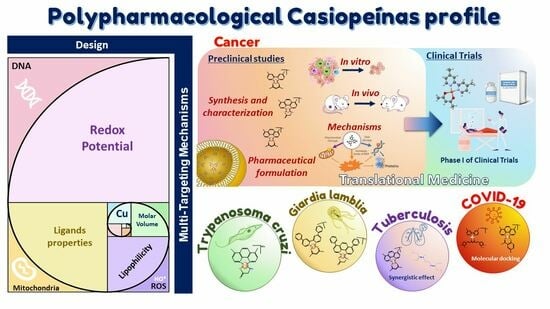
The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities)
Abstract
:1. Introduction

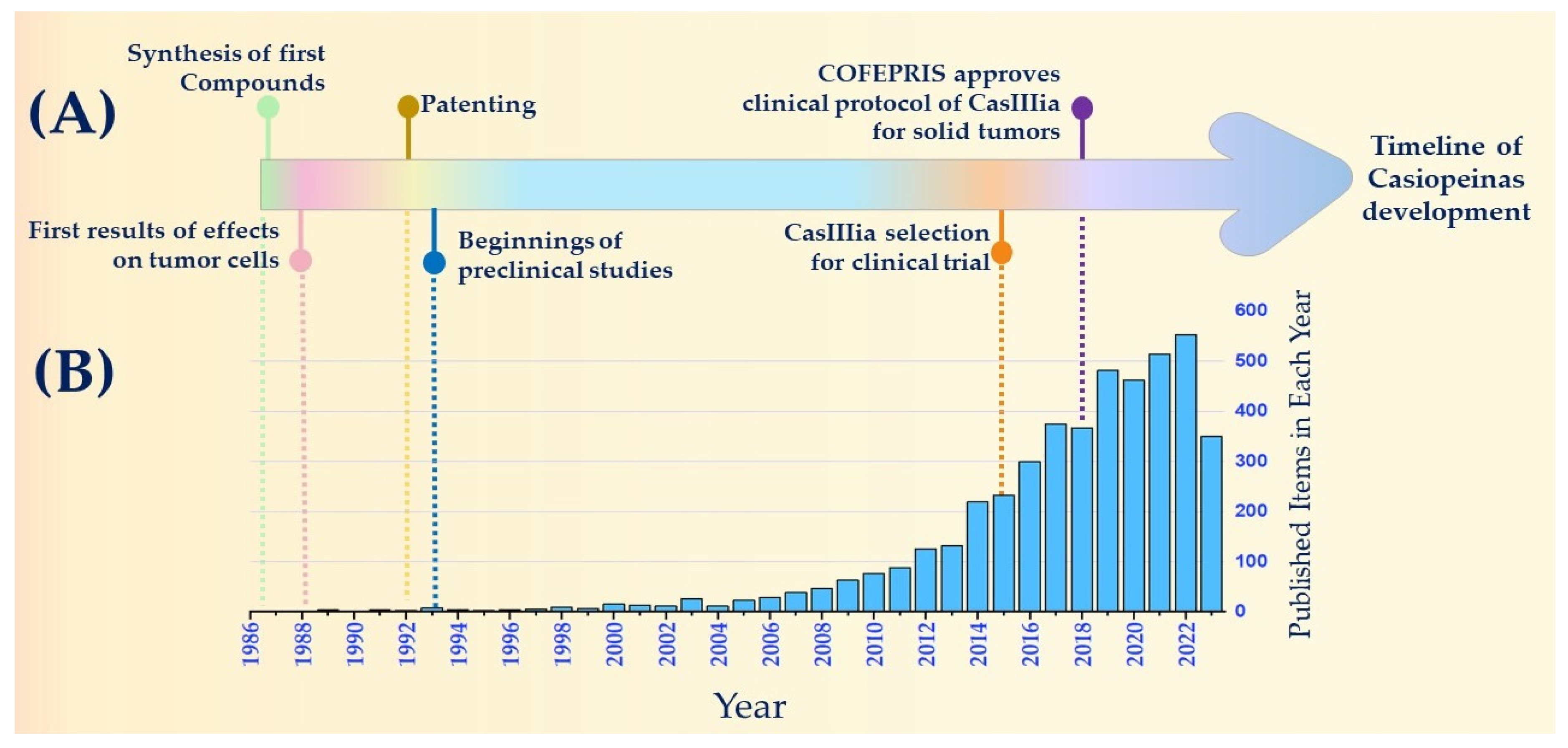
2. Historical Timeline of Anticancer Drugs: Casiopeínas®
- Given DNA’s status as a cancer target at the time, the synthesis of compounds that induce DNA damage was sought. Thus, molecules were designed with ligands situated in the equatorial plane of the metal’s center to facilitate intercalation interactions with DNA;
- Molecules were devised to incorporate a copper atom, which, being an endogenous metal, was expected to mitigate compound toxicity. In addition, we explored whether the redox properties of the metal could be relevant in tumor cells;
- Small cationic molecules were proposed to enhance solubility in biological environments.
3. Activity and Properties
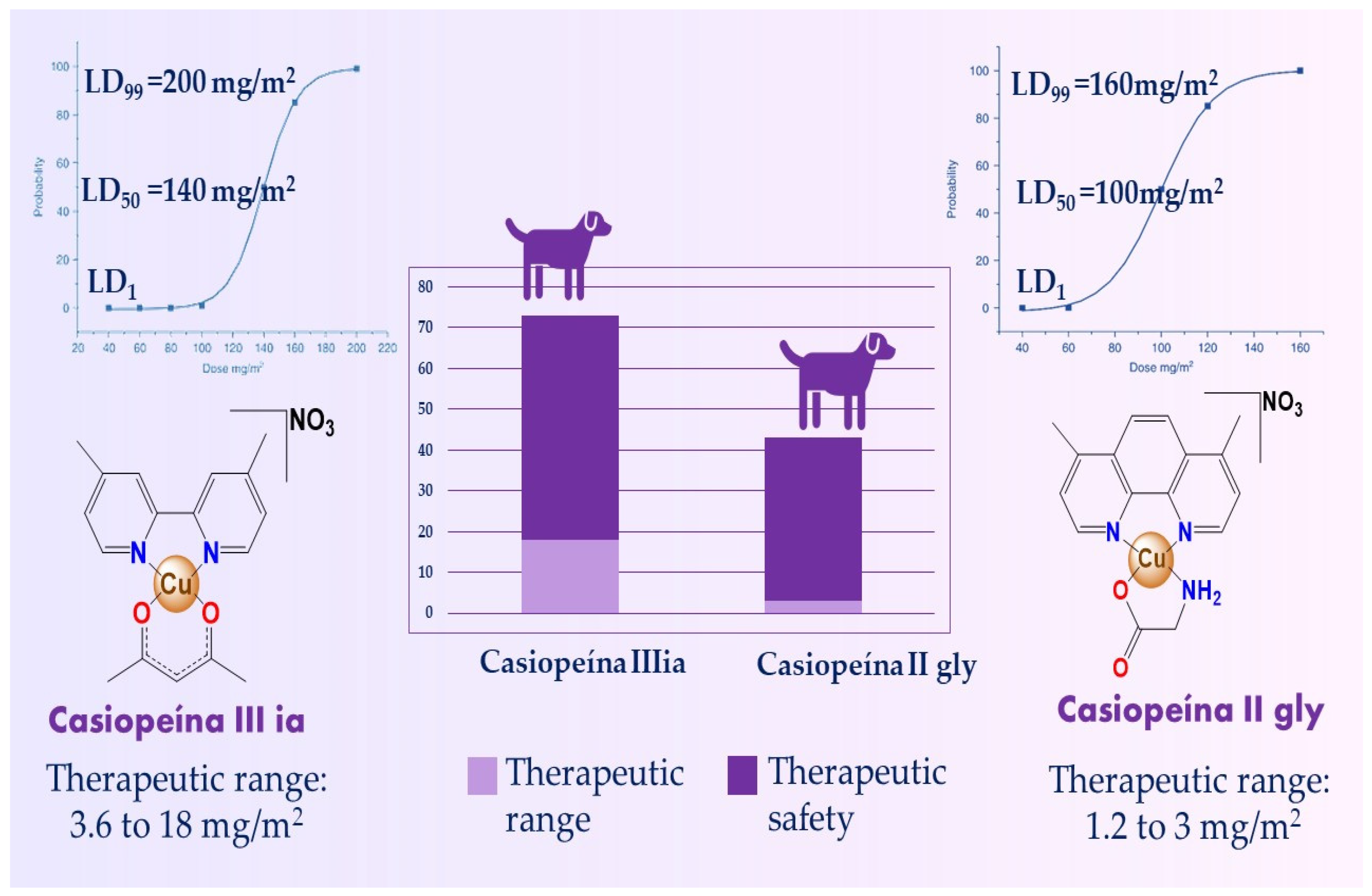
4. Toxicity
Hematoxicity
5. Pharmacokinetics
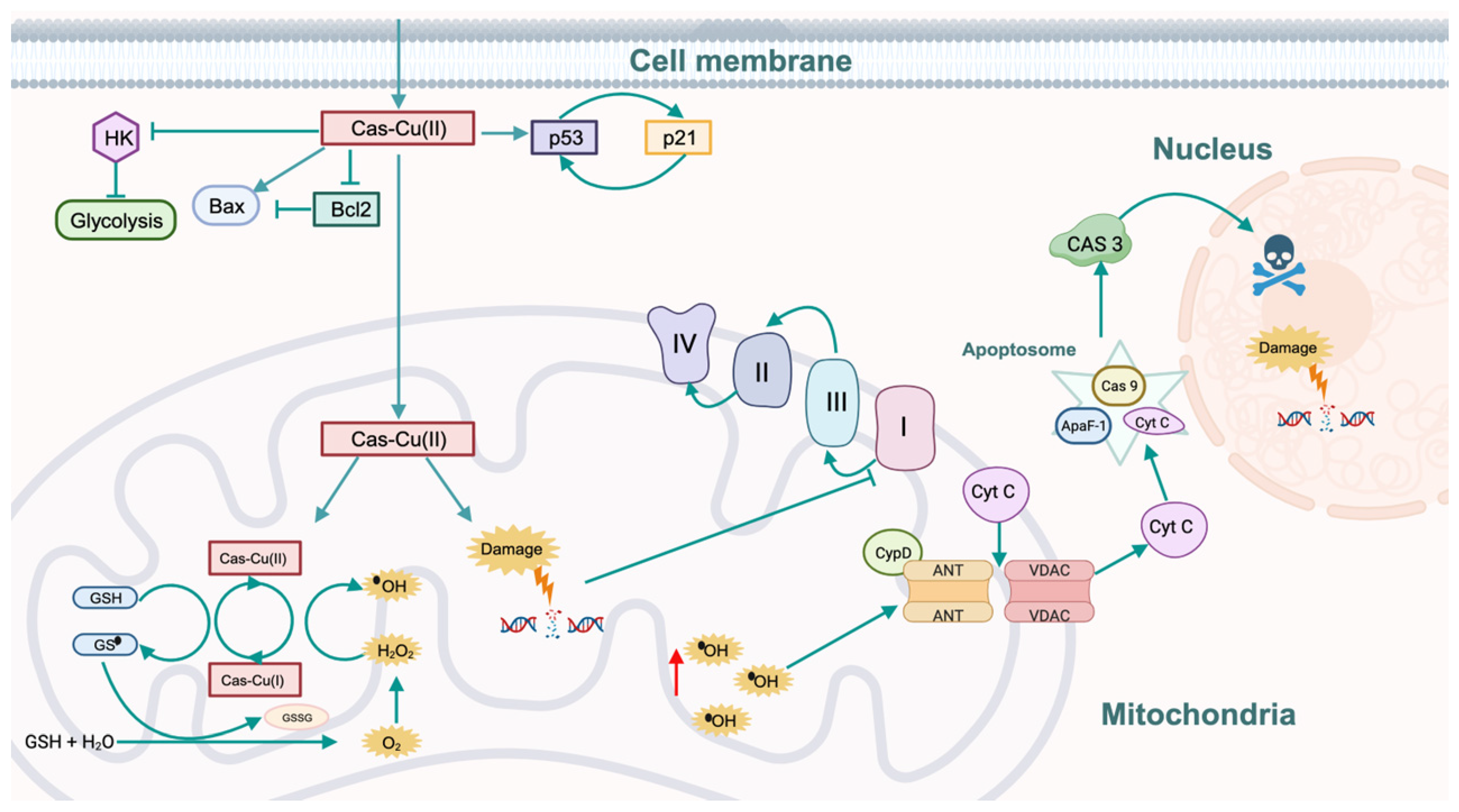
6. Mechanism of Action
7. Metabolomics: CasIIgly and Its Effect on Triple-Negative Breast Cancer (TNBC) Metabolism
8. Encapsulation
9. Casiopeínas-like Compounds
10. Casiopeínas and Other Activities
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guo, Z.; Sadler, P.J. Metals in Medicine. Angew. Chem. Int. Ed. 1999, 38, 1512–1531. [Google Scholar] [CrossRef]
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef]
- Sadler, P.J. Inorganic Chemistry and Drug Design. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1991; Volume 36, pp. 1–48. ISBN 0898-8838. [Google Scholar]
- Kaufmann, S.H.E. Paul Ehrlich: Founder of Chemotherapy. Nat. Rev. Drug Discov. 2008, 7, 373. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Nayeem, N.; Contel, M. Exploring the Potential of Metallodrugs as Chemotherapeutics for Triple Negative Breast Cancer. Chem. Eur. J. 2021, 27, 8891–8917. [Google Scholar] [CrossRef]
- Komeda, S.; Casini, A. Next-Generation Anticancer Metallodrugs. Curr. Top. Med. Chem. 2012, 12, 219–235. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- Ndagi, U.; Mhlongo, N.; Soliman, M.E. Metal Complexes in Cancer Therapy—An Update from Drug Design Perspective. Drug Des. Devel Ther. 2017, 11, 599–616. [Google Scholar] [CrossRef]
- Wilson, J.J.; Johnstone, T.C. The Role of Metals in the next Generation of Anticancer Therapeutics. Curr. Opin. Chem. Biol. 2023, 76, 102363. [Google Scholar] [CrossRef]
- Ghosh, S. Cisplatin: The First Metal Based Anticancer Drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef]
- Aldossary, S.A. Review on Pharmacology of Cisplatin: Clinical Use, Toxicity and Mechanism of Resistance of Cisplatin. Biomed. Pharmacol. J. 2019, 12, 7–15. [Google Scholar] [CrossRef]
- Brown, A.; Kumar, S.; Tchounwou, P.B. Cisplatin-Based Chemotherapy of Human Cancers. J. Cancer Sci. Ther. 2019, 11, 97. [Google Scholar]
- Florea, A.M.; Büsselberg, D. Cisplatin as an Anti-Tumor Drug: Cellular Mechanisms of Activity, Drug Resistance and Induced Side Effects. Cancers 2011, 3, 1351–1371. [Google Scholar] [CrossRef]
- Tsvetkova, D.; Ivanova, S. Application of Approved Cisplatin Derivatives in Combination Therapy against Different Cancer Diseases. Molecules 2022, 27, 2466. [Google Scholar] [CrossRef]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Kopacz-Bednarska, A.; Król, T. Cisplatin—Properties and Clinical Application. Oncol. Clin. Pract. 2022, 18, 166–176. [Google Scholar] [CrossRef]
- Barabas, K.; Milner, R.; Lurie, D.; Adin, C. Cisplatin: A Review of Toxicities and Therapeutic Applications. Vet. Comp. Oncol. 2008, 6, 1–18. [Google Scholar] [CrossRef]
- Amable, L. Cisplatin Resistance and Opportunities for Precision Medicine. Pharmacol. Res. 2016, 106, 27–36. [Google Scholar] [CrossRef]
- Galluzzi, L.; Senovilla, L.; Vitale, I.; Michels, J.; Martins, I.; Kepp, O.; Castedo, M.; Kroemer, G. Molecular Mechanisms of Cisplatin Resistance. Oncogene 2012, 31, 1869–1883. [Google Scholar] [CrossRef]
- Czarnomysy, R.; Radomska, D.; Szewczyk, O.K.; Roszczenko, P.; Bielawski, K. Platinum and Palladium Complexes as Promising Sources for Antitumor Treatments. Int. J. Mol. Sci. 2021, 22, 8271. [Google Scholar] [CrossRef]
- Sava, G. Ruthenium Compounds in Cancer Therapy. In Metal Compounds in Cancer Therapy; Fricker, S.P., Ed.; Springer: Dordrecht, The Netherlands, 1994; pp. 65–91. ISBN 978-94-011-1252-9. [Google Scholar]
- Sharma, A.; Sudhindra, P.; Roy, N.; Paira, P. Advances in Novel Iridium (III) Based Complexes for Anticancer Applications: A Review. Inorg. Chim. Acta 2020, 513, 119925. [Google Scholar] [CrossRef]
- Borkow, G.; Gabbay, J. Copper, an Ancient Remedy Returning to Fight Microbial, Fungal and Viral Infections. Curr. Chem. Biol. 2009, 3, 272–278. [Google Scholar] [CrossRef]
- Kardos, J.; Héja, L.; Simon, Á.; Jablonkai, I.; Kovács, R.; Jemnitz, K. Copper Signalling: Causes and Consequences. Cell Commun. Signal. 2018, 16, 71. [Google Scholar] [CrossRef]
- Ruiz, L.M.; Libedinsky, A.; Elorza, A.A. Role of Copper on Mitochondrial Function and Metabolism. Front. Mol. Biosci. 2021, 8, 711227. [Google Scholar] [CrossRef]
- Klotz, L.-O.; Kröncke, K.-D.; Buchczyk, D.P.; Sies, H. Role of Copper, Zinc, Selenium and Tellurium in the Cellular Defense against Oxidative and Nitrosative Stress. J. Nutr. 2003, 133, 1448S–1451S. [Google Scholar] [CrossRef]
- Peña, M.M.O.; Lee, J.; Thiele, D.J. Critical Review A Delicate Balance: Homeostatic Control of Copper Uptake and Distribution. J. Nutr. 1999, 129, 1251–1260. [Google Scholar] [CrossRef]
- Tang, X.; Yan, Z.; Miao, Y.; Ha, W.; Li, Z.; Yang, L.; Mi, D. Copper in Cancer: From Limiting Nutrient to Therapeutic Target. Front. Oncol. 2023, 13, 1209156. [Google Scholar] [CrossRef]
- Nasulewicz, A.; Mazur, A.; Opolski, A. Role of Copper in Tumour Angiogenesis—Clinical Implications. J. Trace Elem. Med. Biol. 2004, 18, 1–8. [Google Scholar] [CrossRef]
- Alem, M.B.; Damena, T.; Desalegn, T.; Koobotse, M.; Eswaramoorthy, R.; Ngwira, K.J.; Ombito, J.O.; Zachariah, M.; Demissie, T.B. Cytotoxic Mixed-Ligand Complexes of Cu(II): A Combined Experimental and Computational Study. Front. Chem. 2022, 10, 1028957. [Google Scholar] [CrossRef]
- Ji, P.; Wang, P.; Chen, H.; Xu, Y.; Ge, J.; Tian, Z.; Yan, Z. Potential of Copper and Copper Compounds for Anticancer Applications. Pharmaceuticals 2023, 16, 234. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate from Methylate Phenathroline Complexes to Be Used as Anticancerigenic Agents. U.S. Patent 5,576,326, 20 December 1990. [Google Scholar]
- Antman, K.H. Introduction: The History of Arsenic Trioxide in Cancer Therapy. Oncologist 2001, 6, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Groner, B.; Schumacher, U.; Superti-Furga, G.; Busslinger, M.; Kralovics, R.; Zielinski, C.; Penninger, J.M.; Kerjaschki, D.; Stingl, G. Paul Ehrlich (1854–1915) and His Contributions to the Foundation and Birth of Translational Medicine. J. Innate Immun. 2016, 8, 111–120. [Google Scholar] [CrossRef] [PubMed]
- De Vita, V.T., Jr.; Chu, E. A History of Cancer Chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Chabner, B.A.; Roberts, T.G., Jr. Chemotherapy and the War on Cancer. Nat. Rev. Cancer 2005, 5, 65–72. [Google Scholar] [CrossRef]
- Hurley, L.H.; Boyd, F.L. DNA as a Target for Drug Action. Trends Pharmacol. Sci. 1988, 9, 402–407. [Google Scholar] [CrossRef]
- Forestier, J. Comparative Results of Copper Salts and Gold Salts in Rheumatoid Arthritis. Ann. Rheum. Dis. 1949, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble Metals in Medicine: Latest Advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Romero-Canelon, I.; Sadler, P.J. Next-Generation Metal Anticancer Complexes: Multitargeting via Redox Modulation. Inorg. Chem. 2013, 52, 12276–12291. [Google Scholar] [CrossRef]
- Bravo-Gómez, M.E.; García-Ramos, J.C.; Gracia-Mora, I.; Ruiz-Azuara, L. Antiproliferative Activity and QSAR Study of Copper (II) Mixed Chelate [ Cu (N − N)(Acetylacetonato)] NO3 and [ Cu (N − N)(Glycinato)] NO3 Complexes, (Casiopeinas). J. Inorg. Biochem. 2009, 103, 299–309. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gomez, M.E. Copper Compounds in Cancer Chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef]
- Rivero-Müller, A.; De Vizcaya-Ruiz, A.; Plant, N.; Ruiz, L.; Dobrota, M. Mixed Chelate Copper Complex, Casiopeina IIgly®, Binds and Degrades Nucleic Acids: A Mechanism of Cytotoxicity. Chem. Biol. Interact. 2007, 165, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Alemón-Medina, R.; Breña-Valle, M.; Muñoz-Sánchez, J.; Gracia-Mora, M.; Ruiz-Azuara, L. Induction of Oxidative Damage by Copper-Based Antineoplastic Drugs (Casiopeínas(R)). Cancer Chemother. Pharmacol. 2007, 60, 219–228. [Google Scholar] [CrossRef]
- Folli, A.; Ritterskamp, N.; Richards, E.; Platts, J.A.; Murphy, D.M. Probing the Structure of Copper(II)-Casiopeina Type Coordination Complexes [Cu(O–O)(N–N)]+ by EPR and ENDOR Spectroscopy. J. Catal. 2021, 394, 220–227. [Google Scholar] [CrossRef]
- Solans, X.; Ruíz-Ramírez, L.; Martínez, A.; Gasque, L.; Moreno-Esparza, R. Mixed Chelate Complexes. II. Structures of L-Alaninato (Aqua)(4, 7-Diphenyl-1, 10-Phenanthroline) Copper (II) Nitrite Monohydrate and Aqua (4, 7-Dimethyl-1, 10-Phenanthroline)(Glycinato)(Nitrato) Copper (II) Monohydrate. Acta Crystallogr. C 1993, 49, 890–893. [Google Scholar] [CrossRef]
- Figueroa-Depaz, Y.; Pérez-Villanueva, J.; Soria-Arteche, O.; Martínez-Otero, D.; Gómez-Vidales, V.; Ortiz-Frade, L.; Ruiz-Azuara, L. Casiopeinas of Third Generations: Synthesis, Characterization, Cytotoxic Activity and Structure–Activity Relationships of Mixed Chelate Compounds with Bioactive Secondary Ligands. Molecules 2022, 27, 3504. [Google Scholar] [CrossRef]
- Novoa-Ramírez, C.S.; Silva-Becerril, A.; González-Ballesteros, M.M.; Gomez-Vidal, V.; Flores-Álamo, M.; Ortiz-Frade, L.; Gracia-Mora, J.; Ruiz-Azuara, L. Biological Activity of Mixed Chelate Copper (II) Complexes, with Substituted Diimine and Tridentate Schiff Bases (NNO) and Their Hydrogenated Derivatives as Secondary Ligands: Casiopeína’s Fourth Generation. J. Inorg. Biochem. 2023, 242, 112097. [Google Scholar] [CrossRef]
- Godínez-Loyola, Y.; Gracia-Mora, J.; Rojas-Montoya, I.D.; Hernández-Ayala, L.F.; Reina, M.; Ortiz-Frade, L.A.; Rascón-Valenzuela, L.A.; Robles-Zepeda, R.E.; Gómez-Vidales, V.; Bernad-Bernad, M.J. Casiopeinas® Third Generation, with Indomethacin: Synthesis, Characterization, DFT Studies, Antiproliferative Activity, and Nanoencapsulation. RSC Adv. 2022, 12, 21662–21673. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Acevedo, K.; García-Aguilera, M.E.; Esturau-Escofet, N.; Ruiz-Azuara, L. 1H-NMR Metabolomics Study of the Effect of Cisplatin and Casiopeina IIgly on MDA-MB-231 Breast Tumor Cells. Front. Mol. Biosci. 2021, 8, 742859. [Google Scholar] [CrossRef]
- Rodrigues, J.H.V.; de Carvalho, A.B.; Silva, V.R.; de Santos, L.S.; Soares, M.B.P.; Bezerra, D.P.; Oliveira, K.M.; Corrêa, R.S. Copper(II)/Diiminic Complexes Based on 2-Hydroxybenzophenones: DNA- and BSA-Binding Studies and Antitumor Activity against HCT116 and HepG2 Tumor Cells. Polyhedron 2023, 239, 116431. [Google Scholar] [CrossRef]
- García-Ramos, J.C.; Gutiérrez, A.G.; Vázquez-Aguirre, A.; Toledano-Magaña, Y.; Alonso-Sáenz, A.L.; Gómez-Vidales, V.; Flores-Alamo, M.; Mejía, C.; Ruiz-Azuara, L. The Mitochondrial Apoptotic Pathway Is Induced by Cu(II) Antineoplastic Compounds (Casiopeínas®) in SK-N-SH Neuroblastoma Cells after Short Exposure Times. BioMetals 2017, 30, 43–58. [Google Scholar] [CrossRef]
- De Paz, F.-Y.; Resendiz-Acevedo, K.; Dávila-Manzanilla, S.G.; García-Ramos, J.C.; Ortiz-Frade, L.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA, a Target of Mixed Chelate Copper(II) Compounds (Casiopeinas®) Studied by Electrophoresis, UV–Vis and Circular Dichroism Techniques. J. Inorg. Biochem. 2022, 231, 111772. [Google Scholar] [CrossRef]
- Eremina, J.A.; Lider, E.V.; Sukhikh, T.S.; Klyushova, L.S.; Perepechaeva, M.L.; Sheven, D.G.; Berezin, A.S.; Grishanova, A.Y.; Potkin, V.I. Water-Soluble Copper (II) Complexes with 4, 5-Dichloro-Isothiazole-3-Carboxylic Acid and Heterocyclic N-Donor Ligands: Synthesis, Crystal Structures, Cytotoxicity, and DNA Binding Study. Inorg. Chim. Acta 2020, 510, 119778. [Google Scholar] [CrossRef]
- Reina, M.; Talavera-Contreras, L.G.; Figueroa-DePaz, Y.; Ruiz-Azuara, L.; Hernández-Ayala, L.F. Casiopeinas® as SARS-CoV-2 Main Protease (M pro) Inhibitors: A Combined DFT, Molecular Docking and ONIOM Approach. New J. Chem. 2022, 46, 12500–12511. [Google Scholar] [CrossRef]
- Huaizhi, Z.; Yuantao, N. China’s Ancient Gold Drugs. Gold Bull. 2000, 46 34, 24–29. [Google Scholar] [CrossRef]
- Spear, M. Silver: An Age-Old Treatment Modality in Modern Times. Plast. Aesthetic Nurs. 2010, 30, 90–93. [Google Scholar] [CrossRef]
- Kapp, R.W. Arsenic: Toxicology and Health Effects. In Encyclopedia of Food and Health; Caballero, B., Finglas, P.M., Toldrá, F., Eds.; Academic Press: Oxford, UK, 2016; pp. 256–265. ISBN 978-0-12-384953-3. [Google Scholar]
- Gibaud, S.; Jaouen, G. Arsenic-Based Drugs: From Fowler’s Solution to Modern Anticancer Chemotherapy. In Medicinal Organometallic Chemistry; Jaouen, G., Metzler-Nolte, N., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 1–20. ISBN 978-3-642-13185-1. [Google Scholar]
- Holman, J.; Kirkhart, B.; Maxon, M.S.; Oliva, C.; Earhart, R. Safety Experience with Trisenox® (Arsenic Trioxide) Injection. Blood 2004, 104, 4521. [Google Scholar] [CrossRef]
- Hoonjan, M.; Jadhav, V.; Bhatt, P. Arsenic Trioxide: Insights into Its Evolution to an Anticancer Agent. J. Biol. Inorg. Chem. 2018, 23, 313–329. [Google Scholar] [CrossRef] [PubMed]
- Proschak, E.; Stark, H.; Merk, D. Polypharmacology by Design: A Medicinal Chemist’s Perspective on Multitargeting Compounds. J. Med. Chem. 2019, 62, 420–444. [Google Scholar] [CrossRef]
- Jørgensen, J.T. The Importance of Predictive Biomarkers in Oncology Drug Development. Expert. Rev. Mol. Diagn. 2016, 16, 807–809. [Google Scholar] [CrossRef] [PubMed]
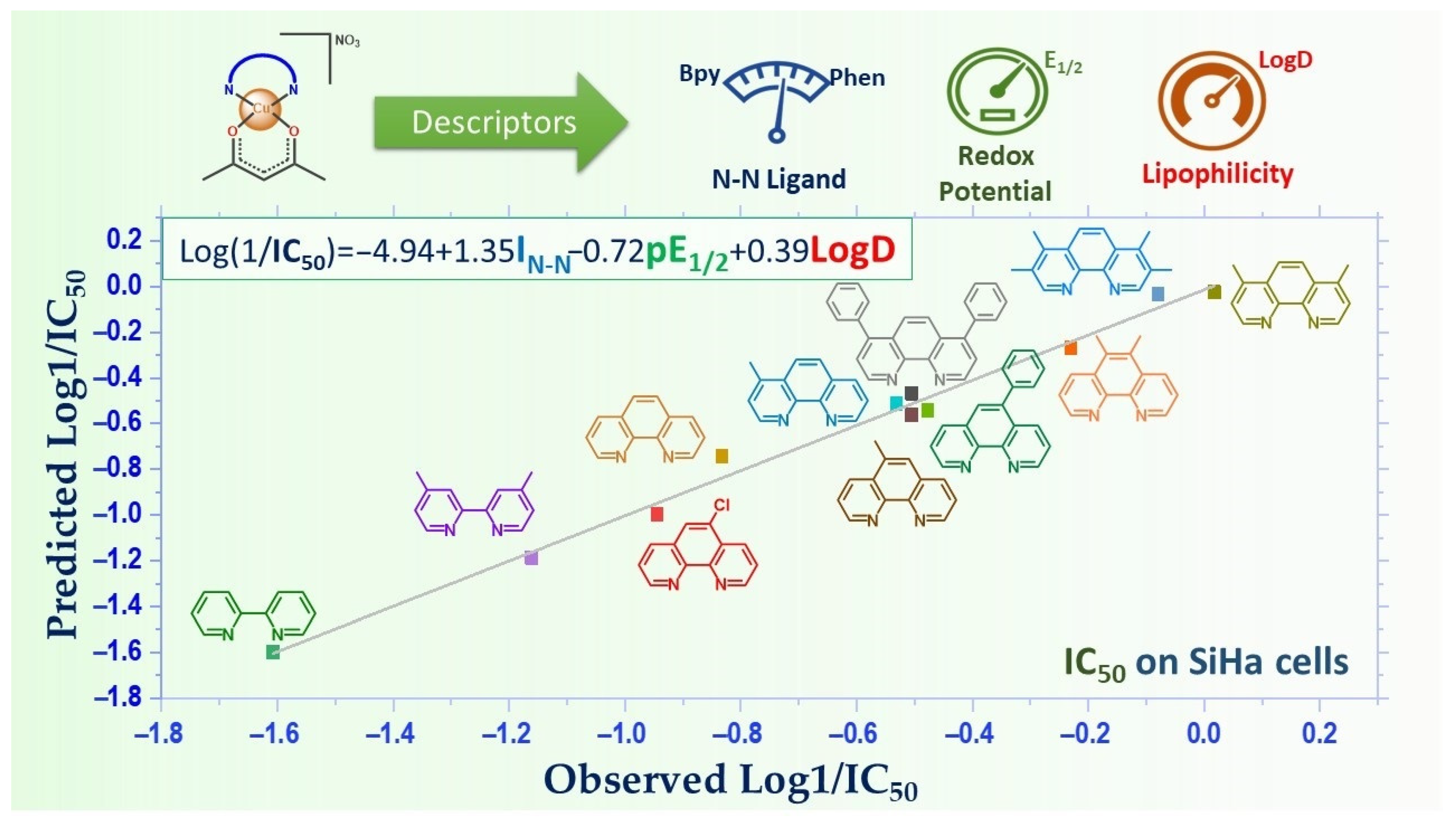
- Reina, M.; Hernández-Ayala, L.F.; Bravo-Gómez, M.E.; Gómez, V.; Ruiz-Azuara, L. Second Generation of Casiopeinas®: A Joint Experimental and Theoretical Study. Inorg. Chim. Acta 2021, 517, 120201. [Google Scholar] [CrossRef]
- Onawumi, O.O.E.; Odunola, O.A.; Suresh, E.; Paul, P. Synthesis, Structural Characterization and Microbial Activities of Mixed Ligand Copper (II) Complexes of 2,2′-Bipyridine and Acetylacetonate. Inorg. Chem. Commun. 2011, 14, 1626–1631. [Google Scholar] [CrossRef]
- Bravo-Gómez, M.E.; Dávila-Manzanilla, S.; Flood-Garibay, J.; Muciño-Hernández, M.Á.; Mendoza, Á.; García-Ramos, J.C.; Moreno-Esparza, R.; Ruiz-Azuara, L. Secondary Ligand Effects on the Cytotoxicity of Several Casiopeína’s Group II Compounds. J. Mex. Chem. Soc. 2012, 56, 85–92. [Google Scholar] [CrossRef]
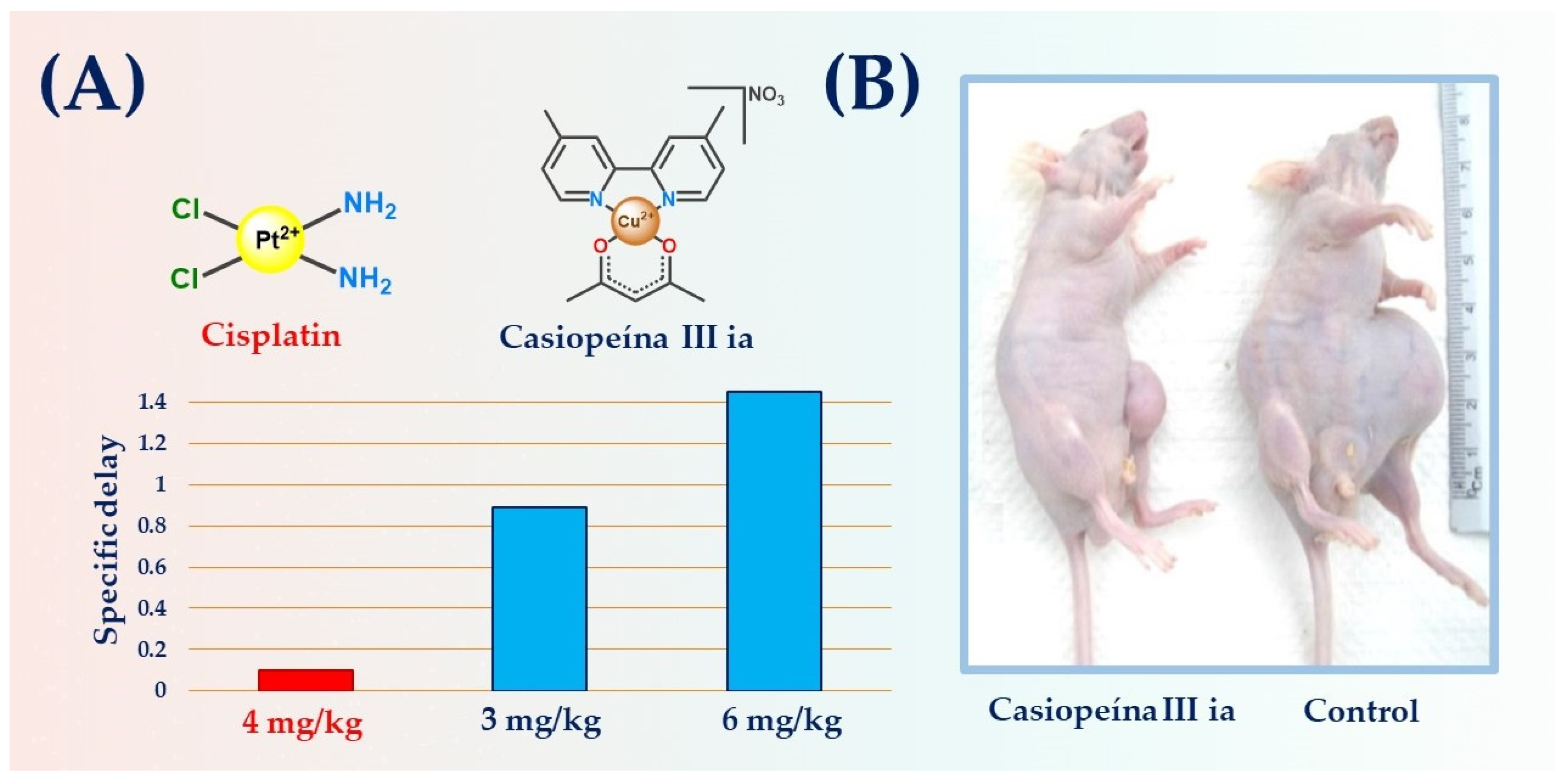
- Carvallo-Chaigneau, F.; Trejo-Solís, C.; Gómez-Ruiz, C.; Rodríguez-Aguilera, E.; MacÍas-Rosales, L.; Cortés-Barberena, E.; Cedillo-Peláez, C.; Gracia-Mora, I.; Ruiz-Azuara, L.; Madrid-Marina, V.; et al. Casiopeina III-Ia Induces Apoptosis in HCT-15 Cells in Vitro through Caspase-Dependent Mechanisms and Has Antitumor Effect in Vivo. BioMetals 2008, 21, 17–28. [Google Scholar] [CrossRef] [PubMed]
- García-Ramos, J.C.; Vértiz-Serrano, G.; Macías-Rosales, L.; Galindo-Murillo, R.; Toledano-Magaña, Y.; Bernal, J.P.; Cortés-Guzmán, F.; Ruiz-Azuara, L. Isomeric Effect on the Pharmacokinetic Behavior of Anticancer CuII Mixed Chelate Complexes: Experimental and Theoretical Approach. Eur. J. Inorg. Chem. 2017, 2017, 1728–1736. [Google Scholar] [CrossRef]
- Davila-Manzanilla, S.G.; Figueroa-de-Paz, Y.; Mejia, C.; Ruiz-Azuara, L. Synergistic Effects between a Copper-Based Metal Casiopeína III-Ia and Cisplatin. Eur. J. Med. Chem. 2017, 129, 266–274. [Google Scholar] [CrossRef]
- Rodríguez-Enríquez, S.; Gallardo-Pérez, J.C.; Hernández-Reséndiz, I.; Marín-Hernández, A.; Pacheco-Velázquez, S.C.; López-Ramírez, S.Y.; Rumjanek, F.D.; Moreno-Sánchez, R. Canonical and New Generation Anticancer Drugs Also Target Energy Metabolism. Arch. Toxicol. 2014, 88, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Leal-García, M.; García-Ortuño, L.; Ruiz-Azuara, L.; Gracia-Mora, I.; Luna-Delvillar, J.; Sumano, H. Assessment of Acute Respiratory and Cardiovascular Toxicity of Casiopeinas in Anaesthetized Dogs. Basic Clin. Pharmacol. Toxicol. 2007, 101, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Silva-Platas, C.; Guerrero-Beltrán, C.E.; Carrancá, M.; Castillo, E.C.; Bernal-Ramírez, J.; Oropeza-Almazán, Y.; González, L.N.; Rojo, R.; Martínez, L.E.; Valiente-Banuet, J. Antineoplastic Copper Coordinated Complexes (Casiopeinas) Uncouple Oxidative Phosphorylation and Induce Mitochondrial Permeability Transition in Cardiac Mitochondria and Cardiomyocytes. J. Bioenerg. Biomembr. 2016, 48, 43–54. [Google Scholar] [CrossRef]
- García, N.; Martínez-Abundis, E.; Pavón, N.; Correa, F.; Chávez, E. Copper Induces Permeability Transition through Its Interaction with the Adenine Nucleotide Translocase. Cell Biol. Int. 2007, 31, 893–899. [Google Scholar] [CrossRef]
- Zazueta, C.; Reyes-Vivas, H.; Zafra, G.; Sánchez, C.A.; Vera, G.; Chávez, E. Mitochondrial Permeability Transition as Induced by Cross-Linking of the Adenine Nucleotide Translocase. Int. J. Biochem. Cell Biol. 1998, 30, 517–527. [Google Scholar] [CrossRef]
- De Vizcaya-Ruiz, A.; Rivero-Müller, A.; Ruiz-Ramirez, L.; Howarth, J.A.; Dobrota, M. Hematotoxicity Response in Rats by the Novel Copper-Based Anticancer Agent: Casiopeina II. Toxicology 2003, 194, 103–113. [Google Scholar] [CrossRef]
- Vértiz, G.; García-Ortuño, L.E.; Bernal, J.P.; Bravo-Gómez, M.E.; Lounejeva, E.; Huerta, A.; Ruiz-Azuara, L. Pharmacokinetics and Hematotoxicity of a Novel Copper-Based Anticancer Agent: Casiopeina III-Ea, after a Single Intravenous Dose in Rats. Fundam. Clin. Pharmacol. 2014, 28, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Cañas-Alonso, R.C.; Fuentes-Noriega, I.; Ruiz-Azuara, L. Pharmacokinetics of Casiopeína IIgly in Beagle Dog: A Copper Based Compound with Antineoplastic Activity. J. Bioanal. Biomed. 2010, 2, 28–34. [Google Scholar] [CrossRef]
- Fuentes-Noriega, I.; Ruiz-Ramırez, L.; Tovar, A.T.; Rico-Morales, H.; Gracia-Mora, I. Development and Validation of a Liquid Chromatographic Method for Casiopeina IIIi in Rat Plasma. J. Chromatogr. B 2002, 772, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Correia, I.; Borovic, S.; Cavaco, I.; Matos, C.P.; Roy, S.; Santos, H.M.; Fernandes, L.; Capelo, J.L.; Ruiz-Azuara, L.; Pessoa, J.C. Evaluation of the Binding of Four Anti-Tumor Casiopeínas® to Human Serum Albumin. J. Inorg. Biochem. 2017, 175, 284–297. [Google Scholar] [CrossRef]
- Serment-Guerrero, J.; Cano-Sanchez, P.; Reyes-Perez, E.; Velazquez-Garcia, F.; Bravo-Gomez, M.E.; Ruiz-Azuara, L. Genotoxicity of the Copper Antineoplastic Coordination Complexes Casiopeinas. Toxicol. Vitr. 2011, 25, 1376–1384. [Google Scholar] [CrossRef]
- Valencia-Cruz, A.I.; Uribe-Figueroa, L.I.; Galindo-Murillo, R.; Baca-Lopez, K.; Gutierrez, A.G.; Vazquez-Aguirre, A.; Ruiz-Azuara, L.; Hernandez-Lemus, E.; Mejía, C. Whole Genome Gene Expression Analysis Reveals Casiopeina-Induced Apoptosis Pathways. PLoS ONE 2013, 8, e54664. [Google Scholar] [CrossRef]
- Kachadourian, R.; Brechbuhl, H.M.; Ruiz-Azuara, L.; Gracia-Mora, I.; Day, B.J. Casiopeína IIgly-Induced Oxidative Stress and Mitochondrial Dysfunction in Human Lung Cancer A549 and H157 Cells. Toxicology 2010, 268, 176–183. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Palencia, G.; Zúniga, S.; Rodríguez-Ropon, A.; Osorio-Rico, L.; Luvia, S.T.; Gracia-Mora, I.; Marquez-Rosado, L.; Sánchez, A.; Moreno-García, M.E. Cas Ilgly Induces Apoptosis in Glioma C6 Cells in Vitro and in Vivo through Caspase-Dependent and Caspase-Independent Mechanisms. Neoplasia 2005, 7, 563–574. [Google Scholar] [CrossRef]
- Gutiérrez, A.G.; Vázquez-Aguirre, A.; García-Ramos, J.C.; Flores-Alamo, M.; Hernández-Lemus, E.; Ruiz-Azuara, L.; Mejía, C. Copper(Ii) Mixed Chelate Compounds Induce Apoptosis through Reactive Oxygen Species in Neuroblastoma Cell Line Chp-212. J. Inorg. Biochem. 2013, 126, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Mao, Y.-P.; OuYang, P.-Y.; Tang, J.; Lan, X.-W.; Xie, F.-Y. Leucopenia and Treatment Efficacy in Advanced Nasopharyngeal Carcinoma. BMC Cancer 2015, 15, 429. [Google Scholar] [CrossRef] [PubMed]
- Pitekova, B.; Uhlikova, E.; Kupcova, V.; Durfinova, M.; Mojto, V.; Turecky, L. Can Alpha-1-Acid Glycoprotein Affect the Outcome of Treatment in a Cancer Patient? Bratisl. Med. J. 2019, 120, 9–14. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does It Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.J.; Miyamoto, S. Hexokinase II Integrates Energy Metabolism and Cellular Protection: Akting on Mitochondria and TORCing to Autophagy. Cell Death Differ. 2015, 22, 248–257. [Google Scholar] [CrossRef]
- Silva, J.A.; Queirós, O.; Baltazar, F.; Ułaszewski, S.; Ko, Y.H.; Pedersen, P.L.; Preto, A.; Casal, M. The Anticancer Agent 3-Bromopyruvate: A Simple but Powerful Molecule Taken from the Lab to the Bedside. J. Bioenerg. Biomembr. 2016, 48, 349–362. [Google Scholar] [CrossRef]
- Rai, Y.; Yadav, P.; Kumari, N.; Kalra, N.; Bhatt, A.N. Hexokinase II Inhibition by 3-Bromopyruvate Sensitizes Myeloid Leukemic Cells K-562 to Anti-Leukemic Drug, Daunorubicin. Biosci. Rep. 2019, 39, BSR20190880. [Google Scholar] [CrossRef]
- Marín-Hernández, A.; Gallardo-Pérez, J.C.; López-Ramírez, S.Y.; García-García, J.D.; Rodríguez-Zavala, J.S.; Ruiz-Ramírez, L.; Gracia-Mora, I.; Zentella-Dehesa, A.; Sosa-Garrocho, M.; Macías-Silva, M. Casiopeina II-Gly and Bromo-Pyruvate Inhibition of Tumor Hexokinase, Glycolysis, and Oxidative Phosphorylation. Arch. Toxicol. 2012, 86, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Boengler, K.; Schulz, R. Inhibition of Mitochondrial Permeability Transition Pore Opening: The Holy Grail of Cardioprotection. Basic Res. Cardiol. 2010, 105, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Arfin, S.; Jha, N.K.; Jha, S.K.; Kesari, K.K.; Ruokolainen, J.; Roychoudhury, S.; Rathi, B.; Kumar, D. Oxidative Stress in Cancer Cell Metabolism. Antioxidants 2021, 10, 642. [Google Scholar] [CrossRef]
- Giles, N.M.; Watts, A.B.; Giles, G.I.; Fry, F.H.; Littlechild, J.A.; Jacob, C. Metal and Redox Modulation of Cysteine Protein Function. Chem. Biol. 2003, 10, 677–693. [Google Scholar] [CrossRef]
- Ramírez-Palma, L.G.; Espinoza-Guillén, A.; Nieto-Camacho, F.; López-Guerra, A.E.; Gómez-Vidales, V.; Cortés-Guzmán, F.; Ruiz-Azuara, L. Intermediate Detection in the Casiopeina–Cysteine Interaction Ending in the Disulfide Bond Formation and Copper Reduction. Molecules 2021, 26, 5729. [Google Scholar] [CrossRef] [PubMed]
- Ong, W.K.; Jana, D.; Zhao, Y. A Glucose-Depleting Silica Nanosystem for Increasing Reactive Oxygen Species and Scavenging Glutathione in Cancer Therapy. Chem. Commun. 2019, 55, 13374–13377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Sun, H.; Xu, H.; Qiu, S.; Wang, X. Cell Metabolomics. OMICS 2013, 17, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Danzi, F.; Pacchiana, R.; Mafficini, A.; Scupoli, M.T.; Scarpa, A.; Donadelli, M.; Fiore, A. To Metabolomics and beyond: A Technological Portfolio to Investigate Cancer Metabolism. Signal Transduct. Target. Ther. 2023, 8, 137. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Li, Q.; Chen, Y.; Yang, Y. Recent Metabolomics Analysis in Tumor Metabolism Reprogramming. Front. Mol. Biosci. 2021, 8, 763902. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Patel, R.; Kirsch, D.G.; Lewis, C.A.; Vander Heiden, M.G.; Locasale, J.W. Metabolomics in Cancer Research and Emerging Applications in Clinical Oncology. CA Cancer J. Clin. 2021, 71, 333–358. [Google Scholar] [CrossRef]
- Vermathen, M.; Paul, L.E.H.; Diserens, G.; Vermathen, P.; Furrer, J. 1H HR-MAS NMR Based Metabolic Profiling of Cells in Response to Treatment with a Hexacationic Ruthenium Metallaprism as Potential Anticancer Drug. PLoS ONE 2015, 10, e0128478. [Google Scholar] [CrossRef]
- De Castro, F.; Stefàno, E.; De Luca, E.; Muscella, A.; Marsigliante, S.; Benedetti, M.; Fanizzi, F.P. A NMR-Based Metabolomic Approach to Investigate the Antitumor Effects of the Novel [Pt(H1-C2H4OMe)(DMSO)(Phen)] + (Phen = 1,10-Phenanthroline) Compound on Neuroblastoma Cancer Cells. Bioinorg. Chem. Appl. 2022, 2022, 8932137. [Google Scholar] [CrossRef]
- De Castro, F.; Benedetti, M.; Del Coco, L.; Fanizzi, F.P. NMR-Based Metabolomics in Metal-Based Drug Research. Molecules 2019, 24, 2240. [Google Scholar] [CrossRef]
- Yang, R.; Li, Y.; Wang, H.; Qin, T.; Yin, X.; Ma, X. Therapeutic Progress and Challenges for Triple Negative Breast Cancer: Targeted Therapy and Immunotherapy. Mol. Biomed. 2022, 3, 8. [Google Scholar] [CrossRef]
- Almansour, N.M. Triple-Negative Breast Cancer: A Brief Review about Epidemiology, Risk Factors, Signaling Pathways, Treatment and Role of Artificial Intelligence. Front. Mol. Biosci. 2022, 9, 836417. [Google Scholar] [CrossRef]
- Kumar, P.; Aggarwal, R. An Overview of Triple-Negative Breast Cancer. Arch. Gynecol. Obstet. 2016, 293, 247–269. [Google Scholar] [CrossRef]
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic Reprogramming in Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 428. [Google Scholar] [CrossRef]
- Nong, S.; Han, X.; Xiang, Y.; Qian, Y.; Wei, Y.; Zhang, T.; Tian, K.; Shen, K.; Yang, J.; Ma, X. Metabolic Reprogramming in Cancer: Mechanisms and Therapeutics. MedComm 2023, 4, e218. [Google Scholar] [CrossRef]
- Scatena, C.; Naccarato, A.G.; Ozsvari, B.; Scumaci, D. Metabolic Reprogramming in Breast Cancer. Front. Oncol. 2022, 12, 1081171. [Google Scholar] [CrossRef]
- Maria, R.M.; Altei, W.F.; Selistre-de-Araujo, H.S.; Colnago, L.A. Impact of Chemotherapy on Metabolic Reprogramming: Characterization of the Metabolic Profile of Breast Cancer MDA-MB-231 Cells Using 1H HR-MAS NMR Spectroscopy. J. Pharm. Biomed. Anal. 2017, 146, 324–328. [Google Scholar] [CrossRef]
- Gupta, S.; Roy, A.; Dwarakanath, B.S. Metabolic Cooperation and Competition in the Tumor Microenvironment: Implications for Therapy. Front. Oncol. 2017, 7, 68. [Google Scholar] [CrossRef]
- Tan, L.T.-H.; Chan, K.-G.; Pusparajah, P.; Lee, W.-L.; Chuah, L.-H.; Khan, T.M.; Lee, L.-H.; Goh, B.-H. Targeting Membrane Lipid a Potential Cancer Cure? Front. Pharmacol. 2017, 8, 12. [Google Scholar] [CrossRef]
- Hill, D.P.; Harper, A.; Malcolm, J.; McAndrews, M.S.; Mockus, S.M.; Patterson, S.E.; Reynolds, T.; Baker, E.J.; Bult, C.J.; Chesler, E.J. Cisplatin-Resistant Triple-Negative Breast Cancer Subtypes: Multiple Mechanisms of Resistance. BMC Cancer 2019, 19, 1039. [Google Scholar] [CrossRef]
- Sulaiman, A.; McGarry, S.; Chambers, J.; Al-Kadi, E.; Phan, A.; Li, L.; Mediratta, K.; Dimitroulakos, J.; Addison, C.; Li, X.; et al. Targeting Hypoxia Sensitizes TNBC to Cisplatin and Promotes Inhibition of Both Bulk and Cancer Stem Cells. Int. J. Mol. Sci. 2020, 21, 5788. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Raimondi, V.; Ciccarese, F.; Ciminale, V. Oncogenic Pathways and the Electron Transport Chain: A DangeROS Liaison. Br. J. Cancer 2020, 122, 168–181. [Google Scholar] [CrossRef]
- Phan, L.M.; Yeung, S.-C.J.; Lee, M.-H. Cancer Metabolic Reprogramming: Importance, Main Features, and Potentials for Precise Targeted Anti-Cancer Therapies. Cancer Biol. Med. 2014, 11, 1. [Google Scholar]
- Wang, Z.; Dong, C. Gluconeogenesis in Cancer: Function and Regulation of PEPCK, FBPase, and G6Pase. Trends Cancer 2019, 5, 30–45. [Google Scholar] [CrossRef]
- Ma, Y.; Temkin, S.M.; Hawkridge, A.M.; Guo, C.; Wang, W.; Wang, X.-Y.; Fang, X. Fatty Acid Oxidation: An Emerging Facet of Metabolic Transformation in Cancer. Cancer Lett. 2018, 435, 92–100. [Google Scholar] [CrossRef]
- Ali, I.; Rahis-Uddin, K.; Salim, K.; Rather, M.; Wani, W.; Haque, A. Advances in Nano Drugs for Cancer Chemotherapy. Curr. Cancer Drug Targets 2011, 11, 135–146. [Google Scholar] [CrossRef]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Miranda-Calderón, J.E.; Macías-Rosales, L.; Gracia-Mora, I.; Ruiz-Azuara, L.; Faustino-Vega, A.; Gracia-Mora, J.; Bernad-Bernad, M.J. Effect of Casiopein III-Ia Loaded into Chitosan Nanoparticles on Tumor Growth Inhibition. J. Drug Deliv. Sci. Technol. 2018, 48, 1–8. [Google Scholar] [CrossRef]
- Zagalo, D.M.; Sousa, J.; Simões, S. Quality by Design (QbD) Approach in Marketing Authorization Procedures of Non-Biological Complex Drugs: A Critical Evaluation. Eur. J. Pharm. Biopharm. 2022, 178, 1–24. [Google Scholar] [CrossRef]
- Rawal, M.; Singh, A.; Amiji, M.M. Quality-by-Design Concepts to Improve Nanotechnology-Based Drug Development. Pharm. Res. 2019, 36, 153. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, Z.; González-Ballesteros, M.; Dávila-Manzanilla, S.G.; Espinoza-Guillén, A.; Ruiz-Azuara, L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper (II) Compound (Casiopeina III-Ia) Loaded in Nonionic Vesicles Using Quality by Design. Int. J. Mol. Sci. 2022, 23, 12756. [Google Scholar] [CrossRef]
- Nave, M.; Castro, R.E.; Rodrigues, C.M.P.; Casini, A.; Soveral, G.; Gaspar, M.M. Nanoformulations of a Potent Copper-Based Aquaporin Inhibitor with Cytotoxic Effect against Cancer Cells. Nanomedicine 2016, 11, 1817–1830. [Google Scholar] [CrossRef] [PubMed]
- Malekshah, R.E.; Salehi, M.; Kubicki, M.; Khaleghian, A. Synthesis, Structure, Computational Modeling and Biological Activity of Two New Casiopeínas® Complexes and Their Nanoparticles. J. Coord. Chem. 2019, 72, 2233–2250. [Google Scholar] [CrossRef]
- Chen, M.; Huang, Z.; Xia, M.; Ding, Y.; Shan, T.; Guan, Z.; Dai, X.; Xu, X.; Huang, Y.; Huang, M.; et al. Glutathione-Responsive Copper-Disulfiram Nanoparticles for Enhanced Tumor Chemotherapy. J. Control. Release 2022, 341, 351–363. [Google Scholar] [CrossRef]
- Fan, Y.; Zhang, J.; Shi, M.; Li, D.; Lu, C.; Cao, X.; Peng, C.; Mignani, S.; Majoral, J.-P.; Shi, X. Poly(Amidoamine) Dendrimer-Coordinated Copper(II) Complexes as a Theranostic Nanoplatform for the Radiotherapy-Enhanced Magnetic Resonance Imaging and Chemotherapy of Tumors and Tumor Metastasis. Nano Lett. 2019, 19, 1216–1226. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, L.; Jiang, Q.; Huo, M.; Lin, H.; Wang, L.; Chen, Y.; Shi, J. Enhanced Tumor-Specific Disulfiram Chemotherapy by In Situ Cu2+ Chelation-Initiated Nontoxicity-to-Toxicity Transition. J. Am. Chem. Soc. 2019, 141, 11531–11539. [Google Scholar] [CrossRef]
- Kheirolomoom, A.; Mahakian, L.M.; Lai, C.-Y.; Lindfors, H.A.; Seo, J.W.; Paoli, E.E.; Watson, K.D.; Haynam, E.M.; Ingham, E.S.; Xing, L.; et al. Copper−Doxorubicin as a Nanoparticle Cargo Retains Efficacy with Minimal Toxicity. Mol. Pharm. 2010, 7, 1948–1958. [Google Scholar] [CrossRef]
- Peña, Q.; Wang, A.; Zaremba, O.; Shi, Y.; Scheeren, H.W.; Metselaar, J.M.; Kiessling, F.; Pallares, R.M.; Wuttke, S.; Lammers, T. Metallodrugs in Cancer Nanomedicine. Chem. Soc. Rev. 2022, 51, 2544–2582. [Google Scholar] [CrossRef]
- Santini, C.; Pellei, M.; Gandin, V.; Porchia, M.; Tisato, F.; Marzano, C. Advances in Copper Complexes as Anticancer Agents. Chem. Rev. 2014, 114, 815–862. [Google Scholar] [CrossRef]
- Gallagher, J.; Chen, C.B.; Pan, C.Q.; Perrin, D.M.; Cho, Y.-M.; Sigman, D.S. Optimizing the Targeted Chemical Nuclease Activity of 1, 10-Phenanthroline−Copper by Ligand Modification. Bioconjug. Chem. 1996, 7, 413–420. [Google Scholar] [CrossRef]
- Hirohama, T.; Kuranuki, Y.; Ebina, E.; Sugizaki, T.; Arii, H.; Chikira, M.; Tamil Selvi, P.; Palaniandavar, M. Copper(II) Complexes of 1,10-Phenanthroline-Derived Ligands: Studies on DNA Binding Properties and Nuclease Activity. J. Inorg. Biochem. 2005, 99, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Lozada-García, M.C.; Enríquez, R.G.; Ramírez-Apán, T.O.; Nieto-Camacho, A.; Palacios-Espinosa, J.F.; Custodio-Galván, Z.; Soria-Arteche, O.; Pérez-Villanueva, J. Synthesis of Curcuminoids and Evaluation of Their Cytotoxic and Antioxidant Properties. Molecules 2017, 22, 633. [Google Scholar] [CrossRef]
- Kunwar, A.; Simon, E.; Singh, U.; Chittela, R.K.; Sharma, D.; Sandur, S.K.; Priyadarsini, I.K. Interaction of a Curcumin Analogue Dimethoxycurcumin with DNA. Chem. Biol. Drug Des. 2011, 77, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.; Gordon, O.N.; Edwards, R.L.; Luis, P.B. Degradation of Curcumin: From Mechanism to Biological Implications. J. Agric. Food Chem. 2015, 63, 7606–7614. [Google Scholar] [CrossRef]
- Meza-Morales, W.; Estévez-Carmona, M.M.; Alvarez-Ricardo, Y.; Obregón-Mendoza, M.A.; Cassani, J.; Ramírez-Apan, M.T.; Escobedo-Martínez, C.; Soriano-García, M.; Reynolds, W.F.; Enríquez, R.G. Full Structural Characterization of Homoleptic Complexes of Diacetylcurcumin with Mg, Zn, Cu, and Mn: Cisplatin-Level Cytotoxicity in Vitro with Minimal Acute Toxicity in Vivo. Molecules 2019, 24, 1598. [Google Scholar] [CrossRef]
- Prisecaru, A.; McKee, V.; Howe, O.; Rochford, G.; McCann, M.; Colleran, J.; Pour, M.; Barron, N.; Gathergood, N.; Kellett, A. Regulating Bioactivity of Cu2+ Bis-1,10-Phenanthroline Artificial Metallonucleases with Sterically Functionalized Pendant Carboxylates. J. Med. Chem. 2013, 56, 8599–8615. [Google Scholar] [CrossRef] [PubMed]
- Kubešová, K.; Dořičáková, A.; Trávníček, Z.; Dvořák, Z. Mixed-Ligand Copper (II) Complexes Activate Aryl Hydrocarbon Receptor AhR and Induce CYP1A Genes Expression in Human Hepatocytes and Human Cell Lines. Toxicol. Lett. 2016, 255, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Q.; Lin, F.; Zhu, L.; Huang, F.; Zhao, L.; Ou, R. Casiopeina II-gly Acts on LncRNA MALAT1 by MiR-17-5p to Inhibit FZD2 Expression via the Wnt Signaling Pathway during the Treatment of Cervical Carcinoma. Oncol. Rep. 2019, 42, 1365–1379. [Google Scholar] [CrossRef] [PubMed]
- Rajalakshmi, S.; Kiran, M.S.; Nair, B.U. DNA Condensation by Copper (II) Complexes and Their Anti-Proliferative Effect on Cancerous and Normal Fibroblast Cells. Eur. J. Med. Chem. 2014, 80, 393–406. [Google Scholar] [CrossRef]
- Correia, I.; Roy, S.; Matos, C.P.; Borovic, S.; Butenko, N.; Cavaco, I.; Marques, F.; Lorenzo, J.; Rodríguez, A.; Moreno, V. Vanadium (IV) and Copper (II) Complexes of Salicylaldimines and Aromatic Heterocycles: Cytotoxicity, DNA Binding and DNA Cleavage Properties. J. Inorg. Biochem. 2015, 147, 134–146. [Google Scholar] [CrossRef]
- Kordestani, N.; Rudbari, H.A.; Fernandes, A.R.; Raposo, L.R.; Baptista, P.V.; Ferreira, D.; Bruno, G.; Bella, G.; Scopelliti, R.; Braun, J.D. Antiproliferative Activities of Diimine-Based Mixed Ligand Copper (II) Complexes. ACS Comb. Sci. 2020, 22, 89–99. [Google Scholar] [CrossRef]
- Zoroddu, M.A.; Aaseth, J.; Crisponi, G.; Medici, S.; Peana, M.; Nurchi, V.M. The Essential Metals for Humans: A Brief Overview. J. Inorg. Biochem. 2019, 195, 120–129. [Google Scholar] [CrossRef]
- Komarnicka, U.K.; Kozieł, S.; Zabierowski, P.; Kruszyński, R.; Lesiow, M.K.; Tisato, F.; Porchia, M.; Kyzioł, A. Copper (I) Complexes with Phosphines P (p-OCH3-Ph) 2CH2OH and P (p-OCH3-Ph) 2CH2SarGly. Synthesis, Multimodal DNA Interactions, and Prooxidative and in Vitro Antiproliferative Activity. J. Inorg. Biochem. 2020, 203, 110926. [Google Scholar] [CrossRef]
- Alvarez, N.; Viña, D.; Leite, C.M.; Mendes, L.F.S.; Batista, A.A.; Ellena, J.; Costa-Filho, A.J.; Facchin, G. Synthesis and Structural Characterization of a Series of Ternary Copper (II)-L-Dipeptide-Neocuproine Complexes. Study of Their Cytotoxicity against Cancer Cells Including MDA-MB-231, Triple Negative Breast Cancer Cells. J. Inorg. Biochem. 2020, 203, 110930. [Google Scholar] [CrossRef]
- Martínez-Valencia, B.; Corona-Motolinia, N.D.; Sánchez-Lara, E.; Noriega, L.; Sanchez-Gaytan, B.L.; Castro, M.E.; Melendez-Bustamante, F.; González-Vergara, E. Cyclo-Tetravanadate Bridged Copper Complexes as Potential Double Bullet pro-Metallodrugs for Cancer Treatment. J. Inorg. Biochem. 2020, 208, 111081. [Google Scholar] [CrossRef]
- Chen, Z.-F.; Tan, M.-X.; Liu, L.-M.; Liu, Y.-C.; Wang, H.-S.; Yang, B.; Peng, Y.; Liu, H.-G.; Liang, H.; Orvig, C. Cytotoxicity of the Traditional Chinese Medicine (TCM) Plumbagin in Its Copper Chemistry. Dalton Trans. 2009, 48, 10824–10833. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, M.; Kellett, A.; McCann, M.; Rosair, G.; McNamara, M.; Howe, O.; Creaven, B.S.; McClean, S.; Foltyn-Arfa Kia, A.; O’Shea, D.; et al. Copper(II) Complexes of Salicylic Acid Combining Superoxide Dismutase Mimetic Properties with DNA Binding and Cleaving Capabilities Display Promising Chemotherapeutic Potential with Fast Acting in Vitro Cytotoxicity against Cisplatin Sensitive and Resista. J. Med. Chem. 2012, 55, 1957–1968. [Google Scholar] [CrossRef]
- Zhang, Z.; Bi, C.; Schmitt, S.M.; Fan, Y.; Dong, L.; Zuo, J.; Dou, Q.P. 1,10-Phenanthroline Promotes Copper Complexes into Tumor Cells and Induces Apoptosis by Inhibiting the Proteasome Activity. JBIC J. Biol. Inorg. Chem. 2012, 17, 1257–1267. [Google Scholar] [CrossRef] [PubMed]
- Levín, P.; Ruiz, M.C.; Romo, A.I.B.; Nascimento, O.R.; Di Virgilio, A.L.; Oliver, A.G.; Ayala, A.P.; Diógenes, I.C.N.; León, I.E.; Lemus, L. Water-Mediated Reduction of [Cu(Dmp)2(CH3CN)]2+: Implications of the Structure of a Classical Complex on Its Activity as an Anticancer Drug. Inorg. Chem. Front. 2021, 8, 3238–3252. [Google Scholar] [CrossRef]
- Itoh, S. Chemical Reactivity of Copper Active-Oxygen Complexes. In Copper-Oxygen Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 225–282. ISBN 9781118094365. [Google Scholar]
- Denoyer, D.; Masaldan, S.; La Fontaine, S.; Cater, M.A. Targeting Copper in Cancer Therapy: “Copper That Cancer”. Metallomics 2015, 7, 1459–1476. [Google Scholar] [CrossRef]
- Balsa, L.M.; Baran, E.J.; León, I.E. Copper Complexes as Antitumor Agents: In Vitro and In Vivo Evidence. Curr. Med. Chem. 2023, 30, 510–557. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.R.; Caleffi-Ferracioli, K.R.; Leite, C.Q.F.; García-Ramos, J.C.; Toledano-Magaña, Y.; Ruiz-Azuara, L.; Siqueira, V.L.D.; Pavan, F.R.; Cardoso, R.F. Potential of Casiopeínas® Copper Complexes and Antituberculosis Drug Combination against Mycobacterium Tuberculosis. Chemotherapy 2016, 61, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Becco, L.; Rodríguez, A.; Bravo, M.E.; Prieto, M.J.; Ruiz-Azuara, L.; Garat, B.; Moreno, V.; Gambino, D. New Achievements on Biological Aspects of Copper Complexes Casiopeínas®: Interaction with DNA and Proteins and Anti-Trypanosoma Cruzi Activity. J. Inorg. Biochem. 2012, 109, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Masuri, S.; Vaňhara, P.; Cabiddu, M.G.; Moráň, L.; Havel, J.; Cadoni, E.; Pivetta, T. Copper(Ii) Phenanthroline-Based Complexes as Potential Anticancer Drugs: A Walkthrough on the Mechanisms of Action. Molecules 2022, 27, 49. [Google Scholar] [CrossRef]
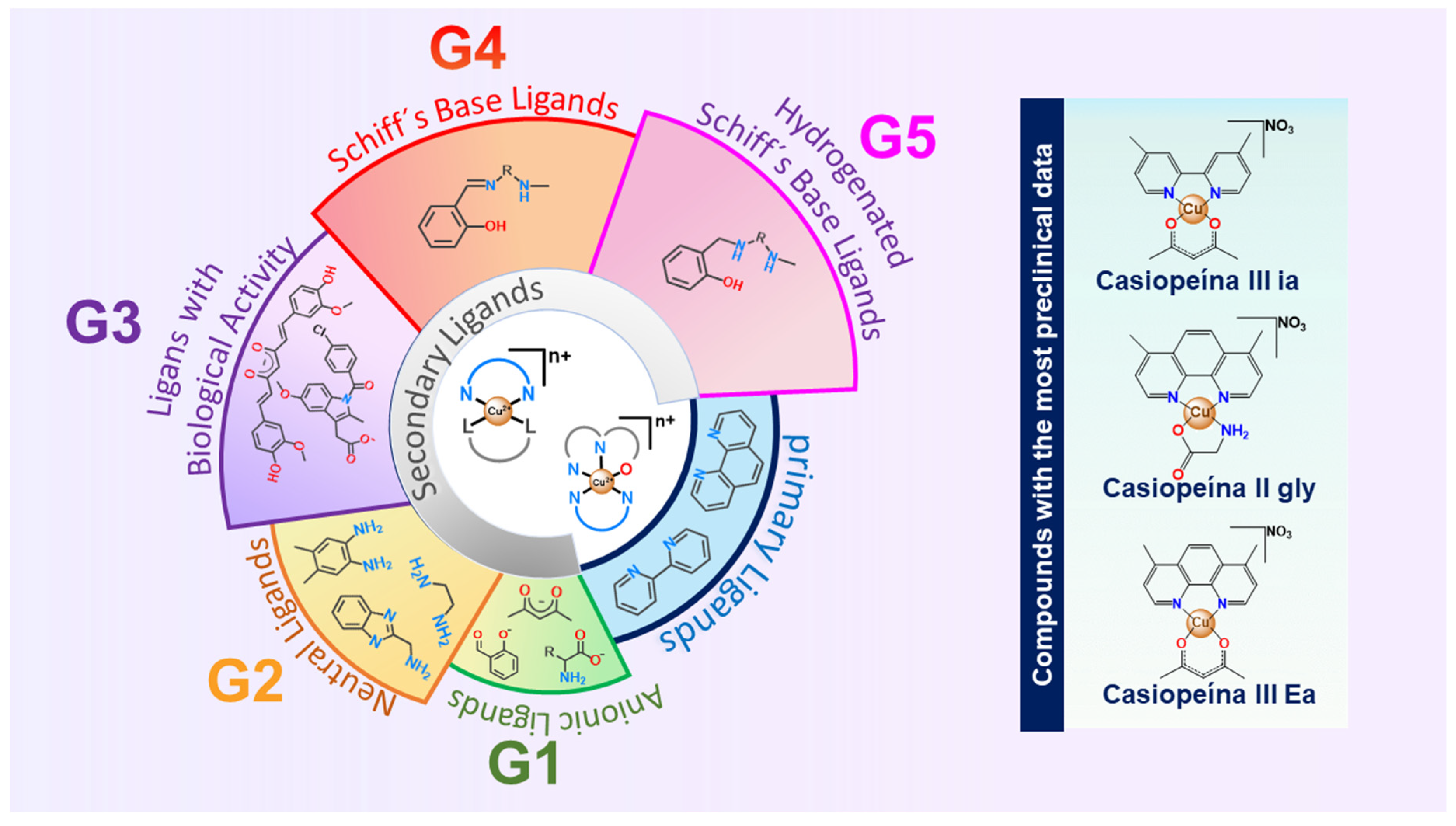
| Common Name | Formula | Short Common Name |
|---|---|---|
| Casiopeína I gly | [Cu(4,7-diphenyl-1,10-phenanthroline)(glycinate)]NO3 | CasIgly |
| Casiopeína II gly | [Cu(4,7-dimethyl-1,10-phenanthroline)(glycinate)]NO3 | CasIIgly |
| Casiopeína III ia | [Cu(4,4′ Dimethyl 2,2′ dipyridyl)(acac)]NO3 | CasIIIia |
| Casiopeína III Ja | [Cu(3,4,7,8-Tetramethyl-1,10-phenanthroline)(acac)]NO3 | CasIIIJa |
| Casiopeína III Ea | [Cu(4,7-dimethyl-1,10-phenanthroline)(acac)]NO3 | CasIIIEa |
| Casiopeína III La | [Cu(5,6-dimethyl-1,10-phenanthroline)(acac)]NO3 | CasIIILa |
| Casiopeína IV gly | [Cu(4,4′ Dimethyl 2,2′ dipyridyl)(glycinate)]NO3 | CasIVgly |
| Casiopeína VIII gly | [Cu(3,4,7,8-Tetramethyl-1,10-phenanthroline)(glycinate)]NO3 | CasVIIIgly |
| Cisplatin | cis-[Pt(NH3)2Cl2] | CDDP |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Jiménez, Z.; Espinoza-Guillén, A.; Resendiz-Acevedo, K.; Fuentes-Noriega, I.; Mejía, C.; Ruiz-Azuara, L. The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities). Inorganics 2023, 11, 394. https://doi.org/10.3390/inorganics11100394
Aguilar-Jiménez Z, Espinoza-Guillén A, Resendiz-Acevedo K, Fuentes-Noriega I, Mejía C, Ruiz-Azuara L. The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities). Inorganics. 2023; 11(10):394. https://doi.org/10.3390/inorganics11100394
Chicago/Turabian StyleAguilar-Jiménez, Zenayda, Adrián Espinoza-Guillén, Karen Resendiz-Acevedo, Inés Fuentes-Noriega, Carmen Mejía, and Lena Ruiz-Azuara. 2023. "The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities)" Inorganics 11, no. 10: 394. https://doi.org/10.3390/inorganics11100394