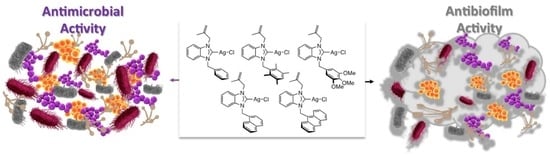
Benzimidazol-2-ylidene Silver Complexes: Synthesis, Characterization, Antimicrobial and Antibiofilm Activities, Molecular Docking and Theoretical Investigations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Ag(I)-NHC Complexes
2.2. Single-Crystal X-ray Diffraction Studies
2.3. Antimicrobial and Antibiofilm Activities
2.4. Molecular Docking
3. Materials and Methods
3.1. General Procedure for the Synthesis of Silver Complexes
3.2. X-ray Crystallography
3.3. Biological Assays
3.3.1. Antimicrobial Activity
3.3.2. Reduction in Biofilm Formation
3.4. Theoretical Methods
3.4.1. Molecular Docking
3.4.2. DFT-Based Calculation Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Igau, A.; Grutzmacher, H.; Baceiredo, A.; Bertrand, G. Analogous α,α‘-bis-carbenoid triply bonded species: Synthesis of a stable λ3-phosphinocarbene-λ5-phosphaacetylene. J. Am. Chem. Soc. 1988, 110, 6463–6466. [Google Scholar] [CrossRef]
- Arduengo III, A.J.; Harlow, R.L.; Kline, M. A stable crystalline carbine. J. Am. Chem. Soc. 1991, 113, 361–363. [Google Scholar] [CrossRef]
- Gao, P.; Szostak, M. Hydration reactions catalyzed by transition metal-NHC (NHC = N-heterocyclic carbene) complexes. Coord. Chem. Rev. 2023, 485, 215110. [Google Scholar] [CrossRef]
- Proetto, M.T.; Alexander, K.; Melaimi, M.; Bertrand, G.; Gianneschi, N.C. Cyclic (alkyl)(amino)carbene (CAAC) gold(I) complexes as chemotherapeutic agents. Chem. Eur. J. 2021, 27, 3772–3778. [Google Scholar] [CrossRef] [PubMed]
- Heravi, M.M.; Heidari, B.; Ghavidel, M.; Ahmadi, T. Non-conventional green strategies for NHC catalyzed carbon-carbon coupling reactions. Curr. Org. Chem. 2017, 21, 2249–2313. [Google Scholar] [CrossRef]
- Danopoulos, A.A.; Simler, T.; Braunstein, B. N-Heterocyclic carbene complexes of copper, nickel, and cobalt. Chem. Rev. 2019, 119, 3730–3961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Meng, M.; Szostak, M.; Nolan, S.P. N-Heterocyclic carbene complexes in C-H activation reactions. Chem. Rev. 2020, 120, 1981–2048. [Google Scholar] [CrossRef]
- Budagumpi, S.; Keri, R.S.; Achar, G.; Brinda, K.N. Coinage metal complexes of chiral N-heterocyclic carbene ligands: Syntheses and applications in asymmetric catalysis. Adv. Synth. Catal. 2020, 362, 970–997. [Google Scholar] [CrossRef]
- Mora, M.; Gimeno, M.C.; Visbal, R. Recent advances in gold–NHC complexes with biological properties. Chem. Soc. Rev. 2019, 48, 447–462. [Google Scholar] [CrossRef]
- Scattolin, T.; Bortolamiol, E.; Palazzolo, S.; Caligiuri, I.; Perin, T.; Canzonieri, V.; Demitri, N.; Rizzolio, F.; Cavallo, L.; Dereli, B.; et al. The anticancer activity of an air-stable Pd(I)-NHC (NHC = N-heterocyclic carbene) dimer. Chem. Commun. 2020, 56, 12238–12241. [Google Scholar] [CrossRef]
- Jakob, C.H.G.; Muñoz, A.W.; Schlagintweit, J.F.; Weiß, V.; Reich, R.M.; Sieber, S.A.; Correira, J.D.G.; Kühn, F.E. Anticancer and antibacterial properties of trinuclear Cu(I), Ag(I) and Au(I) macrocyclic NHC/urea complexes. J. Organomet. Chem. 2021, 932, 121643. [Google Scholar] [CrossRef]
- Üstün, E.; Şahin, N.; Çelik, C.; Tutar, U.; Özdemir, N.; Gürbüz, N.; Özdemir, İ. Synthesis, characterization, antimicrobial and antibiofilm activity, and molecular docking analysis of NHC precursors and their Ag-NHC complexes. Dalton Trans. 2021, 50, 15400–15412. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, L.; Chakchouk-Mtiba, A.; Naouali, O.; Mellouli, L.; Mansour, L.; Özdemir, İ.; Yaser, S.; Sauthier, M.; Hamdi, N. Ruthenium(II) complexes bearing benzimidazole-based N-heterocyclic carbene (NHC) ligands as potential antimicrobial, antioxidant, enzyme inhibition, and antiproliferative agents. J. Coord. Chem. 2022, 75, 645–667. [Google Scholar] [CrossRef]
- Patil, S.A.; Hoagland, A.P.; Patil, S.A.; Bugarin, A. N-heterocyclic carbene-metal complexes as bio-organometallic antimicrobial and anticancer drugs, an update (2015–2020). Future Med. Chem. 2020, 12, 2239–2275. [Google Scholar]
- Ronga, L.; Varcamonti, M.; Tesauro, D. Structure-activity relationships in NHC-silver complexes as antimicrobial agents. Molecules 2023, 28, 4435. [Google Scholar] [CrossRef] [PubMed]
- Barras, F.; Aussel, L.; Ezraty, B. Silver and antibiotic, new facts to an old Story. Antibiotics 2018, 7, 79. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Wu, R.; Xiong, Y.H.; Ren, H.M.; Lei, C.; Zhao, Y.Q.; Zhang, X.Y.; Xu, F.J. Multifunctional antimicrobial materials: From rational design to biomedical applications. Prog. Mater. Sci. 2022, 125, 100887. [Google Scholar] [CrossRef]
- Habash, M.; Reid, G. Microbial biofilms: Their development and significance for medical device-related infections. J. Clin. Pharmacol. 1999, 39, 887–898. [Google Scholar] [CrossRef]
- Livermore, D.M. Discovery research: The scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66, 1941–1944. [Google Scholar] [CrossRef]
- Wohlleben, W.; Mast, Y.; Stegmann, E.; Ziemert, N. Antibiotic drug discovery. Microb. Biotechnol. 2016, 9, 541–548. [Google Scholar] [CrossRef]
- Kascatan-Nebioglu, A.; Panzner, M.J.; Tessier, C.A.; Cannon, C.L.; Youngs, W.J. N-Heterocyclic carbene–silver complexes: A new class of antibiotics. Coord. Chem. Rev. 2007, 251, 884–895. [Google Scholar] [CrossRef]
- Hindi, K.M.; Ditto, A.J.; Panzner, M.J.; Medvetz, D.A.; Han, D.S.; Hovis, C.E.; Hilliard, J.K.; Taylor, J.B.; Yun, Y.H.; Connon, C.L.; et al. The antimicrobial efficacy of sustained release silver-carbene complex-loaded L-tyrosine polyphosphate nanoparticles: Characterization, in vitro and in vivo studies. Biomaterials 2009, 30, 3771–3779. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, T.; Badel, S.; Mayer, P.; Groelly, J.; de Frémont, P.; Jacques, B.; Braunstein, P.; Teyssot, M.L.; Gaulier, C.; Cisnetti, F.; et al. High-throughput screening of metal-N-heterocyclic carbene complexes against biofilm formation by pathogenic bacteria. ChemMedChem 2014, 9, 1140–1144. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.; O’Beirne, C.; Beato, Z.; Tacke, M.; Kavanagh, K. Exposure of Candida parapsilosis to the silver(I) compound SBC3 induces alterations in the proteome and reduced virulence. Metallomics 2022, 14, mfac046. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Ahmadi, S.; Akhavan, O.; Luque, R. Silver and gold nanoparticles for antimicrobial purposes against multi-drug resistance bacteria. Materials 2022, 15, 1799. [Google Scholar] [CrossRef]
- Algotiml, R.; Gab-Alla, A.; Seoudi, R.; Abulreesh, H.H.; El-Readi, M.Z.; Elbanna, K. Anticancer and antimicrobial activity of biosynthesized Red Sea marine algal silver nanoparticles. Sci. Rep. 2022, 12, 2421. [Google Scholar] [CrossRef]
- Rao, S.Q.; Zhang, R.Y.; Chen, R.; Gao, Y.J.; Gao, L.; Yang, Z.Q. Nanoarchitectonics for enhanced antibacterial activity with Lactobacillus buchneri S-layer proteins-coated silver nanoparticles. J. Hazard. Mater. 2022, 426, 128029. [Google Scholar] [CrossRef]
- Şahin, N.; Üstün, E.; Tutar, U.; Çelik, C.; Gürbüz, N.; Özdemir, İ. Antimicrobial activity, inhibition of biofilm formation, and molecular docking study of novel Ag-NHC complexes. J. Organomet. Chem. 2021, 954, 122082. [Google Scholar] [CrossRef]
- Çelik, C.; Üstün, E.; Şahin, N.; Tutar, U. Antimicrobial and antibiofilm activities and bovine serum albumin binding properties of benzimidazolium derivative NHC salts and their Ag(I)-NHC complexes. Appl. Organomet. Chem. 2022, 36, e6891. [Google Scholar] [CrossRef]
- Muscat, J.; Wander, A.; Harrison, N.M. On the prediction of band gaps from hybrid functional theory. Chem. Phys. Lett. 2001, 342, 397–401. [Google Scholar] [CrossRef]
- Üstün, E.; Şahin, N. Density functional theory and molecular docking analysis of newly synthesized and characterized benzimidazolium salts. Ordu Univ. J. Sci. Tech. 2022, 12, 52–63. [Google Scholar] [CrossRef]
- Kumar, V.S.; Mary, Y.S.; Pradhan, K.; Brahman, D.; Mary, Y.S.; Serdaroğlu, G.; Roxy, M.S. Conformational analysis and quantum descriptors of two new imidazole derivatives by experimental, DFT, AIM, molecular docking studies and adsorption activity on grapheme. Heliyon 2020, 6, e05182. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, M.; Mohanty, P.S. Molecular docking in organic, inorganic, and hybrid systems: A tutorial review. Monatsh. Chem. 2023, 154, 683–707. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, A.Y.; Ammar, Y.A.; Salem, M.A.; Abu-Elghait, M.; Ragab, A. Design, synthesis, molecular modeling, and antimicrobial potential of novel 3-[(1H-pyrazol-3-yl)imino]indolin-2-one derivatives as DNA gyrase inhibitors. Arch. Pharm. 2022, 355, 2100266. [Google Scholar] [CrossRef] [PubMed]
- Üstün, E.; Özdemir, N.; Şahin, N. Activity analysis of new N-heterocyclic carbenes and silver N-heterocyclic carbene molecules against novel coronavirus by UV-vis, fluorescence spectroscopy and molecular docking. J. Coord. Chem. 2021, 74, 3109–3126. [Google Scholar] [CrossRef]
- Şahin, N. Synthesis, structural characterization, catalytic activity on Suzuki-Miyaura and Mizoroki-Heck coupling reaction of dichloro[1-(2-metil-2-propenil)-3-(3,4,5-trimethoxybenzyl)benzimidazol-2-ylidene] pyridine palladium(II) complex. J. Nat. Appl. Sci. 2018, 22, 105–109. [Google Scholar]
- Şahin, N. PEPPSI-type 2-methyl-2-propenyl-functionalized N-heterocyclic carbene-palladium complexes: Synthesis, structural characterization and catalytic activity on Suzuki–Miyaura reaction. J. Mol. Struct. 2019, 1177, 193–198. [Google Scholar] [CrossRef]
- Şahin, N.; Gürbüz, N.; Karabıyık, H.; Karabıyık, H.; Özdemir, İ. Arylation of heterocyclic compounds by benzimidazole-based N-heterocyclic carbene-palladium(II) complexes. J. Organomet. Chem. 2020, 907, 121076. [Google Scholar] [CrossRef]
- Wang, H.M.J.; Lin, I.J.B. Facile synthesis of silver(I)-carbene complexes. Useful carbene transfer agents. Organometallics 1998, 17, 972–975. [Google Scholar] [CrossRef]
- Garrison, J.C.; Youngs, W.J. Ag(I) N-heterocyclic carbene complexes: synthesis, structure, and application. Chem. Rev. 2005, 105, 3978–4008. [Google Scholar] [CrossRef]
- Lin, I.J.B.; Vasam, C.S. Solver(I) N-heterocyclic carbenes. Comment Inorg. Chem. 2004, 25, 75–129. [Google Scholar] [CrossRef]
- Chen, W.; Liu, F. Synthesis and characterization of oligomeric and polymeric silver-imidazol-2-ylidene iodide complexes. J. Organomet. Chem. 2003, 673, 5–12. [Google Scholar] [CrossRef]
- Tulloch, A.A.D.; Danopoulos, A.A.; Winston, S.; Kleinhenz, S.; Eastham, G. N-Functionalised heterocyclic carbene complexes of silver. J. Chem. Soc. Dalton Trans. 2000, 24, 4499–4506. [Google Scholar] [CrossRef]
- Asekunowo, P.O.; Haque, R.A.; Razali, M.R.; Avicor, S.W.; Wajidi, M.F.F. Dose-, time-and lipophilicity-dependent silver(I)–N-heterocyclic carbene complexes: Synthesis, characterization and interaction with plasmid and Aedes albopictus DNA. Appl. Organomet. Chem. 2017, 31, e3655. [Google Scholar] [CrossRef]
- Özdemir, N. Unpublished Structure. The data can be obtained from The Cambridge Crystallographic Data Centre. CCDC 2071691: Experimental Crystal Structure Determination. 2021. Available online: https://www.ccdc.cam.ac.uk/structures/search?id=doi:10.5517/ccdc.csd.cc25nf09&sid=DataCite (accessed on 8 May 2023).
- Paas, M.; Wibbeling, B.; Fröhlich, R.; Hahn, F.E. Silver and rhodium complexes of stable, monomeric imidazolidin-2-ylidenes: Synthesis, reactivity and decomposition pathway. Eur. J. Inorg. Chem. 2006, 2006, 158–162. [Google Scholar] [CrossRef]
- Winkelmann, O.; Näther, C.; Lüning, U. Bimacrocyclic NHC transition metal complexes. J. Organomet. Chem. 2008, 693, 923–932. [Google Scholar] [CrossRef]
- Makino, T.; Yamasaki, R.; Azumaya, I.; Masu, H.; Saito, S. Synthesis and characterization of silver and palladium complexes with xanthene-based N-heterocyclic carbine-oxazoline ligands. Organometallics 2010, 29, 6291–6297. [Google Scholar] [CrossRef]
- Wang, W.; Zhao, L.; Lv, H.; Zhang, G.; Xia, C.; Hahn, F.E.; Li, F. Modular “click” preparation of bifunctional polymeric heterometallic catalysts. Angew. Chem. Int. Ed. 2016, 55, 7665–7670. [Google Scholar] [CrossRef]
- Niu, H.; Mangan, R.J.; Protchenko, A.V.; Phillips, N.; Unkrig, W.; Friedmann, C.; Kolychev, E.L.; Tirfoin, R.; Hicks, J.; Aldridge, S. Experimental and quantum chemical studies of anionic analogues of N-heterocyclic carbenes. Dalton Trans. 2018, 47, 7445–7455. [Google Scholar] [CrossRef]
- Laidlaw, G.; Wood, S.H.; Kennedy, A.R.; Nelson, D.J. An N-heterocyclic carbene with a saturated backbone and spatially-defined steric impact. Z. Anorg. Allg. Chem. 2019, 645, 105–112. [Google Scholar] [CrossRef]
- Kollef, M.H.; Torres, A.; Shorr, A.F.; Martin-Loeches, I.; Micek, S.T. Nosocomial infection. Crit. Care Med. 2021, 49, 169–187. [Google Scholar] [CrossRef] [PubMed]
- Aulakh, J.K.; Lobana, T.S.; Sood, H.; Arora, D.S.; Garcia-Santos, I.; Kaur, M.; Jasinski, J.P. Synthesis, structures, and novel antimicrobial activity of silver(I) halide complexes of imidazolidine-2-thiones. Polyhedron 2020, 175, 114235. [Google Scholar] [CrossRef]
- Jamal, M.; Ahmad, W.; Andleeb, S.; Jalil, F.; Imran, M.; Nawaz, M.A.; Hussain, T.; Ali, M.; Rafiq, M.; Kamil, M.A. Bacterial biofilm and associated infections. J. Chin. Med. Assoc. 2018, 81, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dalvie, E.D.; Stacy, J.C.; Neuman, K.C.; Osheroff, N. Recognition of DNA supercoil handedness during catenation catalyzed by type II topoisomerases. Biochemistry 2022, 61, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Battini, N.; Ou, J.M.; Zhang, S.L.; Zhang, L.; Zhou, C.H. New efforts toward aminothiazolylquinolones with multitargeting antibacterial potential. J. Agric. Food Chem. 2023, 71, 2322–2332. [Google Scholar] [CrossRef] [PubMed]
- Samir, M.; Ramadan, M.; Abdelrahman, M.H.; Elbastawesy, M.A.; Halby, H.M.; Abdel-Aziz, M.; Abuo-Rahma, G.E.D.A. New potent ciprofloxacin-uracil conjugates as DNA gyrase and topoisomerase IV inhibitors against methicillin-resistant Staphylococcus aureus. Bioorg. Med. Chem. 2022, 73, 117004. [Google Scholar] [CrossRef] [PubMed]
- Patil, M.; Poyil, A.N.; Joshi, S.D.; Patil, S.A.; Patil, S.A.; Bugarin, A. Design, synthesis, and molecular docking study of new piperazine derivative as potential antimicrobial agents. Bioorg. Chem. 2019, 92, 103217. [Google Scholar] [CrossRef]
- Düşünceli, S.D.; Ayaz, D.; Üstün, E.; Günal, S.; Özdemir, N.; Dinçer, M.; Özdemir, İ. Synthesis, antimicrobial properties, and theoreticalanalysis of benzimidazol-2-ylidene silver(I) complexes. J. Coord. Chem. 2020, 73, 1967–1986. [Google Scholar] [CrossRef]
- Anastasiadou, D.; Geromichalou, E.; Tsavea, E.; Psomas, G.; Hatzidimitriou, A.G.; Kalogiannis, S.; Aslanidis, P. Silver complexes with heterocyclic thioamide and tertiary arylphosphane ligands: Synthesis, crystal structures, in vitro and in silico antibacterial and cytotoxic activity, and interaction with DNA. J. Inorg. Biochem. 2020, 210, 111167. [Google Scholar] [CrossRef]
- Imberty, A.; Hardman, K.D.; Carver, J.P.; Perez, S. Molecular modelling of protein-carbohydrate interactions. Docking of monosaccharides in the binding site of concanavalin A. Glycobiology 1991, 1, 631–642. [Google Scholar] [CrossRef]
- Nagaraj, S.; Manivannan, S.; Narayan, S. Potent antifungal agents and use of nanocarriers to improve delivery to the infected site: A systematic review. J. Basic Microbiol. 2021, 61, 849–873. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Zhang, Y.; Cui, H.; Jiang, H.; Liu, L.; Zheng, Y.; Cheng, M. Design, synthesis and evaluation of novel 5-phenylthiophene derivatives as potent fungicidal of Candida albicans and antifungal reagents of fluconazole-resistant fung. Eur. J. Med. Chem. 2021, 225, 113740. [Google Scholar] [CrossRef]
- Kartsev, V.; Geronikaki, A.; Zubenko, A.; Petrou, A.; Ivanov, M.; Glamočlija, J.; Klimenko, A. Synthesis and antimicrobial activity of new heteroaryl(aryl) thiazole derivatives molecular docking studies. Antibiotics 2022, 11, 1337. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.; Abdel-Rahman, L.H.; Abou El-ezz, D.; Ahmed, E.A.-H.; Nafady, A. Synthesis, structural characterization, and biological studies of ATBS–M complexes (M(II) = Cu, Co, Ni, and Mn): Access for promising antibiotics and anticancer agents. Arch. Pharm. 2021, 354, 2000241. [Google Scholar] [CrossRef] [PubMed]
- Souza, R.A.; Cunha, V.L.; de Souza, J.H.; Martins, C.H.; Franca, E.D.F.; Pivatto, M.; Oliveira, C.G. Zinc(II) complexes bearing N,N,S ligands: Synthesis, crystal structure, spectroscopic analysis, molecular docking and biological investigations about its antifungal activity. J. Inorg. Biochem. 2022, 237, 111995. [Google Scholar] [CrossRef] [PubMed]
- Serdaroğlu, G.; Şahin, N.; Üstün, E.; Tahir, M.N.; Arıcı, C.; Gürbüz, N.; Özdemir, İ. PEPPSI type complexes: Synthesis, x-ray structures, spectral studies, molecular docking and theoretical investigations. Polyhedron 2021, 204, 115281. [Google Scholar] [CrossRef]
- Stoe, C. X-AREA (Version 1.18) and X-RED32 (Version 1.04); Stoe & Cie: Darmstadt, Germany, 2002. [Google Scholar]
- Burla, M.C.; Caliandro, R.; Carrozzini, B.; Cascarano, G.L.; Cuocci, C.; Giacovazzo, C.; Mallamo, M.; Mazzone, A.; Polidori, G. Crystal structure determination and refinement via SIR2014. J. Appl. Cryst. 2015, 48, 306–309. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Wayne, P.A. Clinical and Laboratory Standards Institute (CLSI), Performance Standards for Antimicrobial Susceptibility Testing, Twenty-Fourth Informational Supplement, CLSI Document M100-S24. 2014. Available online: https://webstore.ansi.org/standards/clsi/clsim100s24 (accessed on 2 February 2023).
- Ali, I.A.; Matinlinna, J.P.; Lévesque, C.M.; Neelakantan, P. Trans-cinnamaldehyde attenuates Enterococcus faecalis virulence and inhibits biofilm formation. Antibiotics 2021, 10, 702. [Google Scholar] [CrossRef]
- Lafitte, D.; Lamour, V.; Tsvetkov, P.O.; Makarov, A.A.; Klich, M.; Deprez, P.; Gilli, R. DNA Gyrase interaction with coumarin-based inhibitors: the role of the hydroxybenzoate isopentenyl moiety and the 5‘-methyl group of the noviose. Biochemistry 2002, 41, 7217–7223. [Google Scholar] [CrossRef] [PubMed]
- Keniya, M.V.; Sabherwal, M.; Wilson, R.K.; Woods, M.A.; Sagatova, A.A.; Tyndall, J.D.; Monk, B.C. Crystal structures of full-length lanosterol 14α-demethylases of prominent fungal pathogens Candida albicans and Candida glabrata provide tools for antifungal discovery. Antimicrob. Agents Chemother. 2018, 62, e01134-18. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Üstün, E.; Şahin, N. Synthesis, characterization, and in silico analysis against SARS CoV-2 of novel benzimidazolium salts. Ovidius Univ. Ann. Chem. 2021, 32, 137–144. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. Comput. Mol. Sci. 2018, 8, e1327. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Perdew, J.P. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. I. The effect of the exchange-only gradient correction. J. Chem. Phys. 1992, 96, 2155–2160. [Google Scholar] [CrossRef]
- Tsuneda, T.; Song, J.-W.; Suzuki, S.; Hirao, K. On Koopmans’ theorem in density functional theory. J. Chem. Phys. 2010, 133, 174101. [Google Scholar] [CrossRef]







| Benzimidazolium Salt | Silver Complex | |||||
|---|---|---|---|---|---|---|
| FT-IR ν(CN) (cm−1) 1 | 1H NMR NCHN (ppm) | 13C NMR NCHN (ppm) | FT-IR ν(CN) (cm−1) 1 | 13C NMR NC(Ag)N (ppm) | ||
| 1 | 1557 | 11.86 | 144.2 | 6 | 1397 | 189.3 |
| 2 | 1557 | 11.29 | 144.1 | 7 | 1396 | 187.8 |
| 3 | 1558 | 11.74 | 144.1 | 8 | 1392 | 189.0 |
| 4 | 1556 | 11.94 | 144.5 | 9 | 1392 | Not observed |
| 5 | 1561 | 9.21 | 142.2 | 10 | 1394 | Not observed |
| Drug | Microorganism | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Escherichia coli | Acinetobacter baumannii | Candida albicans | |
| 1 | 125.0 | 62.5 | 62.5 | 62.5 | 62.5 |
| 2 | 31.2 | 62.5 | 62.5 | 62.5 | 62.5 |
| 3 | 125.0 | 62.5 | 62.5 | 62.5 | 62.5 |
| 4 | 15.6 | 62.5 | 62.5 | 62.5 | 31.2 |
| 5 | 7.8 | 31.2 | 62.5 | 62.5 | 31.2 |
| 6 | 15.6 | 15.6 | 7.8 | 7.8 | 7.8 |
| 7 | 15.6 | 15.6 | 7.8 | 7.8 | 7.8 |
| 8 | 7.8 | 7.8 | 3.9 | 7.8 | 7.8 |
| 9 | 7.8 | 7.8 | 3.9 | 7.8 | 7.8 |
| 10 | 1.9 | 7.8 | 3.9 | 7.8 | 1.9 |
| Ciprofloxacin | <1.9 | <1.9 | <1.9 | <1.9 | / |
| Fluconazole | / | / | / | / | <1.9 |
| Drug | Microorganism | ||||
|---|---|---|---|---|---|
| Staphylococcus aureus | Enterococcus faecalis | Escherichia coli | Acinetobacter baumannii | Candida albicans | |
| 1 | 62.5 μg/mL 36.5 ± 1.1% | 31.2 μg/mL 48.5 ± 0.3% | 31.2 μg/mL 59.7 ± 0.6% | 31.2 μg/mL 46.5 ± 0.7% | 31.2 μg/mL 40.9 ± 0.5% |
| 2 | 15.6 μg/mL 47.7 ± 0.4% | 31.2 μg/mL 44.6 ± 0.8% | 31.2 μg/mL 63.4 ± 0.5% | 31.2 μg/mL 49.2 ± 0.8% | 31.2 μg/mL 43.4 ± 0.4% |
| 3 | 62.5 μg/mL 43.2 ± 0.6% | 31.2 μg/mL 50.2 ± 0.1% | 31.2 μg/mL 54.8 ± 1.0% | 31.2 μg/mL 57.3 ± 0.2% | 31.2 μg/mL 7.6 ± 0.7% |
| 4 | 7.8 μg/mL 57.9 ± 0.4% | 31.2 μg/mL 42.7 ± 0.2% | 31.2 μg/mL 59.3 ± 0.4% | 31.2 μg/mL 58.0 ± 0.3% | 15.6 μg/mL 45.6 ± 0.2% |
| 5 | 3.9 μg/mL 71.8 ± 0.3% | 15.6 μg/mL 55.4 ± 0.3% | 31.2 μg/mL 62.6 ± 0.3% | 31.2 μg/mL 55.8 ± 0.3% | 15.6 μg/mL 50.8 ± 0.2% |
| 6 | 7.8 μg/mL 61.6 ±0.4% | 7.8 μg/mL 54.6 ± 0.1% | 3.9 μg/mL 75.9 ± 0.3% | 3.9 μg/mL 77.1 ± 0.7% | 3.9 μg/mL 81.2 ± 0.2% |
| 7 | 7.8 μg/mL 68.4 ± 0.3% | 7.8 μg/mL 59.0 ± 0.1% | 3.9 μg/mL 86.6 ± 0.2% | 3.9 μg/mL 73.9 ± 0.4% | 3.9 μg/mL 78.0 ± 0.3% |
| 8 | 3.9 μg/mL 76.1 ± 0.1% | 3.9 μg/mL 63.3 ± 0.2 | 1.9 μg/mL 90.1 ± 0.1 | 3.9 μg/mL 83.2 ± 0.2% | 3.9 μg/mL 76.5 ± 0.3% |
| 9 | 3.9 μg/mL 67.3 ± 0.2% | 3.9 μg/mL 59.6 ± 0.1% | 1.9 μg/mL 86.5 ± 0.3% | 3.9 μg/mL 80.7 ± 0.1% | 3.9 μg/mL 84.3 ± 0.3% |
| 10 | Not tested | 3.9 μg/mL 70.8 ± 0.4% | 1.9 μg/mL 92.9 ± 0.2% | 3.9 μg/mL 84.0 ± 0.2% | Not tested |
| Drugs | Binding Energy (kcal/mol) | Amino Acids Residue 1 |
|---|---|---|
| Escherichia coli DNA gyrase | ||
| 6 | −7.26 | Thr165, Glu50, Val43, Val47, Arg76, Ile78, Pro79, Ile90, Val167, … |
| 7 | −7.50 | Asn45, Thr165, Ala47, Arg76, Ile78, Pro79, Ile90, Val120, … |
| 8 | −6.21 | Asn46, Asp49, Thr165, Glu50, Arg76, Gly77, Pro79, Ile78, … |
| 9 | −8.11 | Asn46, Glu50, Thr165, Ala47, Ile78, Ile90, Met91, Val120, Val167 |
| 10 | −8.01 | Asn46, Glu50, Ile78, Met91, Val120, Thr165, Ala47, Val167, Val43 |
| Ciprofloxacin | −6.89 | Asp73, Arg76, Arg136, Thr165, Glu50, Gly77, Ile78, Pro79, … |
| CYP51 from the pathogen Candida albicans | ||
| 6 | −7.94 | Gly303, Ile131, Leu139, Lys143, Ala146, Leu150, Leu300, Ile304, Phe126, … |
| 7 | −8.25 | Ile131, Leu139, Lys143, Ala146, Leu150, Leu204, Leu276, Leu300, Ile304, … |
| 8 | −8.52 | Cys470, Gly472, Ile304, Ile471, Ile131, Tyr132, Leu139, Lys143, … |
| 9 | −8.51 | Leu150, Ile304, Ile131, Ala146, Leu204, Leu276, Leu300, Cys470, Ile471, … |
| 10 | −9.26 | Ile131, Leu300, Lys143, Ala146, Leu150, Leu204, Leu276, Ile304, Cys470, … |
| Fluconazole | −5.65 | Ile304, Gly307, Cys470, Gly303, Ile471, Ile131, Lys143, Leu204, … |
| 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|
| HOMO-LUMO Gap (ΔE) | 2.474 | 2.534 | 2.452 | 2.314 | 1.731 |
| Ionization Potential (IP) | 4.854 | 4.831 | 4.735 | 4.809 | 4.856 |
| Electron Affinity (EA) | 2.379 | 2.297 | 2.283 | 2.495 | 3.126 |
| Electronegativity (χ) | 3.616 | 3.564 | 3.509 | 3.652 | 3.991 |
| Chemical Hardness (η) | 1.237 | 1.267 | 1.226 | 1.157 | 0.865 |
| Global Softness (S) | 0.404 | 0.395 | 0.408 | 0.432 | 0.578 |
| CCDC Depository | 2018964 | Color/Shape | Colorless/Prism |
| Chemical formula | AgClC22H26N2 | Formula weight | 461.77 |
| Temperature (K) | 296(2) | Wavelength (Å) | 0.71073 Mo Kα |
| Crystal system | Monoclinic | Space group | C2/c |
| Unit cell parameters | Z | 8 | |
| a (Å) | 28.1169(14) | Dcalc. (g/cm3) | 1.432 |
| b (Å) | 9.3573(6) | μ (mm−1) | 1.073 |
| c (Å) | 17.0595(8) | θ range for data collection (°) | 2.305 ≤ θ ≤ 27.570 |
| α (°) | 90 | Absorption correction | Integration |
| β (°) | 107.331(4) | F000 | 1888 |
| γ (°) | 90 | Diffractometer | STOE IPDS II |
| Index ranges | −33 ≤ h ≤ 36 | Reflections collected | 14053 |
| −10 ≤ k ≤ 12 | Rint. | 0.0365 | |
| −22 ≤ l ≤ 22 | Data/restraints/parameters | 4899/1/235 | |
| Independent reflections | 4899 | Observed reflections | 3493 |
| Refinement method | Full-matrix least-squares on F2 | Final R indices [I > 2σ(I)] | R1 = 0.0327, wR2 = 0.0788 |
| Goodness-of-fit on F2 | 0.918 | R indices (all data) | R1 = 0.0523, wR2 = 0.0842 |
| Δρmax., Δρmin. (e/Å3) | 0.603, −0.410 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tutar, U.; Çelik, C.; Üstün, E.; Özdemir, N.; Şahin, N.; Sémeril, D.; Gürbüz, N.; Özdemir, İ. Benzimidazol-2-ylidene Silver Complexes: Synthesis, Characterization, Antimicrobial and Antibiofilm Activities, Molecular Docking and Theoretical Investigations. Inorganics 2023, 11, 385. https://doi.org/10.3390/inorganics11100385
Tutar U, Çelik C, Üstün E, Özdemir N, Şahin N, Sémeril D, Gürbüz N, Özdemir İ. Benzimidazol-2-ylidene Silver Complexes: Synthesis, Characterization, Antimicrobial and Antibiofilm Activities, Molecular Docking and Theoretical Investigations. Inorganics. 2023; 11(10):385. https://doi.org/10.3390/inorganics11100385
Chicago/Turabian StyleTutar, Uğur, Cem Çelik, Elvan Üstün, Namık Özdemir, Neslihan Şahin, David Sémeril, Nevin Gürbüz, and İsmail Özdemir. 2023. "Benzimidazol-2-ylidene Silver Complexes: Synthesis, Characterization, Antimicrobial and Antibiofilm Activities, Molecular Docking and Theoretical Investigations" Inorganics 11, no. 10: 385. https://doi.org/10.3390/inorganics11100385