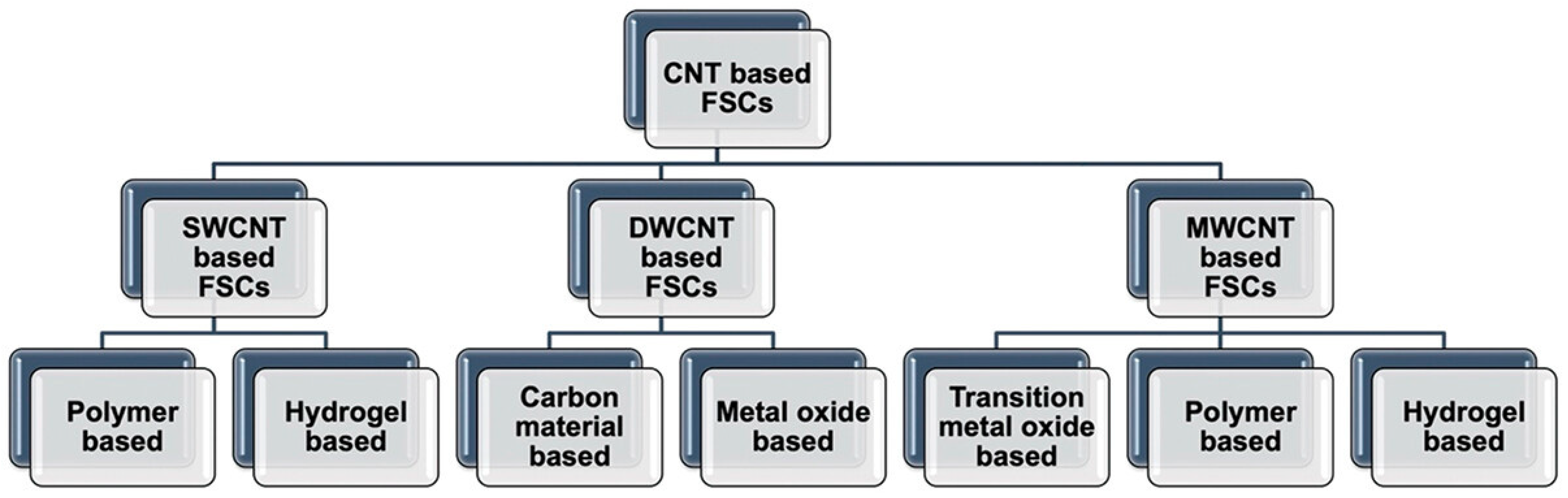
2. Dispersion Stability of Carbon Nanotubes
3. Mechanisms and Strategies for Enhancing Dispersion
3.1. Covalent and Non-Covalent Functionalization
3.1.1. Covalent Functionalization
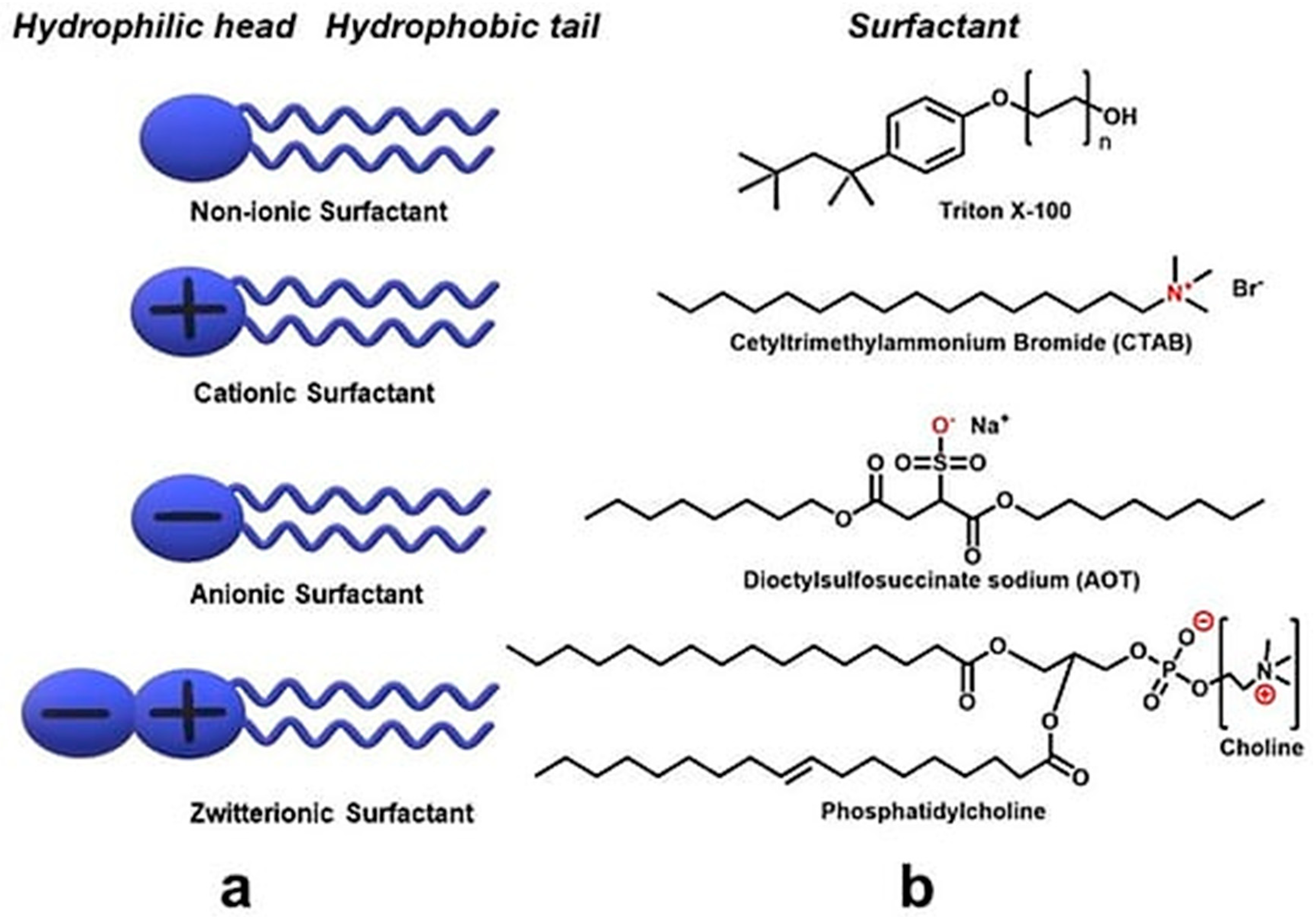
3.1.2. Non-Covalent Functionalization
4. Impact on Energy Storage Devices
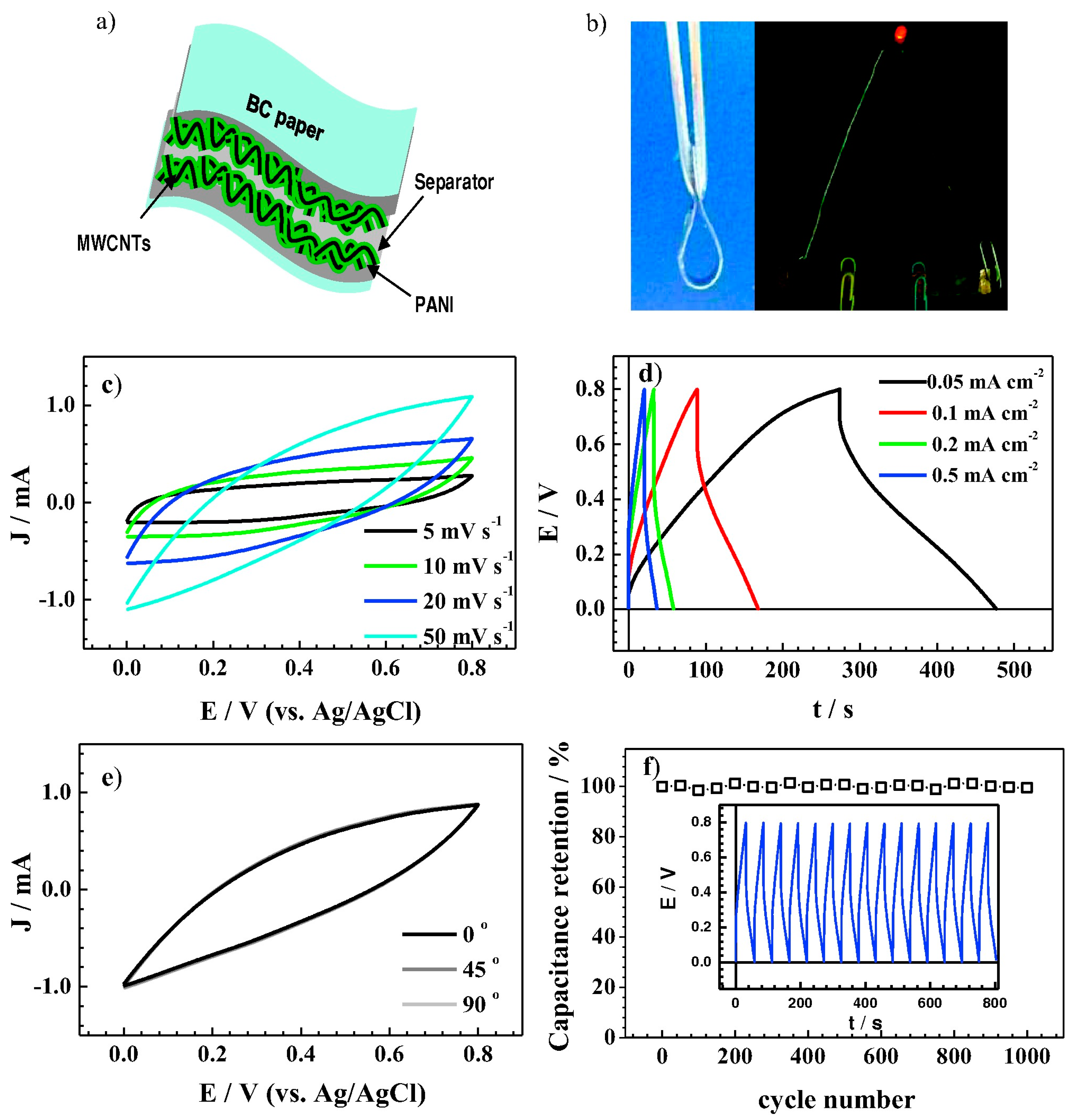
4.1. Supercapacitors Enhance Electrode Performance through Dispersion Stability
4.2. Batteries
5. Future Trajectories and Navigating Challenges
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Li, F.; Ma, L.-P.; Cheng, H.-M. Advanced Materials for Energy Storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Rahman, M.M.; Oni, A.O.; Gemechu, E.; Kumar, A. Assessment of energy storage technologies: A review. Energy Convers. Manag. 2020, 223, 113295. [Google Scholar] [CrossRef]
- Ibrahim, H.; Ilinca, A.; Perron, J. Energy storage systems—Characteristics and comparisons. Renew. Sustain. Energy Rev. 2008, 12, 1221–1250. [Google Scholar] [CrossRef]
- Goodenough, J.B. Energy storage materials: A perspective. Energy Storage Mater. 2015, 1, 158–161. [Google Scholar] [CrossRef]
- Kim, H.; Qaiser, N.; Hwang, B. Electro-mechanical response of stretchable pdms composites with a hybrid filler system. Facta Univ. Ser. Mech. Eng. 2023, 21, 51–61. [Google Scholar] [CrossRef]
- Sears, K.; Dumée, L.; Schütz, J.; She, M.; Huynh, C.; Hawkins, S.; Duke, M.; Gray, S. Recent Developments in Carbon Nanotube Membranes for Water Purification and Gas Separation. Materials 2010, 3, 127–149. [Google Scholar] [CrossRef]
- He, J.-H.; Elazem, N.Y.A. The carbon nanotube-embedded boundary layer theory for energy harvesting. Facta Univ. Ser. Mech. Eng. 2022, 20, 211–235. [Google Scholar] [CrossRef]
- Bhadra, R.; Jana, T.; Mitra, A.; Sahoo, P. Effect of CNT radius on flattening contact behaviour of CNT-Al nanocomposite: A numerical approch. Rep. Mech. Eng. 2023, 4, 121–130. [Google Scholar] [CrossRef]
- Avouris, P. Carbon nanotube electronics. Chem. Phys. 2002, 281, 429–445. [Google Scholar] [CrossRef]
- Hwang, B.; Han, Y.; Matteini, P. Bending fatigue behavior of Ag nanowire/Cu thin-film hybrid interconnects for wearable electronics. Facta Univ. Ser. Mech. Eng. 2022, 20, 553–560. [Google Scholar] [CrossRef]
- Hashemi Kachapi, S.H. Nonlinear vibration response of piezoelectric nanosensor: Influences of surface/interface effects. Facta Univ. Ser. Mech. Eng. 2023, 21, 259–272. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef]
- De Volder, M.F.L.; Tawfick, S.H.; Baughman, R.H.; Hart, A.J. Carbon Nanotubes: Present and Future Commercial Applications. Science 2013, 339, 535–539. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Hecht, D.S.; Grüner, G. Carbon Nanotube Thin Films: Fabrication, Properties, and Applications. Chem. Rev. 2010, 110, 5790–5844. [Google Scholar] [CrossRef] [PubMed]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon Nanotubes—The Route toward Applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Rastogi, R.; Kaushal, R.; Tripathi, S.K.; Sharma, A.L.; Kaur, I.; Bharadwaj, L.M. Comparative study of carbon nanotube dispersion using surfactants. J. Colloid Interface Sci. 2008, 328, 421–428. [Google Scholar] [CrossRef]
- Yoon, H.; Kim, H.; Matteini, P.; Hwang, B. Research Trends on the Dispersibility of Carbon Nanotube Suspension with Surfactants in Their Application as Electrodes of Batteries: A Mini-Review. Batteries 2022, 8, 254. [Google Scholar] [CrossRef]
- Yoon, H.; Thompson, R.; Hwang, B. Dispersibility study of carbon nanotubes using multiple light scattering: A mini-review. Colloid Interface Sci. Commun. 2023, 52, 100686. [Google Scholar] [CrossRef]
- Onyibo, E.C.; Safaei, B. Application of finite element analysis to honeycomb sandwich structures: A review. Rep. Mech. Eng. 2022, 3, 192–209. [Google Scholar] [CrossRef]
- Schneider, S.; Lefebvre, J.; Diercks, N.J.; Berger, F.J.; Lapointe, F.; Schleicher, J.; Malenfant, P.R.L.; Zaumseil, J. Phenanthroline Additives for Enhanced Semiconducting Carbon Nanotube Dispersion Stability and Transistor Performance. ACS Appl. Nano Mater. 2020, 3, 12314–12324. [Google Scholar] [CrossRef]
- Forney, M.W.; Poler, J.C. Significantly Enhanced Single-Walled Carbon Nanotube Dispersion Stability in Mixed Solvent Systems. J. Phys. Chem. C 2011, 115, 10531–10536. [Google Scholar] [CrossRef]
- Lee, J.; Kim, M.; Hong, C.K.; Shim, S.E. Measurement of the dispersion stability of pristine and surface-modified multiwalled carbon nanotubes in various nonpolar and polar solvents. Meas. Sci. Technol. 2007, 18, 3707. [Google Scholar] [CrossRef]
- Banerjee, S.; Sutradhar, G.; Sahoo, P. Design of experiment analysis of elevated temperature wear of Mg-WC nano-composites. Rep. Mech. Eng. 2021, 2, 202–211. [Google Scholar] [CrossRef]
- Lee, C.; Kim, H.; Hwang, B. Fracture behavior of metal oxide/silver nanowire composite electrodes under cyclic bending. J. Alloys Compd. 2019, 773, 361–366. [Google Scholar] [CrossRef]
- Soleimani Zohr Shiri, M.; Henderson, W.; Mucalo, M.R. A Review of The Lesser-Studied Microemulsion-Based Synthesis Methodologies Used for Preparing Nanoparticle Systems of The Noble Metals, Os, Re, Ir and Rh. Materials 2019, 12, 1896. [Google Scholar] [CrossRef]
- Ha, H.; Müller, S.; Baumann, R.-P.; Hwang, B. Peakforce quantitative nanomechanical mapping for surface energy characterization on the nanoscale: A mini-review. Facta Univ. Ser. Mech. Eng. 2023. [Google Scholar] [CrossRef]
- Rajendran, D.; Ramalingame, R.; Adiraju, A.; Nouri, H.; Kanoun, O. Role of Solvent Polarity on Dispersion Quality and Stability of Functionalized Carbon Nanotubes. J. Compos. Sci. 2022, 6, 26. [Google Scholar] [CrossRef]
- Li, S.; Huang, D.; Zhang, B.; Xu, X.; Wang, M.; Yang, G.; Shen, Y. Flexible Supercapacitors Based on Bacterial Cellulose Paper Electrodes. Adv. Energy Mater. 2014, 4, 1301655. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Li, Y.; Feng, Y.; Feng, W. Carbon-based functional nanomaterials: Preparation, properties and applications. Compos. Sci. Technol. 2019, 179, 10–40. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Yong, T.; Xu, N.; Guan, R.; Yue, L. Multiwalled carbon nanotube webs welded with Si nanoparticles as high-performance anode for lithium-ion batteries. J. Alloys Compd. 2016, 688, 216–224. [Google Scholar] [CrossRef]
- Ren, J.; Li, L.; Chen, C.; Chen, X.; Cai, Z.; Qiu, L.; Wang, Y.; Zhu, X.; Peng, H. Twisting Carbon Nanotube Fibers for Both Wire-Shaped Micro-Supercapacitor and Micro-Battery. Adv. Mater. 2013, 25, 1155–1159. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Tang, C.-h.; Gong, H. A High Energy Density Asymmetric Supercapacitor from Nano-architectured Ni(OH)2/Carbon Nanotube Electrodes. Adv. Funct. Mater. 2012, 22, 1272–1278. [Google Scholar] [CrossRef]
- Obreja, V.V.N. On the performance of supercapacitors with electrodes based on carbon nanotubes and carbon activated material—A review. Phys. E Low-Dimens. Syst. Nanostruct. 2008, 40, 2596–2605. [Google Scholar] [CrossRef]
- Zakaria, M.R.; Omar, M.F.; Zainol Abidin, M.S.; Md Akil, H.; Abdullah, M.M.A.B. Recent progress in the three-dimensional structure of graphene-carbon nanotubes hybrid and their supercapacitor and high-performance battery applications. Compos. Part A: Appl. Sci. Manuf. 2022, 154, 106756. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, J.; Yin, Z.; Cui, C.; Qian, W.; Wei, F. Carbon nanotube- and graphene-based nanomaterials and applications in high-voltage supercapacitor: A review. Carbon 2019, 141, 467–480. [Google Scholar] [CrossRef]
- Pan, H.; Li, J.; Feng, Y. Carbon Nanotubes for Supercapacitor. Nanoscale Res. Lett. 2010, 5, 654. [Google Scholar] [CrossRef]
- Kim, H.; Cheong, J.Y.; Hwang, B. Mini Review of Technological Trends of Flexible Supercapacitors Using Carbon Nanotubes. J. Nat. Fibers 2023, 20, 2204455. [Google Scholar] [CrossRef]
- Frackowiak, E.; Metenier, K.; Bertagna, V.; Beguin, F. Supercapacitor electrodes from multiwalled carbon nanotubes. Appl. Phys. Lett. 2000, 77, 2421–2423. [Google Scholar] [CrossRef]
- Hu, S.; Rajamani, R.; Yu, X. Flexible solid-state paper based carbon nanotube supercapacitor. Appl. Phys. Lett. 2012, 100, 104103. [Google Scholar] [CrossRef]
- Choi, C.; Lee, J.A.; Choi, A.Y.; Kim, Y.T.; Lepró, X.; Lima, M.D.; Baughman, R.H.; Kim, S.J. Flexible Supercapacitor Made of Carbon Nanotube Yarn with Internal Pores. Adv. Mater. 2014, 26, 2059–2065. [Google Scholar] [CrossRef]
- Gund, G.S.; Dubal, D.P.; Shinde, S.S.; Lokhande, C.D. Architectured Morphologies of Chemically Prepared NiO/MWCNTs Nanohybrid Thin Films for High Performance Supercapacitors. ACS Appl. Mater. Interfaces 2014, 6, 3176–3188. [Google Scholar] [CrossRef] [PubMed]
- de las Casas, C.; Li, W. A review of application of carbon nanotubes for lithium ion battery anode material. J. Power Sources 2012, 208, 74–85. [Google Scholar] [CrossRef]
- Xiong, Z.; Yun, Y.S.; Jin, H.-J. Applications of Carbon Nanotubes for Lithium Ion Battery Anodes. Materials 2013, 6, 1138–1158. [Google Scholar] [CrossRef] [PubMed]
- Kiebele, A.; Gruner, G. Carbon nanotube based battery architecture. Appl. Phys. Lett. 2007, 91, 144104. [Google Scholar] [CrossRef]
- Chen, W.X.; Lee, J.Y.; Liu, Z. The nanocomposites of carbon nanotube with Sb and SnSb0.5 as Li-ion battery anodes. Carbon 2003, 41, 959–966. [Google Scholar] [CrossRef]
- Guo, B.; Wang, X.; Fulvio, P.F.; Chi, M.; Mahurin, S.M.; Sun, X.-G.; Dai, S. Soft-Templated Mesoporous Carbon-Carbon Nanotube Composites for High Performance Lithium-ion Batteries. Adv. Mater. 2011, 23, 4661–4666. [Google Scholar] [CrossRef]
- Ng, S.H.; Wang, J.; Guo, Z.P.; Chen, J.; Wang, G.X.; Liu, H.K. Single wall carbon nanotube paper as anode for lithium-ion battery. Electrochim. Acta 2005, 51, 23–28. [Google Scholar] [CrossRef]
- Dörfler, S.; Hagen, M.; Althues, H.; Tübke, J.; Kaskel, S.; Hoffmann, M.J. High capacity vertical aligned carbon nanotube/sulfur composite cathodes for lithium–sulfur batteries. Chem. Commun. 2012, 48, 4097–4099. [Google Scholar] [CrossRef]
- Kim, P.J.H.; Kim, K.; Pol, V.G. Towards highly stable lithium sulfur batteries: Surface functionalization of carbon nanotube scaffolds. Carbon 2018, 131, 175–183. [Google Scholar] [CrossRef]
- Liang, W.; Tang, Y.; Liu, L.; Zhu, C.; Sheng, R. Effective Trapping of Polysulfides Using Functionalized Thin-Walled Porous Carbon Nanotubes as Sulfur Hosts for Lithium–Sulfur Batteries. Inorg. Chem. 2020, 59, 8481–8486. [Google Scholar] [CrossRef]
- Ma, L.; Zhuang, H.L.; Wei, S.; Hendrickson, K.E.; Kim, M.S.; Cohn, G.; Hennig, R.G.; Archer, L.A. Enhanced Li–S Batteries Using Amine-Functionalized Carbon Nanotubes in the Cathode. ACS Nano 2016, 10, 1050–1059. [Google Scholar] [CrossRef] [PubMed]
- Evanoff, K.; Khan, J.; Balandin, A.A.; Magasinski, A.; Ready, W.J.; Fuller, T.F.; Yushin, G. Towards Ultrathick Battery Electrodes: Aligned Carbon Nanotube—Enabled Architecture. Adv. Mater. 2012, 24, 533–537. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Zhang, Z.; Zhou, C.; Lai, Y.; Li, J. Multi-walled carbon nanotubes @ mesoporous carbon hybrid nanocomposites from carbonized multi-walled carbon nanotubes @ metal–organic framework for lithium sulfur battery. J. Power Sources 2014, 248, 570–576. [Google Scholar] [CrossRef]


| Functionalization Type | Materials | Advantages | Disadvantages | |
|---|---|---|---|---|
| Covalent | Oxidation | Nitric acid or potassium permanganate | Excellent dispersion stability | Toxic process |
| Cycloaddition | Aziridines or cyclooctynes | Good dispersion stability | Sensitive reaction results | |
| Radical addition | UV irradiation or plasma treatment | Easiness of process | Insufficient dispersion stability | |
| Polymer grafting | Polyurethane, PMMA-OH, or polyvinyl alcohol | Excellent dispersion stability | Complex synthesis process | |
| Non-covalent | Surfactant wrapping | Sodium dodecyl sulfate (SDS) or Triton X-100 | Good dispersion stability, easiness of process | Some chemicals are toxic, less stable than other methods |
| Polymer wrapping | Polyvinylpyrrolidone (PVP) or polyethylene glycol (PEG) | Excellent dispersion stability | Reducing electrical properties | |
| DNA wrapping | Single-stranded DNA or double-stranded DNA | Good dispersion stability | Sensitive synthesis, short shelf life | |
| Application Type | Functionalization Method | Materials | Ref. |
|---|---|---|---|
| Supercapacitor | PANI-MWCNT/BC | 656 F/g at 10 A/g | [28] |
| MWCNTs with NiO NPs | 1727 F/g at 5 mA/cm2 | [41] | |
| SWCNTs functionalized with sulfonic acid groups | 1183 at 5 A/g | [29] | |
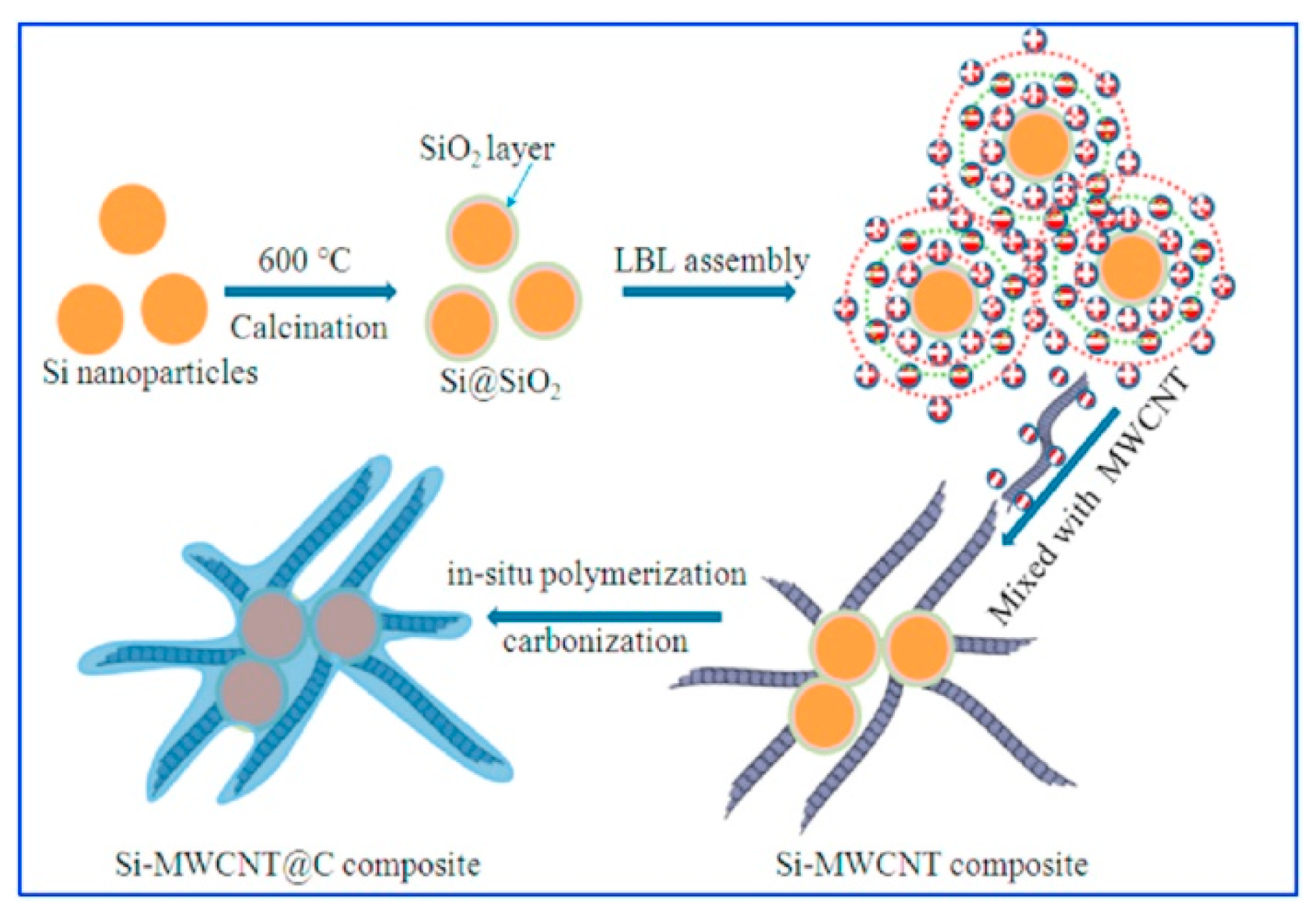
| Battery | MWCNTs coated with Si NPs | 937 mAh/g | [30] |
| CNT-S functionalization | 800 mAh/g | [48] | |
| CNT- polyethylenimine (PEI) | 838 mAh/g | [51] | |
| CNT-oxygen functionalization | 798.5 mAh/g | [50] | |
| Oxygen treatment of CNT | 1239.8 mAh/g | [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, C.; Yun, T.G.; Hwang, B. Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices. Inorganics 2023, 11, 383. https://doi.org/10.3390/inorganics11100383
Choi C, Yun TG, Hwang B. Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices. Inorganics. 2023; 11(10):383. https://doi.org/10.3390/inorganics11100383
Chicago/Turabian StyleChoi, Chunghyeon, Tae Gwang Yun, and Byungil Hwang. 2023. "Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices" Inorganics 11, no. 10: 383. https://doi.org/10.3390/inorganics11100383





